Abstract
To date, hematopoietic development of human embryonic stem cells (hESCs) has been limited to cell lines cultured in the presence of either mouse embryonic fibroblasts (MEFs) or MEF-conditioned media (MEF-CM). Anonymous xenogenic factors from MEFs or MEF-CM complicate studies of hESC self-renewal and also raise concerns for the potential clinical applications of generating primitive hematopoietic cells from hESC lines maintained under these ambiguous conditions. Here, we demonstrate that hESCs can be cultured over 30 passages in defined conditions in the absence of MEFs or MEF-CM using only serum replacement (SR) media and high concentrations of basic fibroblast growth factor (SR-bFGF). Similar to hESCs cultured in MEF-CM, hESCs cultured in SR-bFGF sustained characteristics of undifferentiated hESCs, proliferative potential, normal karyotype, in vitro and in vivo 3 germ-layer specification and gave rise to hemogenic-endothelial precursors required for subsequent primitive hematopoietic development. Our report demonstrates that anonymous factors produced by feeder cells are not necessary for hESC maintenance and subsequent hematopoietic specification, thereby providing a defined system for studies of hESC self-renewal and hESC-derived hematopoiesis. (Blood. 2005;105:4598-4603)
Introduction
Human embryonic stem cell (hESC) lines have been derived from the inner cell mass of human embryos by coculture on either mouse or human embryonic feeder layers.1,2 To better define the conditions to propagate and differentiate hESC lines, Xu et al maintained hESCs in feeder-free conditions by feeding them with mouse embryonic fibroblast-conditioned media (MEF-CM).3 However, significant variations in the production of MEFs and harvesting of MEF-CM have hampered reproducibility of these conditions to culture hESCs stably.4 Moreover, several unknown substances and combinations of factors contained in MEF-CM5 further complicate fundamental studies of hESC self-renewal, impact future studies in defining factors that specify lineage maturation, and also become a concern for future clinical applications. Although hESCs have been documented to be capable of hematopoietic specification, maintenance of these hESCs required either MEFs or MEF-CM.6-14 In this report, we demonstrate that anonymous factors produced by feeder cells are not necessary for hESC maintenance and subsequent hematopoietic development, thereby providing a more defined system for further characterization of hESC self-renewal and lineage maturation.
Materials and methods
Human ESC culture and human embryoid body (hEB) formation
The hESC lines H9 and H11 cultured in MEF-CM3,7 were passed into either MEF-CM or serum replacement (SR) media containing a high concentration of basic fibroblast growth factor (SR-bFGF). MEF-CM was collected as previously described3,7 and contained 12 ng/mL bFGF. SR-bFGF media consisted of 80% knock-out Dulbecco modified Eagle medium (KO-DMEM; Gibco, Burlington, ON, Canada), 20% SR, 24 to 36 ng/mL bFGF (variants among batches), 1% nonessential amino acids, 1 mM l-glutamine (all from Gibco), and 0.1 mM β-mercaptoethanol (Sigma, Oakville, ON, Canada). Maintenance of hESC lines was performed as previously described.3,7 In brief, hESC colonies were dissociated with collagenase IV (Gibco) for 5 minutes and passed every 6 to 7 days. Occasionally, hESC colonies maintained either in MEF-CM or SR-bFGF were physically dissociated during passaging to reduce the numbers of fibroblast-like cells. The effect of bFGF on maintenance of hESCs was determined by use of bFGF neutralization antibody (Abcam, Cambridge, MA).15 Formation of human embryoid bodies (hEBs) and preparation of single cells from hESCs and hEBs were performed as previously described.7
Immunocytochemical and histochemical staining of Oct4 and alkaline phosphatase (AP)
Cultured hESCs were fixed in the culture plates with 10% buffered formalin. Permeabilized hESCs were incubated for 30 minutes with 3 μg/mL of either primary goat anti-Oct4 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or normal goat immunoglobulin G (IgG) isotype (Sigma, St Louis, MO) or antibody diluent alone and visualized with Alexa Fluor 488-conjugated donkey anti-goat IgG secondary antibody (1:400; Molecular Probes, Eugene, OR). AP activity staining was then performed as previously described7 with Vector Red substrate (Vector Laboratories, Burlingame, CA) and followed by DAPI (4,6 diamidino-2-phenylindole) counter-staining. The cells were examined with an Olympus fluorescent microscope (Olympus, Tokyo, Japan).
