Abstract
Anticoagulants are pivotal agents for prevention and treatment of thromboembolic disorders. Limitations of existing anticoagulants, vitamin K antagonist and heparins, have led to the development of newer anticoagulant therapies. These anticoagulants have been designed to target specific coagulation enzymes or steps in the coagulation pathway. New anticoagulants that are under evaluation in clinical trials include: (1) inhibitors of the factor VIIa/tissue factor pathway; (2) factor Xa inhibitors, both indirect and direct; (3) activated protein C and soluble thrombomodulin; and (4) direct thrombin inhibitors. Although most of these are parenteral agents, several of the direct inhibitors of factor Xa and thrombin are orally active. Clinical development of these therapies often starts with studies in the prevention of venous thrombosis before evaluation for other indications, such as prevention of cardioembolism in patients with atrial fibrillation or prosthetic heart valves. At present, the greatest clinical need is for an oral anticoagulant to replace warfarin for long-term prevention and treatment of patients with venous and arterial thrombosis. Ximelagatran, an oral direct thrombin inhibitor, is the first of a series of promising new agents that might fulfill this need. Large phase 3 trials evaluating ximelagatran for the secondary prevention of venous thromboembolism and prevention of cardioembolic events in patients with atrial fibrillation have been completed.
Introduction
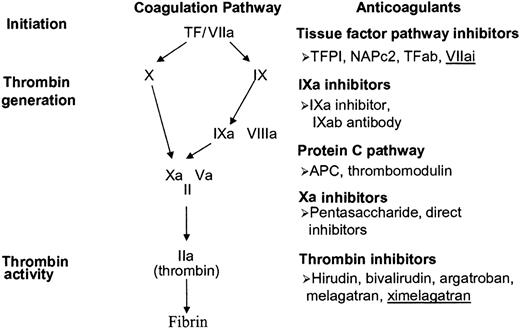
Anticoagulants are pivotal agents for prevention and treatment of thromboembolic disorders.1,2 Heparin and coumarins were discovered more than 60 years ago, long before their mechanism of action was completely understood.3,4 Low-molecular-weight heparin (LMWH), a derivative of heparin, was in clinical development for at least a decade before its mechanistic advantages over heparin were elucidated.2 In contrast, the new anticoagulants have been designed to target specific coagulation enzymes or steps in the coagulation pathway (Figure 1).5 These anticoagulants have been developed from hematophagous organisms, by the application of recombinant DNA technology, or by structure-based drug design.5
New anticoagulants under clinical development. TF indicates tissue factor; r-TFPI, recombinant tissue factor pathway inhibitor; NAPc2, nematode anticoagulant peptide.
New anticoagulants under clinical development. TF indicates tissue factor; r-TFPI, recombinant tissue factor pathway inhibitor; NAPc2, nematode anticoagulant peptide.
The characteristics of an ideal anticoagulant are listed in Table 1. Of these, by far the most important is a high efficacy-to-safety index. Despite claims that inhibition of 1 clotting enzyme may cause less bleeding than inhibition of another enzyme, these assertions have yet to be substantiated in humans.
The cost to develop a new anticoagulant is high, largely reflecting the expense of phase 3 clinical trials. Therefore, the selection of the first clinical indication is often based on cost considerations rather than unmet medical needs. For example, although the greatest need for new anticoagulants is for prevention of cardioembolism in patients with atrial fibrillation or prosthetic heart valves, 6 drug development often starts with studies in the prevention of venous thrombosis because the required sample size is much smaller and the duration of follow-up is shorter. Such an approach presupposes that efficacy in the prevention of venous thromboembolism (VTE) predicts success for other indications.
Inhibitors of the factor VIIa/tissue factor pathway (extrinsic pathway)
Three compounds that target the factor VIIa/tissue factor pathway have been evaluated in clinical trials. These include recombinant tissue factor pathway inhibitor (TFPI), nematode anticoagulant peptide (NAPc2), and active-site blocked factor VIIa (factor VIIai).
