Abstract
Dendritic cells (DCs) are involved in the initiation and regulation of innate and adaptive immune responses. Several molecular mechanisms regulate these diverse DC functions, and we have previously reported that mouse dendritic cells (mDCs) can produce interleukin-2 (IL-2) in vitro and in vivo, in response to microbial activation and T-cell-mediated stimuli. This property is shared by different DC subtypes, including Langerhans cells. Here we show that, on appropriate stimulation, human DCs, both plasmacytoid and myeloid subtypes, also express IL-2. Interestingly, the production of IL-2 by myeloid DCs is induced by T-cell-mediated stimuli and depends on the presence of IL-15. The key role of this cytokine in regulating IL-2 production was also confirmed in the mouse system. In particular, we could show that DCs from IL-15-deficient mice were strongly impaired in the ability to produce IL-2 after interactions with different microbial stimuli. Our results indicate that DC-produced IL-2 is tightly coregulated with the expression of IL-15.
Introduction
Dendritic cells (DCs) are antigen-presenting cells (APCs) involved in the initiation and regulation of innate and adaptive immune responses. The key role of DCs in regulating innate responses has been recently investigated, and it has been shown that DCs can activate NK cells in humans and in mice.1,2
With regard to T-cell priming, the unique effectiveness of DCs has been demonstrated in many different experimental systems. The high efficiency of DCs as APCs cannot be attributed to a particular DC-specific surface molecule or secreted molecule; rather, it can be attributed to the high level of expression of cell membrane costimulatory proteins, to the efficient antigen-processing machinery, and to the secretion of cytokines, properties acquired by DCs during the maturation process.3
Recently, we have demonstrated that mouse DCs can produce IL-2 after activation with different inflammatory stimuli.4 The production of this cytokine is tightly regulated and is induced in a narrow timeframe after microbial and T-cell-mediated activation.4 IL-2 production can be observed in vitro and in vivo in different DC subtypes, such as CD8α+ and CD8α- splenic DCs and epidermal Langerhans cells.4 IL-2 produced by DCs represents an additional relevant molecule conferring on DCs a unique T-cell priming capacity. Early bacterially activated IL-2-deficient DCs are, indeed, severely impaired in the ability to induce allogeneic CD8+ and CD4+ T-cell proliferation if compared with wild-type (WT) DCs.5 Moreover, cytomegalovirus is an immunosuppressive virus that establishes persistent infection, blocks IL-2 production by DCs, and affects the capacity of DCs to activate T cells.6 In addition to the role in T-cell priming, we have found that DC-derived IL-2 is also required for DC-mediated NK cell activation (manuscript in preparation33 ). Given the function played by DC-derived IL-2 in activating NK and T cells in the mouse system and given the fact that IL-2 and DCs are used in human tumor therapy to prime antimetastatic NK cell activities,7 it was relevant to establish whether human DCs could produce IL-2 and to determine the signals required to induce IL-2 production.
Materials and methods
Antibodies and reagent used for human cells
Lipopolysaccharide (LPS; Escherichia coli 026:B6, used at 1 μg/mL) was obtained from Sigma Chemical (St Louis, MO). Influenza virus (strain A/Beijing/353/89) was kindly provided by I. Julkunen. CpG oligodeoxynucleotide 2216 was obtained from Microsynth (Balgach, Switzerland), and rCD40L was obtained from Alexis Biochemicals (Lausen, Switzerland). Phycoerythrin (PE)-conjugated anti-CD86, PE-conjugated anti-CD80, PE-conjugated anti-CD83, PE-conjugated anti-CD40, PE-conjugated anti-IL-2, and PE-conjugated rat isotype control were obtained from Becton Dickinson (San Jose, CA). Biotin-conjugated anti-CD40L (Serotec, Oxford, United Kingdom) and PE-conjugated streptavidin were obtained from Sigma Chemical. Fluorescein isothiocyanate (FITC)-conjugated anti-CD11c was obtained from DAKO Cytomation (Glostrup, Denmark). PE-conjugated anti-Langerin was obtained from Schering-Plough (Kenilworth, NJ). Recombinant human granulocyte macrophage-colony-stimulating factor (rhGM-CSF) was obtained from (Mielogen, Schering-Plough), rhIL-4 was from Becton Dickinson, rhIL-15 was from R&D Systems (Minneapolis, MN; Serotec, Oxford, United Kingdom), rhIL-13 was from Schering-Plough, and recombinant tumor necrosis factor-α (rhTNF-α), rhIL-1β, rhIL-6, and rhIL-3 were from R&D Systems.