Assessment of undifferentiated markers by flow cytometry
Expression of Oct4, Tra-1-60, Tra-1-81, and stage-specific embryonic antigen-4 (SSEA-4) was considered as undifferentiated indicators of hESCs and analyzed by flow cytometry. For intracellular Oct4 assay, 100 μL dissociated hESCs (1 × 105 cells) were fixed for 15 minutes by addition of 100 μL fixation solution (Caltag Laboratories, Burlingame, CA). After washing with 1 mL phosphate-buffered saline (PBS) and centrifugation at 1800g for 3 minutes, the cells were treated with permeabilization solution for 5 minutes and then stained with 1 μL primary mouse anti-Oct4 antibody or normal mouse IgG isotype (BD, San Jose, CA) for 20 minutes. The cells were washed once prior to staining with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Sigma, Oakville, ON, Canada) for 20 minutes and washed twice prior to flow cytometry acquisition. For detection of Tra-1-60 and Tra-1-81, primary Tra-1-60 monoclonal antibody (mAb) (3.3 μL) or Tra-1-81 mAb (2.6 μL) (both from Chemicon, Temecula, CA) was preincubated for 30 minutes at 4°C with 2.5 μL FITC-conjugated goat anti-mouse IgG antibody (Immunotech, Marseille, France) in 100 μL PBS containing 3% fetal bovine serum (FBS). Dissociated hESCs were suspended in PBS containing 3% FBS at a concentration of 2 × 104 to 5 × 104 cells per 100 μL and added to the preincubated Tra-1-60 or Tra-1-81. After 30 minutes at 4°C, the cells were washed twice and resuspended in PBS containing 3% FBS and finally stained with 7-aminoactinomycin D (7-AAD) viability dye (Immunotech) at 4 μL/200 μL for 15 minutes at room temperature. Live cells identified by 7-AAD exclusion were analyzed for surface marker expression using FACSCalibur (BD) and Cell Quest software (BD). Cell surface expression of SSEA-4 was determined as previously described.3,7
Cell proliferation and karyotype of hESCs
Characterization of hESC-derived hemogenic precursors and hematopoietic cells
CD45negPFV (CD45-, platelet endothelial cell adhesion molecule-1-positive [PECAM-1+], Flk1-positive, and vascular endothelial [VE]-cadherin-positive) hemogenic precursors and CD45+ hematopoietic cells derived from hESCs were characterized as previously described.9
Colony-forming unit (CFU) assays and Wright Giemsa staining
Human clonogenic progenitor assays were performed by plating hESC derivatives (1 × 104 cells) into methylcellulose H4230 (StemCell Technologies, Vancouver, BC, Canada) supplemented with the following human recombinant growth factors: 50 ng/mL stem cell factor (Amgen, Thousand Oaks, CA), 3 U/mL erythropoietin (Amgen), 10 ng/mL granulocyte monocyte colony-stimulating factor (Novartis, Dorval, QC, Canada), and 10 ng/mL interleukin-3 (IL-3) (R&D, Minneapolis, MN). After incubation for 10 to 14 days at 37°C and 5% CO2 in a humidified atmosphere, colony counts were performed based on standard morphologic characteristics. Isolation of the cells from individual colonies, cytospin preparation, and Wright Giemsa staining were performed as previously described.7
Quantitative real-time PCR (Q-PCR)
Total RNA was extracted and reverse transcribed as described previously.8 An initial regular polyermase chain reaction (PCR) was performed for each gene of interest (GOI) to optimize the conditions and check the amplicon size. The conditions for quantitative real-time (Q-PCR) reactions were reported elsewhere.16 Forward and reverse primer sequences were 5′-gtggctccaggatgttagga-3′ and 5′-gcctgagttcatgttgctga-3′ for HNF3α; 5′-ccggcagaagattgtagagc-3′ and 5′-cgttggacacgttttgattg-3′ for Pax-6; and 5′-tgcaccaccaactgcttagc-3′ and 5′-ggcatggactgtggtcatgag-3′ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The amount of GOI was normalized to the endogenous housekeeping gene GAPDH.