TFPI
TFPI is a bivalent, naturally occurring inhibitor that modulates the initiation of coagulation by inhibiting the factor VIIa/tissue factor complex.7 TFPI first binds and inactivates factor Xa, and the resulting complex then inhibits factor VIIa within the factor VIIa/tissue factor complex. TFPI is found in plasma, in platelet α-granules and on the endothelial cell surface.7 TFPI levels increase after heparin or LMWH administration, but it is uncertain whether released TFPI contributes to the antithrombotic properties of these agents.8
NAPc2
NAPc2, a polypeptide originally isolated from the canine hookworm, Ancylostoma caninum,12 is now expressed in yeast. NAPc2 binds to a noncatalytic site on both factor X and factor Xa13 and the resulting NAPc2/factor Xa complex then inhibits factor VIIa within the factor VIIa/tissue factor complex.12,13 Because NAPc2 binds to factor X, it has a half-life of approximately 50 hours after subcutaneous injection.13 NAPc2 was evaluated in an open-label phase 2 dose-finding study14 of 293 patients undergoing elective knee arthroplasty. Subcutaneous NAPc2 was given 1 to 12 hours after surgery and every second day thereafter to a maximum of 4 doses. A dose of 3.0 μg/kg given 1 hour after surgery was considered most effective.14
The safety of NAPc2 has been evaluated in a randomized, double-blind, placebo-controlled dose-escalation study of 154 subjects undergoing elective coronary angiography.15
Factor VIIai
Factor VIIai exerts its anticoagulant effect by competing with factor VIIa for tissue factor binding.16 Factor VIIai (with or without adjunctive heparin) was reported to be no more effective than heparin alone in a phase 2 trial of 491 patients undergoing elective percutaneous coronary intervention (PCI).17
Factor IXa inhibitors
Factor IXa inhibitors have been shown to be effective in animal models of thrombosis, 18 but have yet to be evaluated in phase 2 clinical trials.
Factor Xa inhibitors
Factor Xa inhibitors act indirectly or directly. New indirect inhibitors, fondaparinux and idraparinux, are synthetic analogs of the unique pentasaccharide sequence that mediates the interaction of heparin with antithrombin.19 Once the pentasaccharide/antithrombin complex binds factor Xa, the pentasaccharide dissociates from antithrombin/Xa complex and can be reutilized. In contrast, direct factor Xa inhibitors bind to the enzyme with 1:1 stoichiometry and block the interaction of factor Xa with its substrates.20 Currently available direct factor Xa inhibitors are reversible and not only inhibit free factor Xa, but also inactivate factor Xa bound to platelets within the prothrombinase complex.21,22
Fondaparinux
Fondaparinux binds antithrombin with high affinity, has excellent bioavailability after subcutaneous injection, and has a plasma half-life of 17 hours that permits once-daily administration.22 The drug is excreted unchanged in the urine and is contraindicated in patients with severe renal impairment (creatinine clearance < 30 mL/min).22 Fondaparinux does not bind to platelet factor 4 (PF4); consequently, it should not cause heparin-induced thrombocytopenia.22,23 Unlike heparin, there is no antidote for fondaparinux. If uncontrolled bleeding occurs with fondaparinux, a procoagulant, such as recombinant factor VIIa, might be effective.24 Fondaparinux has been evaluated for prevention and treatment of VTE and for treatment of arterial thrombosis.
Prevention of VTE
Fondaparinux has been shown to be more effective than enoxaparin in 4 large phase 3 trials for thromboprophylaxis in patients undergoing surgery for hip fracture or elective hip or knee arthroplasty and is licensed for these indications25-28 (Table 3). In a meta-analysis of these studies that included a total of 7344 subjects (4510 undergoing hip arthroplasty, 1670 having surgery for hip fracture, and 1034 undergoing knee arthroplasty) randomly assigned to receive fondaparinux (2.5 mg once daily) or fixed-dose enoxaparin, fondaparinux reduced the risk of VTE by 55% compared with enoxaparin (Table 4).38 Major bleeding occurred more frequently in the fondaparinux-treated group (P = .008), but the incidence of bleeding leading to death, reoperation, or occurring in a critical organ was not significantly different between the 2 groups.38 In these trials the protocol specified that fondaparinux was to be started 4 to 8 hours after surgery, whereas enoxaparin therapy was to be initiated 12 to 24 hours after surgery, with or without a dose 12 hours prior to surgery (Table 3). Because of the asymmetric study design, it is not possible to conclude whether differences in efficacy and safety were drug-related or caused by differences in the timing of drug administration after surgery. It is noteworthy, however, that post-hoc subgroup analysis of pooled data from the 4 trials suggested that when the first dose of fondaparinux was administered 6 to 8 hours after surgery, the regimen currently approved by the Food and Drug Administration (FDA), the rate of major bleeding with fondaparinux was similar to that with enoxaparin.43
Extended fondaparinux thromboprophylaxis has been evaluated in a phase 3 trial (The PENTasaccharide in HIp-FRActure Surgery-Plus [PENTHIFRA-Plus]) in 656 patients undergoing surgery for hip fracture.29 Patients received 2.5 mg fondaparinux subcutaneously once daily for 7 days and were then randomized to receive continuing fondaparinux or placebo for an additional 3 weeks, at which time routine venography was performed. Fondaparinux treatment decreased the rate of deep vein thrombosis (DVT) from 35% to 1.4%, (P < .001) and reduced the rate of symptomatic VTE from 2.7% to 0.3% (P = .021)29 (Table 3). These results support the notion that extended anticoagulant prophylaxis should be used in high-risk patients undergoing hip surgery.44,45 Accordingly, fondaparinux has been approved by regulatory authorities for extended prophylaxis after surgery for hip fracture.