Complete RPMI medium consisted of RPMI 1640, 2 mM L-glutamine, 100 U/mL penicillin, 50 μg/mL streptomycin, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids (BioWhittaker, Walkersville, MD), and heat-inactivated 10% fetal bovine serum (FBS; Hyclone, South Logan, UT).
Generation of monocyte-derived DCs from PBMCs
Monocytes were positively selected from peripheral blood mononuclear cells (PBMCs by magnetic bead separation using CD14 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). The CD14+ cells were cultured in 24-well plates (5 × 105/well) for 5 days in the presence of GM-CSF (800 U/mL)/IL-4 (1000 U/mL) or GM-CSF (800 U/mL)/IL-13 (20 U/mL) or GM-CSF (800 U/mL)/IL-15 (400 U/mL). On day 3, half the supernatant was replenished with fresh complete medium and cytokines.
Purification and activation of human CD4+ T cells
Purified CD4+ T cells were obtained from PBMCs of the same donors from which we derived the DCs by positive magnetic selection after incubation with beads coated with anti-CD4 antibody (Miltenyi Biotec), following the manufacture's instructions. CD4+ T cells (1 × 106 cells/mL) and autologous monocytes (0.25 × 106 cells/mL), as antigen-presenting cells, were cultured in 24-well plates in RPMI 1640 medium supplemented with 10% heat-inactivated human serum (BioWhittaker) in the presence of phytohemagglutinin (10 μg/mL) (Sigma Aldrich, Milan, Italy) for 3 days. Expression of CD40L was determined by fluorescence-activated cell sorter (FACS) analysis.
Analysis of IL-2 production by human MoDCs
Monocyte-derived DCs (MoDCs) generated with GM-CSF and IL-15, GM-CSF and IL-13, or GM-CSF and IL-4 were incubated for 6 hours with TNF-α, IL-1β, and IL-6 plus LPS or J558 CD40L cells or for 12 hours with LPS, TNF-α, IL-1β, and IL-6 and for 6 hours with J558 CD40L cells. MoDCs generated with GM-CSF and IL-15 were also incubated for 6 hours with autologous activated CD4+ T cells. MoDCs were incubated with Brefeldin A (10 μg/mL; Sigma Chemical) for 5 hours, and cells were fixed with 2% paraformaldehyde, permeabilized with phosphate-buffered saline (PBS) containing 5% fetal calf serum (FCS) and 0.5% saponin, and stained. Cells were then analyzed through FACScan (Becton Dickinson).
IL-12p70 enzyme-linked immunosorbent assay
The presence of IL-12p70 in the supernatants from human DCs obtained in the presence of GM-CSF and IL-4, IL-13, IL-15 and induced to maturation with LPS or J558 CD40L cells was determined with the use of a commercially available kit (Bender MedSystems GmbH, Vienna, Austria) according to the manufacturer's instructions.
Purification of human pDCs
BDCA4+ cells stained with PE-conjugated anti-BDCA4 (Miltenyi Biotec) were positively selected from PBMCs by magnetic bead separation anti-PE (Miltenyi Biotec), following the manufacture's instructions. The BDCA4+ CD14- cells were then sorted on a FACSVantage (Becton Dickinson).
IL-2 transcript in pDCs
Plasmacytoid DCs (pDCs) were cultured in 96-well plates (5 × 104/well) in complete RPMI medium in the presence of rhIL-3 (10 ng/mL) for 2, 6, and 16 hours in the presence of CpG, CD40L, or influenza virus, or for 12 hours with influenza virus or with CpG plus 4 hours of CD40L.