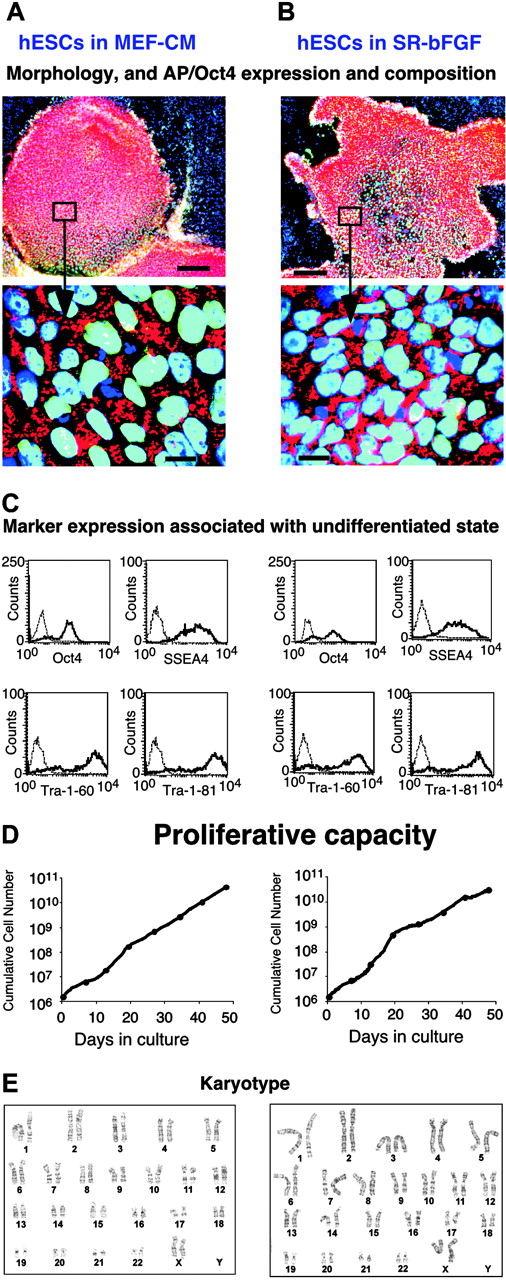
SR-bFGF maintains hESCs in an undifferentiated state. Human ESCs (H9 cell line) were cultured over 14 passages in either MEF-CM (left column) or in SR-bFGF (right column). (A-B) Double alkaline phosphatase (AP) and Oct4 immunostaining. In the undifferentiated area of the colonies, nuclei were costained for Oct4 (green) and DAPI (blue), resulting in a merged color (aqua). The AP (red) was detected in the cytoplasm regions. Bar = 200 μm in row 1 and 20 μm in row 2. (C) Expression of intracellular Oct4 and cell surface markers SSEA-4, Tra-1-60, and Tra-1-81 was analyzed by flow cytometry. Dotted lines indicate isotype controls. (D) Proliferation capacity. (E) Karyotype (21 passage in SR-bFGF culture).
SR-bFGF maintains hESCs in an undifferentiated state. Human ESCs (H9 cell line) were cultured over 14 passages in either MEF-CM (left column) or in SR-bFGF (right column). (A-B) Double alkaline phosphatase (AP) and Oct4 immunostaining. In the undifferentiated area of the colonies, nuclei were costained for Oct4 (green) and DAPI (blue), resulting in a merged color (aqua). The AP (red) was detected in the cytoplasm regions. Bar = 200 μm in row 1 and 20 μm in row 2. (C) Expression of intracellular Oct4 and cell surface markers SSEA-4, Tra-1-60, and Tra-1-81 was analyzed by flow cytometry. Dotted lines indicate isotype controls. (D) Proliferation capacity. (E) Karyotype (21 passage in SR-bFGF culture).
Teratoma formation and histologic analysis
The University of Western Ontario Council On Animal Care approved animal protocols. Male nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice (5 to 7 weeks old) were anesthetized by intraperitoneal injection of 2.5% tribromoethanol (0.14 to 0.18 mL/g of body weight). Small (1 cm) sagittal skin and body wall incisions were made slightly above the penis on the side of the abdominal midline with fine scissors after removing hairs. The testis was positioned fully outside the body on sterile gauze. Fifteen to 20 clumps prepared from SR-bFGF-maintained hESC colonies (each clump containing approximately 200 to 500 cells) were suspended in 20 μL KO-DMEM and injected into one testis capsule of each mouse using an 0.5 mL syringe with a 30 gauge short needle. Incisions were closed with 1 or 2 stitches. All operations were performed in sterile conditions. After surgical operations, each mouse was subcutaneously administered with 0.5 to 1.0 mL buprenorphine (0.05 to 0.1 mg/kg of body weight) and 1 mL of 0.9% saline. Testicular lesions were palpable from about 4 weeks after inoculation. Mice were killed at weeks 6 to 12. Tumors were embedded in frozen tissue embedding gel (Fisher Scientific, Ottawa, ON, Canada), snap-frozen in liquid nitrogen, and stored at -80°C. Cryostat sections (5 μm) were examined histologically after hematoxylin and eosin staining.