Fondaparinux has also been evaluated in general surgery patients30 and in medical patients.31 In both trials, the primary outcome measure was a composite of venographically documented DVT, symptomatic DVT, and nonfatal and fatal pulmonary embolism (PE). In a double-blind trial of 2297 subjects undergoing major abdominal surgery, patients were randomly assigned to receive dalteparin (2500 IU 2 hours preoperatively and 6 hours postoperatively and then 5000 IU postoperatively [once daily]) or fondaparinux (2.5 mg subcutaneously once daily) for 5 to 9 days.30 The outcome, assessed at postoperative day 10, occurred in 4.6% of the fondaparinux group and 6.1% of those given dalteparin (P = .14). Symptomatic VTE occurred in 0.4% and 0.3% of patients, respectively, whereas major bleeding occurred in 3.4% and 2.4%, respectively; these differences are not statistically significant (Table 3).
In a double-blind study, 849 acutely ill, hospitalized, medical patients aged 65 years or older were randomly assigned to receive subcutaneous fondaparinux (2.5 mg once daily) or placebo for 6 to 14 days.31 VTE at day 15 was reported in 5.6% of the fondaparinux group and 10.5% of the placebo group (P = .03). Major bleeding while on therapy was infrequent and occurred in 0.2% of patients in both groups (Table 3).31
Treatment of VTE
Fondaparinux has been evaluated for initial treatment of VTE in 2 double-blind, noninferiority, randomized, phase 3 clinical trials. The MATISSE-DVT trial39 included 2205 patients with DVT who were assigned to receive either fondaparinux (7.5 mg subcutaneously once daily) or enoxaparin (1 mg/kg subcutaneously twice daily) for 5 days followed by a minimum of a 3-month course of treatment with a vitamin K antagonist. At 3 months, recurrent symptomatic VTE was observed in 3.9% and 4.1% of the fondaparinux or enoxaparin groups, respectively, whereas major bleeding rates were 1.1% and 1.2%, respectively39 (Table 4).
The MATISSE-PE trial40 was an open-label, noninferiority trial of 2213 patients with PE who were randomly assigned to receive either fondaparinux (5, 7.5, or 10 mg subcutaneously once daily, depending on patient weight) or unfractionated heparin (by continuous infusion) for 5 days followed by a minimum of a 3-month course of therapy with a vitamin K antagonist. At 3 months, recurrent symptomatic VTE was observed in 3.8% and 5.0% of the fondaparinux or unfractionated heparin groups, respectively, and major bleeding rates were 1.3% and 1.1%, respectively. Thus, fondaparinux is at least as effective and safe as LMWH or unfractionated heparin for initial treatment of VTE (Table 4).39,40
Acute coronary syndromes
Fondaparinux has been evaluated for treatment of acute coronary syndromes in 2 phase 2 studies.46,47 Bleeding was similar in all treatment groups. Based on these results, phase 3 trials with fondaparinux in patients with ST-elevation and non–ST-elevation myocardial infarction (MI) have been initiated.
Idraparinux
Idraparinux is a hyper-methylated derivative of fondaparinux. Its plasma half-life is 80-130 hours.48 Consequently, idraparinux is given once weekly by subcutaneous injection.
Idraparinux was compared with warfarin in a phase 2 trial of 659 patients with proximal DVT.49 The primary outcome measure rates were similar in all idraparinux groups and did not differ from those in the warfarin group. In contrast, there was a clear dose response for major bleeding in patients given idraparinux and there was less bleeding with the 2.5-mg idraparinux dose than with warfarin.49 A phase 3 trial with this dose of idraparinux is under way.