RT-PCR. mRNA was isolated from the cell samples using the Trizol procedure (Invitrogen, Carlsbad, CA). The mRNA was then converted to whole cDNA with random hexamers (Invitrogen). Specific cDNA was amplified by real-time PCR using specific primers.
Real-time PCR. TaqMan probes for IL-2 and 18S (internal control) (Applied Biosystems, Foster City, CA). The normalization to 18S is the result of 2(-ΔCT), where ΔCT = IL-2 18S.
Antibodies and reagents used for mouse cells
LPS (E coli 026:B6, used at 10 μg/mL or 50 μg/mouse; Sigma Chemical), CpG 5′-TCC ATG ACG TTC CTG ATG CT-3′ (1 μM; Primm, Milan, Italy), and zymosan A (used at 10 μg/mL; Sigma Chemical) were the antibodies used. Biotin-conjugated anti-CD3, biotin-conjugated anti-CD19, PE-conjugated anti-B7.2, PE-conjugated anti-IL-2, PE-conjugated rat isotype control, PE-conjugated anti-CD69, and FITC-conjugated anti-CD11c monoclonal antibodies (Becton Dickinson) were the reagents used.
Mice
Pathogen-free C57BL/6 and BALB/c mice were purchased from Harlan-Italy, C57BL/6 IL-15 KO8 were obtain from Taconic (Germantown, NY), and C57BL/6 IL-2Rγ knockout (KO) mice were kept in pathogen-free conditions and were used at 6 to 10 weeks of age. All experiments were performed in compliance with the relevant laws and institutional guidelines.
DCs and culture medium for mouse cells
Bone marrow-derived DCs (BMDCs) were obtained from C57BL/6 or C57BL/6 IL-15 KO or C57BL/6 IL-2Rγ KO mice. Bone marrow cells were cultured for 5 days in Iscove modified Dulbecco medium (IMDM) (Sigma Chemical) containing 10% heat-inactivated fetal bovine serum (Gibco; Invitrogen), 100 IU penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine (all from Sigma Chemical), and 50 μM 2-mercaptoethanol (complete IMDM) in the presence of 10% of the supernatant of GM-CSF-transduced B16 tumor cell9 culture and then frozen. BMDCs were obtained from thawed bone marrow cells after 7 days of culture in the medium described previously. Only immature BMDCs were used for activation, as judged by low B7.2 and CD40 expression. For IL-2 experiments, 2.5 × 105 cells/well were plated in 24-well plates in 0.5 mL medium.
IL-2 and IL-12p70 enzyme-linked immunosorbent assay
The presence of IL-2 and IL-12p70 in mouse DCs was detected by using the DuoSet kit (R&D Systems, Minneapolis, MN) and following the manufacturer's recommendations.
In vivo analysis of IL-2 production by mouse DCs
C57BL/6 WT or IL-15 KO mice were intraperitoneally injected with 50 μg LPS. Three hours after treatment, spleens were removed, unicellular suspensions were made, and CD11c+ cells were enriched by negative selection of T and B cells. These cells were eliminated with the Dynal Simply magnetic system (Dynal AS, Oslo, Norway). The CD11c+ cells were then sorted on a MoFlo (DAKO Cytomation, Glostrup, Denmark). We incubated the CD11c+ cells with Brefeldin A (10 μg/mL; Sigma Chemical) for 1.5 hours. Cells were then fixed with 2% paraformaldehyde, permeabilized with PBS containing 5% FCS and 0.5% saponin, and stained. Cells were analyzed with the use of FACScan (Becton Dickinson).
Mixed lymphocyte reaction
BMDC WT or IL-15 KO (2.5 × 105) were activated with zymosan A for 2 hours, and 4 to 7 hours later they were incubated with 1 × 106 carboxyfluorescein diacetate succidimyl ester (CFSE)-labeled T cells. CD4+ and CD8+ lymphocytes were purified (to 99% and 95% purity, respectively) from BALB/c lymph nodes by negative selection of macrophages, DCs, B cells, and CD4+ or CD8+ T cells. These cell populations were eliminated with the Dynal Simply magnetic system (Dynal AS) after preincubation with Mac1, CD11c, CD19, and CD4 or CD8 antibodies (all from Becton Dickinson). T-cell division was assessed through FACS analysis 48 or 72 hours later.