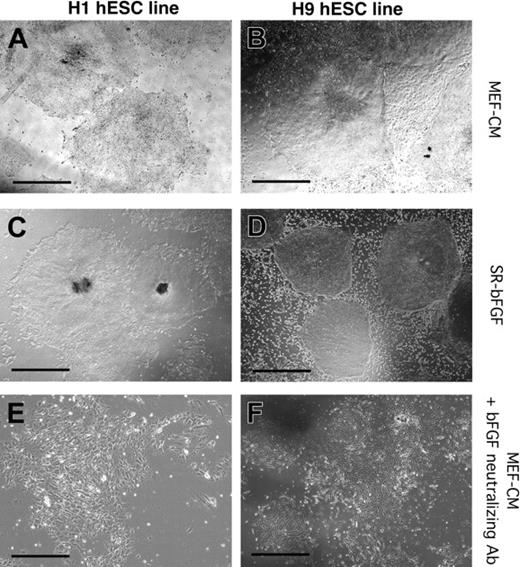
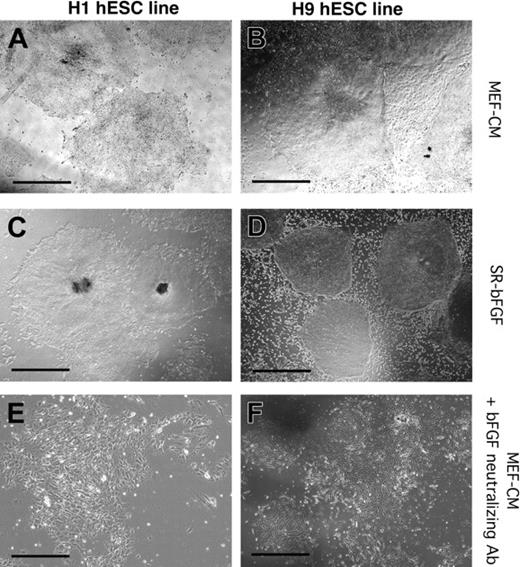
Maintenance of hESC lines H1 and H9 in SR-bFGF conditions and differentiation in response to neutralization of bFGF. H1 (A,C,E) and H9 (B,D,F) hESC lines were cultured in MEF-CM (A-B), SR-bFGF media (C-D), or in the presence of bFGF neutralizing antibody (E-F). The H1 hESC line was maintained in SR-bFGF media for more than 6 passages to date (C) and H9 hESC lines for more than 31 passages to date (D). Addition of bFGF neutralization antibody to hESC lines cultured in MEF-CM induced differentiation and loss of colony integrity. Scale bars = 200 μm.
Maintenance of hESC lines H1 and H9 in SR-bFGF conditions and differentiation in response to neutralization of bFGF. H1 (A,C,E) and H9 (B,D,F) hESC lines were cultured in MEF-CM (A-B), SR-bFGF media (C-D), or in the presence of bFGF neutralizing antibody (E-F). The H1 hESC line was maintained in SR-bFGF media for more than 6 passages to date (C) and H9 hESC lines for more than 31 passages to date (D). Addition of bFGF neutralization antibody to hESC lines cultured in MEF-CM induced differentiation and loss of colony integrity. Scale bars = 200 μm.
Image acquisition
Images in Figures 1, 2, 3 were acquired using an Olympus I×70 microscope (Olympus, Tokyo, Japan) equipped with a CoolSNAP ES digital camera (Roper Scientific, Tucson, AZ). Images in Figures 4 and 5 were acquired using an Olympus I×51 microscope equipped with an Olympus Qcolor 3 digital camera (Olympus, Melville, NY). All images were processed with ImagePro Plus (Media Cybernetics, Silver Spring, MD). The objectives used were Olympus UPlanFl 4× with a numerical aperture (NA) of 0.13, UPlanFl 10×/0.30 NA, or LUCPlanFl 40×/0.60 NA, respectively. The imaging medium was air. The immunofluorescent images in Figure 1 were obtained by taking multiple exposures through bandpass optical filter sets appropriated for Texas Red (AP staining), Endow GFP (Oct4 staining), and DAPI (nuclei staining).
Statistical analysis
Results were expressed as means ± SEM. Statistical significance was determined using an unpaired Student t test. Results were considered significant at P < .05.
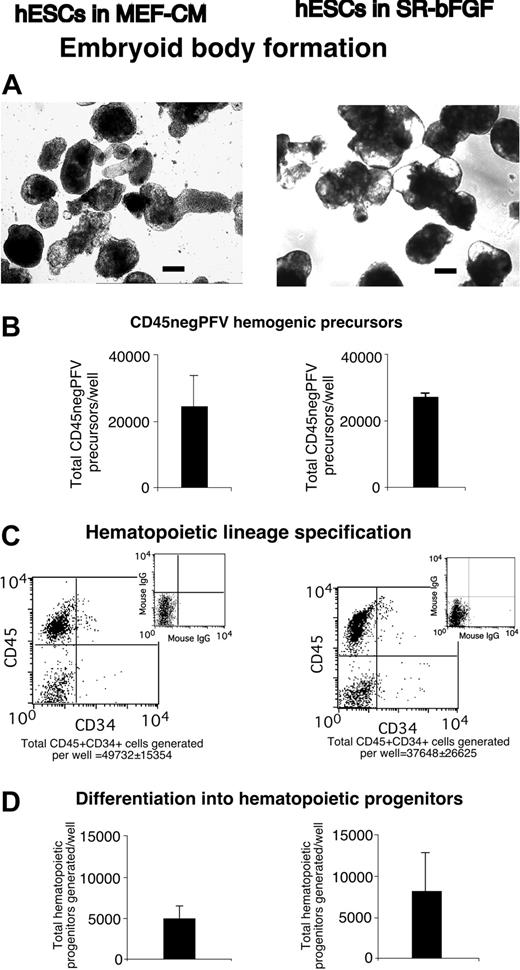
SR-bFGF-maintained hESCs undertake hematopoietic development. In comparison with MEF-CM-cultured hESCs (left column), SR-bFGF-cultured hESCs (right column) displayed the similar following properties after differentiation: (A) formation of hEBs (bar = 100 μm), (B) generation of CD45negPFV hemogenic precursors at day 10 of hEB development,9 (C) expression of the panleukocyte hematopoietic marker CD45 and primitive hematopoietic marker CD34 at day 22 of hEB development, and (D) generation of hematopoietic progenitors determined by clonogenic colony-forming unit assay at day 22 of hEB development.