DX-9065a
DX-9065a is a nonpeptidic arginine derivative that binds to the active site of factor Xa. In a small phase 2 safety study, 50 DX-9065a given as a continuous intravenous infusion was compared with placebo in patients with stable coronary artery disease. There were no major bleeds with DX-9065a.
Razaxaban
Razaxaban (DPC 906), an orally active agent, has been compared with enoxaparin 30 mg twice daily in a phase 2 dose-finding study in 656 patients following elective knee arthroplasty.51 Razaxaban was given at 25-, 50-, 75-, and 100-mg doses twice daily. There was a dose response both for efficacy and safety. There was a trend for the lowest dose (25 mg twice daily) to be more effective than enoxaparin, with similar low rates of major bleeding. The 3 higher dose groups receiving razaxaban were stopped prematurely because of excessive major bleeding.51
Inhibitors of factors Va and VIIIa
Activated protein C (APC) modulates thrombin generation by inactivating factors Va and VIIIa.52 An increase of APC can be produced by direct administration of recombinant APC or by administration of recombinant soluble thrombomodulin.
Activated protein C
In a phase 3 clinical trial, recombinant APC, drotrecogin alfa (activated), given as an infusion over 96 hours, was compared with placebo in 1690 patients with severe sepsis.11 APC reduced mortality at 28 days by 19% (from 30.8% to 24.7%; P = .005) but produced a 1.5% increase in major bleeding (from 2.0% to 3.5%; P = .06; Table 2).11 It is unclear whether the benefits of APC are due exclusively to its anticoagulant effect or whether other non-anticoagulant mechanisms play a role. A number of non-anticoagulant effects of ACP have been reported including its ability to down-regulate inflammatory cytokines (tumor necrosis factor and interleukin 6).53 Based on these results, recombinant APC has been licensed in North America as an adjunct for treatment of severe sepsis.
Soluble thrombomodulin
Soluble thrombomodulin binds thrombin and induces a conformational change in the active site of the enzyme that renders it a potent activator of protein C.54 A recombinant analog of the extracellular portion of soluble thrombomodulin, which has a half-life of 2 to 3 days after subcutaneous injection, has been evaluated in a phase 2 dose-escalating study in 312 subjects undergoing elective hip arthroplasty. Although there was no control group, soluble thrombomodulin reduced the rate of DVT in a dose-dependent fashion.55
Thrombin inhibitors
All of the new thrombin inhibitors bind directly to thrombin and block its interaction with substrates. Unlike heparin, direct thrombin inhibitors inactivate fibrin-bound thrombin, as well as fluid-phase thrombin.56,57 Three parenteral direct thrombin inhibitors (hirudin, argatroban, and bivalirudin) and 1 oral direct thrombin inhibitor (ximelagatran) have been evaluated in phase 3 clinical trials. Specific antidotes are not available to neutralize these compounds.56,57 The parenteral thrombin inhibitors have been licensed in North America for limited indications; hirudin and argatroban are approved for treatment of patients with heparin-induced thrombocytopenia, whereas bivalirudin is licensed as an alternative to heparin in patients undergoing PCI. Ximelagatran is still under investigation.
