Comment on Druhan et al, page 584
An acquired granulocyte colony-stimulating factor receptor (G-CSFR) mutation in severe congenital neutropenia (SCN) severely truncating the carboxyl terminus of the G-CSF receptor may not only explain atypical refractoriness to G-CSF treatment but upsets dogma that ligand binding initiates signal transduction by inducing subunit oligomerization.
Severe congenital neutropenia (SCN), also known as Kostmann syndrome, is a genetically heterogeneous disorder most often caused by heritable mutations of the ELA2 gene encoding neutrophil elastase.1 Most patients respond to granulocyte colony-stimulating factor (G-CSF) treatment, and a subset, primarily those with ELA2 mutations,2 progress to myelodysplasia or acute myeloid leukemia. While germ-line G-CSF receptor (G-CSFR) mutations rarely cause SCN,3,4 acquired mutations of G-CSFR do commonly arise during evolution to leukemia. Because G-CSFR mutations neither invariantly herald the onset of leukemia nor accompany its emergence,5 their role in the disease is not well understood. G-CSFR mutations typically delete the carboxyl tail that is required for signaling, and thereby produce a dominant-negative effect thought to result from heterodimerization of normal and mutant chains appearing together on the cell surface.FIG1
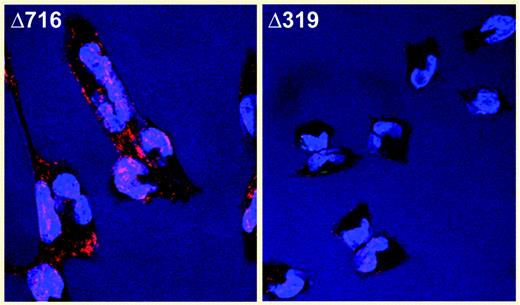
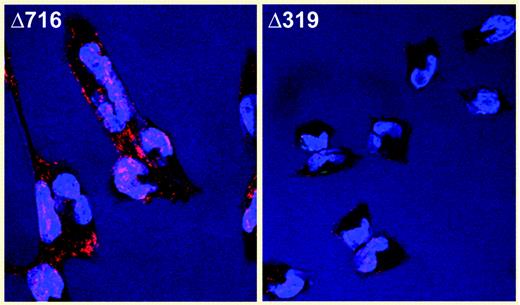
Intracellular accumulation of the Δ319 G-CSFR. See the complete figure in the article beginning on page 584.
Intracellular accumulation of the Δ319 G-CSFR. See the complete figure in the article beginning on page 584.
Druhan and colleagues report the case of an SCN patient who acquired a chain-terminating mutation taking out the distal extracellular domain and entire transmembrane and intracellular domains of the G-CSFR. The same mutation, although apparently constitutional in origin, has previously been reported in another SCN case,3 and both patients are unresponsive to G-CSF therapy. Unexpectedly, the mutation produces a stable transcript (somehow escaping nonsense-mediated decay) whose abbreviated polypeptide heterodimerizes with normal G-CSFR subunits in the endoplasmic reticulum and/or Golgi, thus sequestering receptors before they can traffic to the cell surface. The paradigm for cytokine receptor–induced signal transduction holds that ligand binding stimulates formation of dimers or oligomers from receptor monomers, yet the implication here is that oligomers can form without ligand and a conformational change upon ligand binding instead activates signal transduction (Figure 3B of Druhan et al., “Intracellular accumulation of mutant G-CSCF Receptor.” ▪