SR-bFGF-maintained hESCs undertake hematopoietic development. In comparison with MEF-CM-cultured hESCs (left column), SR-bFGF-cultured hESCs (right column) displayed the similar following properties after differentiation: (A) formation of hEBs (bar = 100 μm), (B) generation of CD45negPFV hemogenic precursors at day 10 of hEB development,9 (C) expression of the panleukocyte hematopoietic marker CD45 and primitive hematopoietic marker CD34 at day 22 of hEB development, and (D) generation of hematopoietic progenitors determined by clonogenic colony-forming unit assay at day 22 of hEB development.
Results
SR-bFGF is capable of maintaining undifferentiated hESCs
To determine whether established hESCs could be grown in the absence of factors produced by feeders, we passed the hESC line H9 previously cultured in MEF-CM for 35 passages into either MEF-CM or defined SR-bFGF media conditions that were never directly or indirectly in contact with MEFs. In side-by-side comparisons with the same passage of hESCs cultured in MEF-CM, hESCs cultured in SR-bFGF possessed similar properties. Using hESCs maintained in MEF-CM as our standard, undifferentiated colonies cultured in SR-bFGF had similar morphology, activity of alkaline phosphatase (AP), and expression of Oct417 (Figure 1B). Flow cytometry analysis showed that SR-bFGF-maintained hESCs had comparable levels of Oct4, SSEA4, Tra-1-60, and Tra-1-81 expression (Figure 1C). The hESCs cultured in SR-bFGF also showed identical proliferation capacity to those cultured in MEF-CM (Figure 1D) and normal karyotype (Figure 1E). To assure the support of hESC growth, using defined SR-FGF conditions was not restricted to one cell line alone; another hESC line, H1,1 was also maintained in an undifferentiated state in SR-bFGF over 8 passages (Figure 2A,C).
To examine which components of SR-bFGF conditions were crucial to hESC maintenance in an undifferentiated state, we cultured hESCs in (a) media without SR but supplemented with bovine serum albumin, insulin, transferrin, and 36 ng/mL bFGF; (b) media supplemented with SR in the absence of bFGF or (c) lower concentrations of bFGF (less than 12 ng/mL); and finally in (d) SR-bFGF media without precoating of the culture plates with Matrigel or laminin (the major component in Matrigel). None of the 4 conditions supported hESCs with undifferentiated properties past day 21 (3 passages) of culture (data not shown). Under conditions a, b, and c, hESCs gradually lost their typical colony morphology and diminished expression of undifferentiated markers. Under condition d, hESC colonies were unable to attach to the plate, resulting in cell death after one passage (7 days). The role of bFGF in hESC maintenance was further examined by use of a neutralizing antibody specific to bFGF. Addition of the bFGF-neutralizing antibody to the MEF-CM induced both H1 and H9 hESC differentiation and loss of hESC colony maintenance (Figure 2E-F), suggesting that bFGF is an essential factor released by MEFs to support hESC lines. Collectively, these experiments indicate that the combination of SR media, high concentrations of bFGF, and Matrigel or laminin are crucial for the maintenance of hESCs. In addition, these comparative analyses underscore the importance of long-term culture to define robust conditions for hESC maintenance because hESCs seemingly possess autonomous survival responsiveness over limited (4 to 7 days) short-term culture.
Human ESCs cultured in SR-bFGF are capable of characteristic hematopoietic development
The ability to form embryoid bodies (EBs), and subsequently undergo differentiation, is an important measure of the overall quality of hESC lines.7,8 To determine whether SR-bFGF-maintained hESCs are capable of EB development and differentiation as previously documented for hESCs cultured in MEF-CM,7,8 hESCs were allowed to aggregate into EBs.7 Compared with EBs formed from MEF-CM-cultured hESCs, SR-bFGF-cultured hESCs produced similar number and size of EBs (Figure 3A).