Hirudin
Hirudin is a bivalent inhibitor; its amino-terminal domain interacts with the active site of thrombin and its carboxy-terminal tail binds to exosite 1.58 Binding is essentially irreversible. The plasma half-life of hirudin is 60 minutes after intravenous injection and 120 minutes after subcutaneous injection.59 Hirudin is cleared via the kidneys so its dose must be adjusted in patients with renal insufficiency.60
Although hirudin has been evaluated in acute coronary syndromes61-70 and for prevention and treatment of DVT, 71-73 its development for these indications is no longer being pursued. For acute coronary indications hirudin was marginally more effective than heparin but caused more bleeding (Table 5).61-70 In contrast, at the lower doses used for the prevention of DVT in patients undergoing elective hip arthroplasty, hirudin was more effective than LMWH and heparin and was not associated with an increased risk of bleeding.71,72 The approval for use of hirudin for treatment of patients with heparin-induced thrombocytopenia is based largely on 2 prospective cohort studies that evaluated hirudin in this setting and reported a significant reduction in the incidence of a composite of death, amputation, and thromboembolic events when compared with historical controls.77,78
Bivalirudin
Bivalirudin is a 20–amino acid synthetic polypeptide analog of hirudin.79 The amino-terminal D-Phe-Pro-Arg-Pro sequence, which binds to the active site of thrombin, is connected via 4 Gly residues to a carboxy-terminal dodecapeptide that interacts with exosite 1 on thrombin.80 Once bound, thrombin cleaves the Pro-Arg bond within the amino terminal of bivalirudin, thereby reducing its antithrombin activity.79-81 Bivalirudin has a plasma half-life of 25 minutes after intravenous injection82 and only a fraction is excreted via the kidneys.83 Bivalirudin has been evaluated in patients undergoing PCI and as an adjunct to streptokinase in patients with ST-elevation MI.73-76,84
Coronary angioplasty
The first phase 3 study compared bivalirudin with heparin in 4098 patients undergoing coronary angioplasty for unstable or postinfarction angina.74 The frequency of the primary outcome measure, a composite of in-hospital death, MI, abrupt vessel closure, or rapid clinical deterioration of cardiac origin, was not significantly lower in the bivalirudin group (Table 5; 11.4% versus 12.2%), whereas bleeding was significantly lower with bivalirudin than with heparin (3.8% and 9.8%, respectively; P < .001).74 In a prospectively stratified, high-risk subgroup of 704 patients with postinfarction angina, bivalirudin significantly reduced the primary outcome (from 14.2% to 9.1%; P = .04).84
Bivalirudin has also been evaluated in Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events (REPLACE-2)75 a phase 3 clinical trial of 6010 PCI patients who were randomly assigned to bivalirudin plus provisional glycoprotein (GP) IIb/IIIa antagonist (either abciximab or eptifibatide), or heparin plus a GPIIb/IIIa antagonist. The primary outcome measure, a composite of death, MI, urgent revascularization, or major bleeding at 30 days, occurred in 9.2% of the patients treated with bivalirudin and in 10% of those given heparin (P = .32). A GPIIb/IIIa antagonist was required in only 7% of patients randomized to bivalirudin. Rates of major bleeding were significantly lower in patients given bivalirudin than in those treated with heparin (2.4% and 4.1%, respectively; P < .001; Table 5).75
Adjunct to thrombolysis
Bivalirudin has been compared with heparin as adjuncts to thrombolytic therapy in a phase 3 randomized trial (Hirulog and Early Reperfusion or Occlusion [HERO-2]) of 17 073 patients with acute ST-elevation MI.76 Patients were randomly assigned to an intravenous bolus followed by a 48-hour infusion of either fixed-dose bivalirudin (n = 8516) or adjusteddose heparin (n = 8557) in conjunction with streptokinase. The primary outcome measure was 30-day mortality, and secondary outcome measures included reinfarction within 96 hours and bleeding. Mortality at 30 days was similar in patients randomized to bivalirudin or heparin (10.8% and 10.9%, respectively; P = .85), but in contrast to the other 2 randomized trials, the rate of bleeding was significantly higher with bivalirudin. In addition, there was a trend for higher major bleeding rates (including severe bleeding) with bivalirudin (Table 5).76
Argatroban
Argatroban, a competitive inhibitor of thrombin, binds noncovalently to the active site of thrombin to form a reversible complex.57,85 The plasma half-life of argatroban is 45 minutes and the drug is metabolized in the liver.57,85,86 Argatroban is licensed for treatment of heparin-induced thrombocytopenia with or without thrombosis. Argatroban has not been evaluated in appropriately designed randomized controlled trials in this setting and, like hirudin, evidence supporting its use is confined to 2 prospective cohort studies of patients treated with argatroban compared with historical controls.87,88
Ximelagatran
Ximelagatran is the first orally active thrombin inhibitor. It is a prodrug of the active site-directed thrombin inhibitor, melagatran.92 After ingestion, ximelagatran is absorbed from the small intestine and undergoes rapid biotransformation to melagatran, the active agent.92 Melagatran is eliminated via the kidneys.92 Ximelagatran has a plasma half-life of 4 to 5 hours and is administered orally twice daily.92 Ximelagatran has been evaluated for thromboprophylaxis in high-risk orthopedic patients, 32-37 treatment of VTE, 41,42 and prevention of cardioembolic events in patients with nonvalvular atrial fibrillation.93,94 Based on its predictable anticoagulant response, ximelagatran was administered in fixed doses without coagulation monitoring.
