Abstract
The long-term follow-up of a study including 214 patients receiving either peripheral blood stem cells (PBSCs) or bone marrow (BM) from an HLA-A, -B, and -DR–compatible unrelated donor is presented. Median follow-up was 4.4 (2.3-7.3) and 5.0 (0.7-8.4) years in the 2 groups, respectively. Cumulative incidence of overall chronic graft-versus-host disease (GVHD) was similar in the 2 groups (78% vs 71%), while extensive chronic GVHD was significantly more common in the PBSC group compared with the BM group (39% vs 24%, P = .03). The 5-year transplant-related mortality (TRM) was 37% in the PBSC group and 35% in the BM controls (P = .7), and overall survival was 42% in both groups. The relapse incidences were 26% and 27% in the 2 groups, respectively, resulting in a disease-free survival of 41% in both groups. In conclusion, PBSCs from HLA-compatible unrelated donors results in similar outcome compared to BM but implies an increased risk for extensive chronic GVHD.
Introduction
Only few patients have been reported who received peripheral blood stem cells (PBSCs) from unrelated donors.1-3 Reasons for the reluctance to use PBSCs from unrelated donors have been the ethics of administering granulocyte colony-stimulating factor (G-CSF) to healthy volunteers and that the 10- to 15-fold higher donor T-cell content of PBSCs would increase the risk of graft-versus-host disease (GVHD). Some reports have indicated a higher incidence of chronic GVHD after sibling PBSCs.4-6 Previously we reported the initial results comparing PBSCs with BM from unrelated donors at 3 centres.2 Here we report the long-term results.
Study design
Patients
The study group consisted of 107 consecutive patients who received PBSCs from unrelated donors between February 1993 and September 1999 (Table 1). Approval for this study was obtained from the institutional review board at Huddinge University Hospital and the local ethics committee at each center. Informed consent was provided according to the Declaration of Helsinki.
BM control group
For each unrelated PBSC transplant recipient, we selected a control who had received unrelated bone marrow (BM) at almost the same time. Controls were matched for diagnosis, stage of disease, age (< 20 years or > 20 years) and GVHD prophylaxis. No difference between the 2 groups existed except that slightly more patients in the BM group received total body irradiation (TBI)–based conditioning (84% vs 70%, P = .03).
Donors
All donors were HLA-A, -B and -DRβ1 compatible with the patients. Before 1997, class I HLA typing was serological. Since then, polymerase chain reaction-sequence-specific-primer (PCR-SSP) low-resolution typing for class I was used. For HLA class II, genomic high resolution DNA-based typing (PCR-SSP) was used.7 All patients from Huddinge (n = 64) were retyped using PCR-SSP for HLA class I (A, B, and C) and II (DR, DP, and DQ).8
Conditioning
Most patients received cyclophosphamid (Cy) 120 mg/kg, combined with TBI, dose ranging from 10 to 13.5 Gy (Table 1).
GVHD prophylaxis
The most common immunosuppression was cyclosporine A (CsA) and 4 doses of methotrexate (MTX) (Table 1). Twelve patients did not receive MTX as part of GVHD prophylaxis.
Graft-versus-host disease
Chronic GVHD was defined as limited or extensive according to standard criteria.11
Statistics
Analysis was performed on December 10, 2003.
Separate statistical analyses were performed for each end point (relapse, transplant-related mortality [TRM], disease-free survival [DFS], and chronic GVHD). Overall survival and disease-free survival were calculated with the Kaplan-Meier method,12 comparing the groups using the log-rank test (Mantel-Haentszel).13 The incidence of TRM, relapse, and chronic GVHD were estimated using a nonparametric estimator of cumulative incidence curves. The Cox regression model was used to analyze predictive factors for chronic GVHD, TRM, survival, relapse, and DFS.14
In analyzing risk factors for relapse and chronic GVHD, only patients surviving more than 90 days after transplantation were included. Four patients relapsed before day 90 and were included in the risk factor analysis of relapse. Factors significant at the 10% level in the univariate analysis were included in the multivariate analysis. The following factors were analyzed: methotrexate, nucleated cell dose, CD34 dose, patient and donor age and sex, antithymocyteglobulin (ATG), granulocyte colony-stimulating factor (G-CSF) after transplant, stem cell source, disease, disease stage, pretransplant cytomegalovirus (CMV) serology, conditioning, and GVHD.