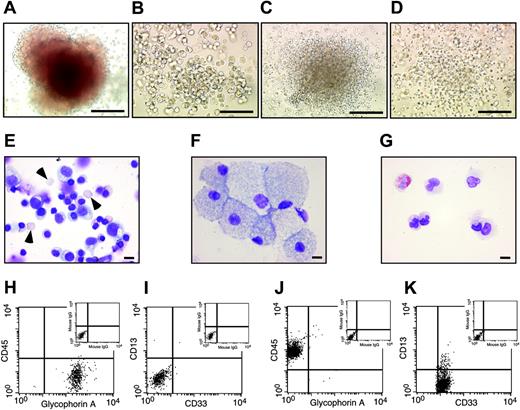
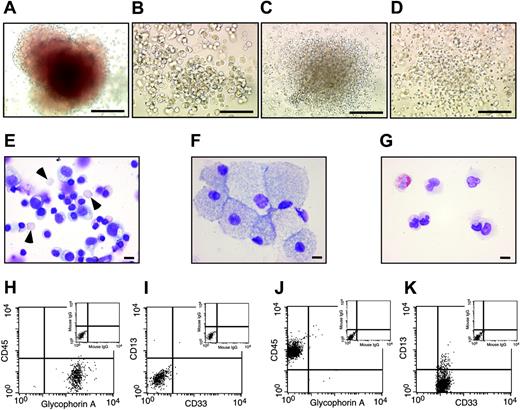
Hematopoietic cells derived from SR-bFGF-maintained hESCs have characteristic hematopoietic progenitor properties. Hematopoietic cells derived from SR-bFGF-maintained hESCs were identical to those derived from MEF-CM-maintained hESCs and produced characteristic hematopoietic CFU subtypes, including erythroid (A), macrophage (B), granulocyte (C), and granulocyte-macrophage colonies (D); scale bars = 100 μm. The cells prepared from an erythroid colony showed mature enucleated erythrocytes (arrowheads) and immature erythrocytes (E), from a macrophage colony showed macrophage morphology (F), and from a granulocyte colony showed mature neutrophils and eosinophils (G); Wright Giemsa staining; scale bars = 10 μm. The cells from erythroid colonies were stained positive for erythroid marker glycophorin A and negative for panleukocyte marker CD45 and myelomonocytic markers CD33 and CD13 (H-I). In contrast, the cells from granulocyte colonies were stained negative for glycophorin A and positive for CD45, CD33, and CD13 (J,K).
Hematopoietic cells derived from SR-bFGF-maintained hESCs have characteristic hematopoietic progenitor properties. Hematopoietic cells derived from SR-bFGF-maintained hESCs were identical to those derived from MEF-CM-maintained hESCs and produced characteristic hematopoietic CFU subtypes, including erythroid (A), macrophage (B), granulocyte (C), and granulocyte-macrophage colonies (D); scale bars = 100 μm. The cells prepared from an erythroid colony showed mature enucleated erythrocytes (arrowheads) and immature erythrocytes (E), from a macrophage colony showed macrophage morphology (F), and from a granulocyte colony showed mature neutrophils and eosinophils (G); Wright Giemsa staining; scale bars = 10 μm. The cells from erythroid colonies were stained positive for erythroid marker glycophorin A and negative for panleukocyte marker CD45 and myelomonocytic markers CD33 and CD13 (H-I). In contrast, the cells from granulocyte colonies were stained negative for glycophorin A and positive for CD45, CD33, and CD13 (J,K).
To further examine whether SR-bFGF-cultured hESCs are capable of hematopoietic lineage specification, EBs were treated with a combination of hematopoietic cytokines (stem cell factor [SCF], Flt3 ligand, IL-3, IL-6, granulocyte colony-stimulating factor [G-CSF]) and the ventral mesoderm inducer bone morphogenetic protein-4 (BMP-4) to specify hematopoietic lineage differentiation.7,8 SR-bFGF-cultured hESCs followed a similar spatial and temporal hematopoietic developmental pattern to MEF-CM-cultured hESCs. Similar numbers of CD45negPFV hemogenic precursors9 were derived after 10 days of EB development from either MEF-CM- or SR-bFGF-cultured hESCs in the presence of hematopoietic cytokines and BMP-4 (Figure 3B). Development of hEBs for 22 days from either MEF-CM- or SR-bFGF-cultured hESCs gave rise to up to 74% of hematopoietic cells expressing panleukocyte marker CD45+ (Figure 3C), with similar primitive CD34+ hematopoietic cell composition (49 732 ± 15 354 versus 37 648 ± 26 625; Figure 3C). Consistent with our previous report,7 hematopoietic cells generated from SR-bFGF-cultured hESCs contained similar hematopoietic progenitor capacity determined by the hematopoietic colony-forming unit (CFU) assay18 (Figure 3D). Hematopoietic colony types included erythyroid, macrophage, granulocyte, and granulocyte-macrophage progenitors (Figure 4A-D). Wright Giemsa staining of cytospin preparations of cells isolated from individual erythroid colonies showed mature enucleated erythrocytes and immature erythrocytes (Figure 4E), whereas cells from monocyte progenitors showed monocytic morphology (Figure 4F) and mature neutrophils and eosinophils from granulocytic progenitors (Figure 4G). Further analysis by flow cytometry of cells from individual erythroid colonies demonstrated expression of glycophorin A and absence of CD45, whereas myelomonocytic markers CD33 and CD13 were absent (Figure 4H-I). In contrast, the cells from granulocyte colonies were stained negative for glycophorin A but positive for CD45 and myeloid markers CD33 and CD13 (Figure 4J-K).