Venous thromboprophylaxis
Ximelagatran has been evaluated for the prevention of VTE in 6 phase 3 trials in patients undergoing either elective hip surgery, major knee surgery, or both32-37 (Table 3). The comparator was enoxaparin in 3 studies32,33,36 and warfarin in the other 3.34,35,37 Enoxaparin was started postoperatively (at a dose of 30 mg twice daily) in the North American study33 and was started 12 hours prior to surgery (at a dose of 40 mg daily) in the European studies.32,36 These 2 regimens reflect current practices in North America and Europe, respectively. The end-point in these studies was a composite of venographically detected DVT and symptomatic PE. The results of these 6 trials are summarized in Table 3.
Ximelagatran was commenced postoperatively in a dose of either 24 mg or 36 mg twice daily. In the North American studies, ximelagatran was started on the day after surgery (ie, 12-24 hours postoperatively33-35,37 ; Table 3) In the 2 European trials32,36 it was preceded by melagatran 3 mg subcutaneously; given postoperatively32 in 1 study and preoperatively in the other.36 In the MElagatran for THRombin inhibition in Orthopedic surgery (METHRO III) trial32 (hip and knee replacement surgery), melagatran/ximelagatran showed similar efficacy and safety to preoperative enoxaparin. In the Platinum Hip33 study, postoperative enoxaparin was more effective and showed a similar incidence of bleeding to the postoperative melagatran/ximelagatran regimen, whereas in the EXpanded PRophylaxis Evaluation Surgery Study (EXPRESS) trial36 (hip and knee replacement surgery), the combination of preoperative melagatran and postoperative ximelagatran was more effective than preoperative enoxaparin, but at the cost of more bleeding.
In the 3 North American trials in which warfarin was the comparator, 34,35,37 it was started postoperatively in a dose adjusted to produce an international normalized ratio (INR) of 2.0 to 3.0. Ximelagatran was commenced postoperatively in a dose of 24 mg or 36 mg twice daily. In the Platinum-Knee trial, 34 ximelagatran (24 mg) showed a nonsignificant trend (P = .07) to be more effective than warfarin, with a similar rate of bleeding. In the EXanta Used to Lessen Thrombosis (EXULT) A-knee trial, 35 which evaluated either 24 mg or 36 mg ximelagatran twice daily, ximelagatran (36 mg) was more effective than warfarin, with a similar rate of bleeding. In the EXULT B-knee trial37 ximelagatran 36 mg was more effective than warfarin with a similar rate of bleeding.
The results of these studies indicate that ximelagatran administered postoperatively is as safe and marginally less effective than enoxaparin and more effective than warfarin, for the prevention of venous thrombosis in patients undergoing elective orthopedic surgical procedures. However, when combined with preoperative melagatran, the prophylactic regimen is associated with an increase in perioperative bleeding (Table 3). At present, melagatran/ximelagatran has been approved in France for prevention of VTE after major orthopedic surgery, based on the results of the METHRO III and EXPRESS trials.32,36
Treatment of VTE
Ximelagatran has been evaluated in 2 phase 3 trials. In the THRombin Inhibitor in Venuous thromboEmbolism (THRIVE III) trial, 41 1233 patients, who had completed a 6-month course of anticoagulant therapy for treatment of VTE, were randomized to ximelagatran (24 mg twice daily) or placebo for an additional 18 months. The cumulative risk of an event during the 18 months was 2.8% in the ximelagratran group and 12.6% in the placebo group (hazard ratio 0.16; P < .001). Major bleeding occurred in 1% of patients in each group. There was no difference between groups in death from any cause (Table 4).
The THRIVE II/V42 trial was a double-dummy, randomized noninferiority trial of 2491 patients with acute VTE. Participants were randomized to receive oral ximelagatran (36 mg twice daily) or subcutaneous enoxaparin (1 mg/kg twice daily for a minimum of 5 days) followed by warfarin (target INR, 2.0-3.0). Treatment was given for 6 months and patients were followed up for an additional 40 days. Recurrent VTE occurred in 2.1% of ximelagatran-treated patients and 2.0% in the enoxaparin/warfarin group and all-cause mortality occurred in 2.3% and 3.4%, respectively. Major bleeding occurred in 1.3% of those given ximelagatran and 2.2% of those treated with enoxaparin/warfarin, a trend that was not significant. These results suggest that ximelagatran is an effective and safe alternative to LMWH followed by warfarin for acute treatment of VTE (Table 4).