Results and discussion
PBSC grafts versus BM grafts showed faster engraftment of neutrophils and platelets, which was presented previously.2 In this long-term follow-up we found no difference in overall chronic GVHD: 78% and 71% in patients receiving PBSCs and BM grafts, respectively. This is in accordance with other studies with unrelated donors.1-3
Using HLA-identical sibling donors, some studies have reported a higher incidence of overall chronic GVHD when using PBSCs.4-6 The reason for absence of a difference may be the higher incidence of chronic GVHD using unrelated donors compared to HLA-identical siblings.15,16
As previously reported, chronic myeloid leukemia (CML) and acute GVHD were correlated to chronic GVHD.17 We also found that patients not receiving ATG had more chronic GVHD. Absence of ATG is correlated to more acute GVHD, and acute GVHD triggers chronic GVHD.17
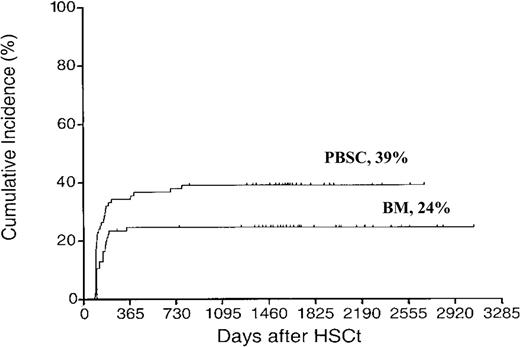
Extensive chronic GVHD was more common in the PBSC group compared with the BM group (39% vs 24%, P = .03) (Figure 1). This has previously been reported using sibling donors4 but not with unrelated donors. In multivariate analysis GVHD grades II-IV (RH 1.88, CI 1.07-3.29, P = .03) and PBSC (RH 1.73, CI 1.00-3.00, P < .05) were correlated to extensive chronic GVHD. As acute GVHD triggers chronic GVHD, it is logical to assume that more severe acute GVHD triggers more severe chronic GVHD. This was previously shown.18 Some information on patients with extensive chronic GVHD is displayed in Table 2.
Cumulative incidence of extensive chronic GVHD. Time to and cumulative incidence of extensive chronic GVHD after unrelated donor stem cell transplantation with peripheral blood stem cells (PBSCs) or bone marrow (BM).
Cumulative incidence of extensive chronic GVHD. Time to and cumulative incidence of extensive chronic GVHD after unrelated donor stem cell transplantation with peripheral blood stem cells (PBSCs) or bone marrow (BM).
TRM was 28% and 29% at 1 year and 37% and 35% at 5 years in the PBSC and BM groups, respectively. This is in line with previous studies with related and unrelated donors. Acute GVHD is one of the main causes for TRM, and most studies comparing PBSCs and BM showed similar incidence of acute GVHD.
In the multivariate analysis, acute GVHD grades II-IV (RH 6.05, CI 3.67-9.97, P < .001), patient age (RH 1.34, CI 1.09-1.63, P = .007), and absence of MTX as GVHD prophylaxis (RH 3.10, CI 1.28-7.46, P = .012) were independent risk factors for TRM.
The 5-year probability of survival was 42% in both groups. Among patients with early disease (CR1/CP1), the probability of survival was 56% and 54%, and for patients in later stages it was 24% and 26% in the PBSC and the BM group, respectively (not statistically significant [ns]).
Primary causes of death were relapse in 19 and 25, infection in 17 and 13, GVHD in 18 and 13, and other causes, 6 and 10 in the PBSC and BM group, respectively (ns). In the multivariate analysis, absence of chronic GVHD (RH 3.29, CI 1.99-5.42 P < .001), late disease (RH 2.32, CI 1.49-3.60, P < .001), and acute GVHD II-IV (RH 2.34, CI 1.42-3.86, P < .001) were independent risk factors for death.
The cumulative incidence of relapse was 26% and 27% in the PBSC and BM groups, respectively. Among patients with early disease the 5-year incidence of relapse was 9% and 15%, and for patients in late disease it was 37% in both groups (ns).
In the multivariate analysis, advanced disease (RH 4.05, CI 2.03-8.08, P < .001), absence of chronic GVHD (RH 3.22, CI 1.77-5.87, P < .001), and non-CML diagnoses (RH 2.25, CI 1.04-4.85, P = .037) were independent risk factors for relapse. Using unrelated donors, the incidence of chronic GVHD is higher than in HLA-identical siblings, which also carries a graft-versus-leukemia effect,19,20 thus reducing the risk of relapse.
DFS at 5 years was 41% in both groups. Among patients with CML, DFS was 57% and 52% in the PBSC and the BM groups, respectively. Corresponding figures for patients with acute leukemia was 26% and 31% (ns).
Two studies and a meta-analysis demonstrated a survival advantage with PBSC limited to patients with advanced disease.21-23 In our material, we found no difference in DFS among patients given PBSC or BM with CML or acute leukemia with early or late disease.
However, among patients not in remission at transplantation (n = 33), we found a trend for a better OS and DFS (24% vs 11%, P = .11) in patients receiving PBSCs compared with BM. However, the number of patients is small.
In the multivariate analysis, chronic GVHD (RH 3.42, CI 2.14-5.47, P < .001), early disease (RH 2.66, CI 1.72-4.14, P < .001), and acute GVHD 0-I (RH 1.80, CI 1.13-2.89, P = .016) were independent prognostic factors for a better DFS.
With regard to TRM, survival, relapse, and disease-free survival, the outcome was the same in patients receiving PBSCs or BM. This accords with most reports comparing PBSCs with BM using related or unrelated donors,1,2,4-6,26 while a study from European Group for Blood and Marrow Transplantation (EBMT) showed better outcome with rich bone marrow.27
In conclusion, this study has shown that the use of PBSCs from unrelated donors is a safe and well-tolerated procedure, but the risk for extensive chronic GVHD is increased.
Prepublished online as Blood First Edition Paper, September 14, 2004; DOI 10.1182/blood-2004-03-1000.
Supported by grants from the Swedish Cancer Society (0070-B02-16XAC), the Children's Cancer Foundation (2002/074), the Swedish Research Council (K03-32X-05971-23A), the Cancer Society in Stockholm (02;181), and the Tobias Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank all nurses at the departments for excellent and skillful care and all volunteer donors for their generous gifts to the patients.