SR-bFGF-cultured hESCs maintain potential to generate derivatives of 3 germ layers
In addition to hematopoietic specification (representing mesoderm development), SR-bFGF culture conditions sustained hESC capacity to undergo ectoderm and endoderm lineage differentiation. Using Q-PCR, expression of germ layer-specific genes was detected from differentiated EBs generated from hESCs cultured in either MEF-CM or SR-bFGF. Human EBs generated from either MEF-CM- or SR-bFGF-cultured hESC lines were capable of differentiating into ectoderm and endoderm, as demonstrated by similar levels of Pax-6 (ectoderm) and HNF3α (endoderm) expression (Figure 5A-B).
To further confirm the potential to form derivatives of all 3 embryonic germ layers and pluripotent differentiation potential of hESCs cultured in SR-bFGF in vivo, we injected SR-bFGF-maintained hESCs into the testis capsules of NOD/SCID mice. All mice receiving hESCs cultured in SR-bFGF produced teratomas (n = 5 independent experiments). Testicular growths were palpable 4 weeks after injection. There was no gross evidence of metastases to other organs or other sites within the peritoneal cavity (not shown). The cut surfaces of the tumors displayed mucinous or serous cysts and solid components. Microscopically, all tumors contained tissues representing all 3 embryonic germ layers, including ectoderm (cystic epithelium, neural rosettes, and squamous epithelium), mesoderm (bone, muscle, and adipocytes [not shown]), and endoderm (glandular epithelium) (Figure 5C-H). These results demonstrate that SR-bFGF-cultured hESCs can be applied not only for studies of hematopoietic specification but also toward fundamental studies of hESC pluripotency in vivo.
Discussion
We report that high concentrations of bFGF in serum-free media are sufficient in sustaining the replicative and differentiative potential of hESCs in the absence of MEFs or MEF-CM. Based on our observations, we suggest that bFGF most likely represents a signaling pathway similar to leukemia inhibitory factor (LIF) for murine ESCs. It will be critical to evaluate the specific downstream targets of bFGF in hESCs cultured in these defined conditions and compare them with potential common targets represented by murine ESCs cultured in the media with serum and LIF alone or in serum-free media with LIF and BMP-4.19
Currently, hESCs have been found to be capable of incorporating the nonhuman sialic acid N-glycolylneuraminic acid (Neu5Gc).20 Animal sources of Neu5Gc might cause potential immunogenic reaction with human complement.20 Although Neu5Gc can be eliminated by replacement with human orthologs or neutralized by pooled human sera, culture of hESCs on MEF feeder layers prevented complete elimination of Neu5Gc.20 These results further emphasize the importance of using less complex culture conditions with defined chemical components. Thus, SR-bFGF provides a foundation for further characterizing self-renewal of the established hESC lines and deriving new hESCs under these defined conditions that are free of potential xenogenic or human allogenic containments by replacing animal sources of growth factors or reagents with human orthologs or recombinant proteins.
SR-bFGF-cultured hESCs maintain potential to generate derivatives of 3 germ layers. (A-B) In vitro, in addition to generation of hematopoietic cells (part of mesoderm development; Figures 3 and 4), SR-bFGF-cultured hESCs also contained capacity to differentiate into endoderm (HNF3α) and ectoderm (Pax-6) derivatives according to gene expression determined by a quantitative real-time PCR. (C-H) In vivo, 5 of 5 NOD/SCID mice produced teratomas 6 weeks after injection of SR-bFGF-maintained hESCs (passage 26) into the testis capsule. Microscopic examination from a representative teratoma revealed that the tumor was composed of tissues representing all 3 embryonic germ layers, including ectoderm ([C] cystic epithelium, [D] neural rosettes, [E] squamous epithelium), mesoderm ([F] muscle, [G] osteoid island showing bony differentiation), and endoderm ([H] glandular epithelium). Hematoxylin and eosin staining. Bars = 50 μm.
SR-bFGF-cultured hESCs maintain potential to generate derivatives of 3 germ layers. (A-B) In vitro, in addition to generation of hematopoietic cells (part of mesoderm development; Figures 3 and 4), SR-bFGF-cultured hESCs also contained capacity to differentiate into endoderm (HNF3α) and ectoderm (Pax-6) derivatives according to gene expression determined by a quantitative real-time PCR. (C-H) In vivo, 5 of 5 NOD/SCID mice produced teratomas 6 weeks after injection of SR-bFGF-maintained hESCs (passage 26) into the testis capsule. Microscopic examination from a representative teratoma revealed that the tumor was composed of tissues representing all 3 embryonic germ layers, including ectoderm ([C] cystic epithelium, [D] neural rosettes, [E] squamous epithelium), mesoderm ([F] muscle, [G] osteoid island showing bony differentiation), and endoderm ([H] glandular epithelium). Hematoxylin and eosin staining. Bars = 50 μm.