Atrial fibrillation
Two phase 3 trials have compared ximelagatran (36 mg twice daily) to warfarin for the prevention of cardioembolic events in patients with nonvalvular atrial fibrillation93,94 (Table 6). The Stroke Prevention and the ORal Thrombin Inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial Fibrillation (SPORTIF III) study randomized 3407 such subjects, in an open-label fashion, to receive either ximelagatran or adjusted-dose warfarin (targeted to an INR of 2.0-3.0).93 The primary outcome, all strokes and systemic embolic events, was reported in 40 subjects (1.6%/year) assigned to ximelagatran and 56 (2.3%/year) assigned to warfarin in an intention-to-treat analysis following a mean duration of follow-up of 21 months. Rates of major and intracranial hemorrhage were comparable in both groups, although major and minor bleeds were reduced in the ximelagatran group (25.5% compared with 29.5%; P = .007). All-cause mortality was 3.2%/year in both groups.
The SPORTIF V trial randomized 3922 participants with nonvalvular atrial fibrillation (and more than 1 additional vascular risk factor) to receive ximelagatran 36 mg twice daily or adjusted-dose warfarin (INR, 2.0-3.0). SPORTIF V was a double-blind, double-dummy trial.94 The primary outcome measure of all strokes (ischemic or hemorrhagic) and systemic embolic events was reported in 51 subjects (1.6%/year) assigned to ximelagatran and 37 (1.2%/year) assigned to warfarin (intention-to-treat analysis) following a mean duration of follow-up of 24 months (P = .13). Rates of major bleeding were 3.1% in those receiving adjusted-dose warfarin and 2.4% in those receiving ximelagatran (P = .16). Intracranial hemorrhage occurred in 0.06% of participants in both groups. The rate of major plus minor bleeding was lower in the ximelagatran group (37% compared with 47%).94 When the results of SPORTIF III and V were combined, ximelagatran was associated with a 16% relative risk reduction in the composite outcome measure of all strokes (ischemic or hemorrhagic), systemic embolic events, major bleeding, and death (P = .038).94
Acute coronary syndrome
In the Efficacy and Safety of oral direct Thrombin inhibitor ximelagatran in patiEnts with rEcent Myocardial infarction (ESTEEM) study, 95 1883 patients with ST-elevation or non–ST-elevation MI within the past 14 days were randomly assigned to receive oral ximelagatran (24, 36, 48, or 60 mg twice daily) or placebo. All patients received aspirin. The primary outcome measures, all-cause mortality, nonfatal MI, and severe recurrent ischemia, were significantly reduced in the ximelagatran group (16.3% and 12.7%, respectively; P = .036). Within the ximelagatran groups, efficacy was comparable among the various dosage groups, but the lowest rate of major bleeding was reported with a dose of 24 mg twice-daily.
Nonhemorrhagic side effects of ximelagatran
Between 6% and 9.6% of patients treated with long-term ximelagatran develop a 3-fold or greater increase in alanine aminotransferase.41,42,93,94 Typically, this side effect occurs between 6 weeks and 6 months of treatment.41,42,93,94 The increase in alanine aminotransferase is usually asymptomatic and reversible, even if the medication is continued. Based on data from clinical trials, the increase in transaminases with ximelagatran has been benign. However, this side effect is of potential concern and, if the drug is approved, its long-term impact on liver function will need to be carefully monitored in clinical practice after the drug is marketed. It is likely that liver function tests will need to be monitored, at least during the initial 6 months of ximelagatran therapy.
Summary of results by clinical indication
Venous thromboprophylaxis
Both LMWH and warfarin are safe and effective agents for thromboprophylaxis in high-risk orthopedic patients.96 Fondaparinux is a new alternative and is approved for this indication. Although ximelagatran also appears to be effective, it is not yet approved in North America. In the 3-month period after a 7- to 10-day course of postoperative prophylaxis with either agent, the incidence of symptomatic VTE is about 2.5% and 1.4% for hip and knee arthroplasty, respectively, and the incidence of fatal PE is about 0.05%.97 The main clinical need in this setting is a thromboprophylactic approach that is safe, effective, and convenient for use after hospital discharge, particularly in patients having hip surgery. LMWH is effective for extended thromboprophylaxis after hip and knee surgery, 45 whereas fondaparinux is effective for extended prophylaxis in patients undergoing surgery for hip fracture.29 Neither agent requires coagulation monitoring, but both must be given by subcutaneous injection. The efficacy of ximelagatran in this setting has yet to be determined.
Treatment of VTE
LMWH is effective and safe for the initial treatment of acute VTE.2 The recently completed MATISSE trials39,40 indicate that fondaparinux is as effective and safe as heparin or LMWH for initial treatment of patients with VTE. The most pressing clinical need in the treatment VTE is for longer-term secondary prevention after the acute event. Although long-term warfarin therapy markedly reduces the risk of recurrence, its benefit is offset by the risk of major bleeding, and the inconvenience of frequent monitoring.98-102 Ximelagatran does not require coagulation monitoring, and based on the results of the THRIVE trials, 41,42 ximelagatran has the potential to replace warfarin for secondary prevention of VTE. Still to be determined is the clinical impact of the abnormalities in liver function tests on the utility of this drug.
Prevention of cardioembolic events
Although warfarin is more effective than aspirin at reducing the risk of embolization in high-risk patients with atrial fibrillation, it has limitations.1,103 Even with monitoring in specialized clinics, the level of anticoagulation with vitamin K antagonists is outside the therapeutic range almost half of the time.1,103 Furthermore, the risk of major bleeding with long-term treatment increases in the elderly, the population where atrial fibrillation presents the greatest risk. Because of these problems, it is estimated that warfarin is not given to almost half of the eligible patients with atrial fibrillation.103 Ximelagatran has the potential to replace warfarin for this indication if the effects of ximelagatran on liver function tests are not limiting.
Acute coronary syndromes
Parenteral anticoagulants continue to have a role in the treatment of acute coronary syndromes. The results of the REPLACE-2 trial74,84 suggest that bivalirudin obviates the need for GPIIb/IIIa antagonists in low-risk to moderate-risk patients undergoing PCI and reduces the risk of bleeding. The role of fondaparinux, NAPc2, and DX9065a for these indications remains to be established. Long-term treatment with the combination of aspirin and clopidogrel104 or with warfarin105 is more effective at reducing the risk of recurrent ischemia than aspirin alone, but at the cost of more bleeding. The relative efficacy and safety of aspirin plus clopidogrel versus warfarin is unknown. The initial results with ximelagatran are promising; eventually, ximelagatran will have to be compared with warfarin or the combination of aspirin and clopidogrel.
Mechanistic insights
Based on the results of these randomized trials it is not possible to conclude with certainty that any 1 anticoagulant has a better efficacy-to-safety index than another. All of the anticoagulants evaluated for the prevention of VTE cause excessive bleeding if given in the immediate perioperative period. Although fondaparinux was more effective than enoxaparin in patients undergoing major orthopedic surgery, it was started sooner after surgery and, if started too soon, was associated with increased bleeding.38,43 In patients undergoing percutaneous coronary interventions, bivalirudin was associated with less bleeding than heparin in 2 studies.74,75 However, when used as adjuncts to streptokinase, there was more bleeding with bivalirudin than with heparin.76 Consequently, the difference in the efficacy-to-safety index between these 2 anticoagulants remains uncertain. Ximelagatran showed a similar efficacy to safety index to dose-adjusted warfarin.93,94 Because anticoagulant control with warfarin is likely to be better in the clinical trial setting than in routine practice, 1,93,94 ximelagatran may prove to be safer, with respect to bleeding, than warfarin. There now are newer parenteral anticoagulants that are more convenient to use than heparin because they produce a more predictable anticoagulant response, thereby obviating the need for routine monitoring. The introduction of fondaparinux and bivalirudin also provides an opportunity to eliminate heparin-induced thrombocytopenia. Although these new agents are a step forward, the greatest clinical need is for an oral anticoagulant that is safe and effective when used in fixed or weight-adjusted doses without routine coagulation monitoring. As the first such agent, ximelagatran is promising. With other oral direct thrombin and factor Xa inhibitors in development, we soon will have a number of oral anticoagulants that have the potential to replace warfarin. So, more than 60 years since the introduction of the coumarins, this class of compounds will likely be replaced by new and more convenient oral anticoagulation in the very near future.
Prepublished online as Blood First Edition Paper, June 10, 2004; DOI 10.1182/blood-2003-12-4195.
M.O. is the recipient of a Research Fellowship from the Canadian Stroke Network, the Heart and Stroke Foundation of Canada, and the Canadian Institute of Health Research in association with AstraZeneca Canada; J.I.W. is a Career Investigator of the Heart and Stroke Foundation of Canada and holds the Heart and Stroke Foundation of Ontario/J. F. Mustard Chair in Cardiovascular Research and the Canada Research Chair in Thrombosis from the Government of Canada. J.H. and J.I.W. have declared a financial interest in a company whose product was reviewed in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.