Prepublished online as Blood First Edition Paper, February 17, 2005; DOI 10.1182/blood-2004-10-4065.
Supported in part by the Krembil Foundation, an operating grant from the Canadian Institutes of Health Research (CIHR) MOP-67207, National Centre of Excellence Program (NCE) StemNet, a Canadian Research Chair in Stem Cell Biology and Regenerative Medicine and Krembil Chair in Stem Cell Biology (M.B.), and CIHR Postdoctoral Fellowships (L.W., P.M.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to David Goodale and Anne Rouleau for their excellent technical assistance.

![Figure 5. SR-bFGF-cultured hESCs maintain potential to generate derivatives of 3 germ layers. (A-B) In vitro, in addition to generation of hematopoietic cells (part of mesoderm development; Figures 3 and 4), SR-bFGF-cultured hESCs also contained capacity to differentiate into endoderm (HNF3α) and ectoderm (Pax-6) derivatives according to gene expression determined by a quantitative real-time PCR. (C-H) In vivo, 5 of 5 NOD/SCID mice produced teratomas 6 weeks after injection of SR-bFGF-maintained hESCs (passage 26) into the testis capsule. Microscopic examination from a representative teratoma revealed that the tumor was composed of tissues representing all 3 embryonic germ layers, including ectoderm ([C] cystic epithelium, [D] neural rosettes, [E] squamous epithelium), mesoderm ([F] muscle, [G] osteoid island showing bony differentiation), and endoderm ([H] glandular epithelium). Hematoxylin and eosin staining. Bars = 50 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/12/10.1182_blood-2004-10-4065/5/m_zh80120579640005.jpeg?Expires=1767814021&Signature=bc9j67HlBQBemfLPy0G13cyUmv1raQCcRKI7IGxmxBY1YhDSFtZyhZxjAcwzNIId-~dyMPnm-tljH3LdhngxwurOMLijEzYR4g8d87R3kSaNtdexbdyXo4KdJF7XHl~k1U1ukZtsmgqJC0vl8ImUXVsAgGDMeS~nkKz033MEl~asL4kd62j6TyIU-VrNvzBbzyD7tC6EWMlcqjAOOAz-iQ~W-n8YJsfuwz6tcbMEnTvGd8bIh7s2MOkd4WEjIoXMBRero8vibRrr~CCLOqM8VRIc5nI4CymJSB4S33b~-IVtPU~IVxp8uePXbPmDoA-POCqeTs3qsp~S2GjH5Mme9A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 5. SR-bFGF-cultured hESCs maintain potential to generate derivatives of 3 germ layers. (A-B) In vitro, in addition to generation of hematopoietic cells (part of mesoderm development; Figures 3 and 4), SR-bFGF-cultured hESCs also contained capacity to differentiate into endoderm (HNF3α) and ectoderm (Pax-6) derivatives according to gene expression determined by a quantitative real-time PCR. (C-H) In vivo, 5 of 5 NOD/SCID mice produced teratomas 6 weeks after injection of SR-bFGF-maintained hESCs (passage 26) into the testis capsule. Microscopic examination from a representative teratoma revealed that the tumor was composed of tissues representing all 3 embryonic germ layers, including ectoderm ([C] cystic epithelium, [D] neural rosettes, [E] squamous epithelium), mesoderm ([F] muscle, [G] osteoid island showing bony differentiation), and endoderm ([H] glandular epithelium). Hematoxylin and eosin staining. Bars = 50 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/12/10.1182_blood-2004-10-4065/5/m_zh80120579640005.jpeg?Expires=1767814022&Signature=rCtrlJaw4Q42j7rSYBDruDEKPdfuFL9QRnHUzzC~y597vuyzu3sM~bJtmPD17dEyn5auxo4GAo5dphCKMKyh4CGQQMmANkeDzrJLfV2cadN8kNPaaLyEJRuadRq6ajwTIoLUT3sEJljiX~R2NmfoYMcNtlLZ-fi3T2l1wgwsIfV51Jg3UsUNNeqIg3elIAxFXco5aY62zn1biW-87VqTWXV9kKRmGnRu0-Bc7tQf-kDn7ga1snpI1klykIhNJ-E6DE4HLOtuUsAEow6KthGE1Qe2rKghGi3XuobSyfwZswy-~xPuOCuFnZqmKSd0V~-lmFHY6ffxuRvwy-i68uzkvA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)