Results
Human MoDCs generated in the presence of IL-15 produce IL-2 after CD40-mediated stimulation
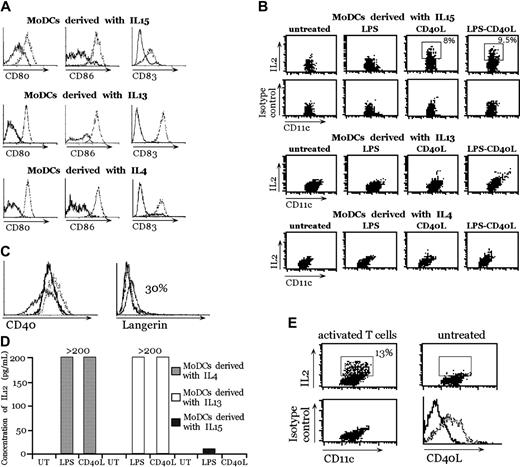
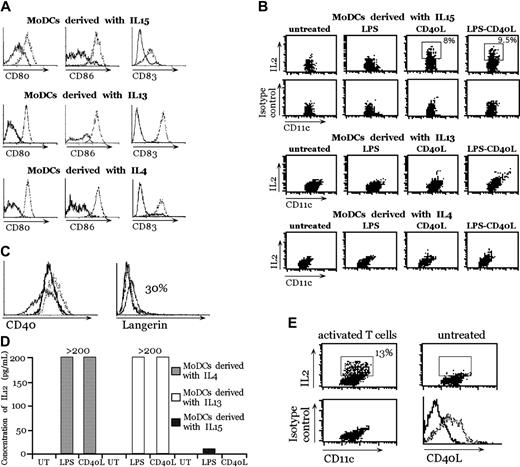
MoDCs were generated in vitro from human peripheral blood CD14+ cells by using 3 different procedures that yielded DCs functionally similar in their ability to prime T cells:10-12 in the presence of GM-CSF and IL-410 ; in the presence of GM-CSF and IL-1311 ; or in the presence of GM-CSF and IL-15.12 These MoDCs were equally able to undergo maturation after interaction with LPS and CD40L, as assessed by the up-regulation of activation markers, such as CD80, CD86, and CD83 (Figure 1A). Therefore, we tested the ability of MoDCs to produce IL-2 after stimulation with LPS, CD40L, and LPS plus CD40L. To potentially increase DC activation13 and to create a physiologic inflammatory environment, a cocktail of cytokines composed of IL-6, TNF-α, and IL-1β was added. These cytokines alone were unable to induce IL-2 production in MoDCs (data not shown). MoDCs were also stimulated with zymosan A, which is the strongest inducer of IL-2 in mouse bone marrow-derived DCs.4 As shown in Figure 1B, the only type of MoDCs competent for IL-2 production after activation were MoDCs derived in the presence of IL-15, and the only stimulus capable of inducing IL-2 was CD40L (Figure 1B). A clear IL-2-positive population was detectable by intracellular staining in MoDCs generated in the presence of IL-15 6 hours after stimulation with CD40L. In contrast, LPS alone (Figure 1B) or zymosan A or Streptococcus pyogenes (data not shown) did not induce IL-2 in any of the 3 MoDC types. We tested then, whether, in the absence of inflammatory cytokines, CD40L was able to promote IL-2 production. Indeed, CD40L was able to induce IL-2 expression in the absence of these cytokines (data not shown).
IL-2 production by human MoDCs derived with GM-CSF + IL-15, GM-CSF + IL-13, or GM-CSF + IL-4 after stimulation. (A) Expression of maturation markers (CD80, CD86, and CD83) by MoDCs after stimulation with LPS + CD40L. Unstimulated DCs (solid lines) or DCs stimulated for 24 hours with LPS + CD40L (dotted lines). (B) IL-2 production by MoDCs derived with GM-CSF + IL-15, GM-CSF + IL-13, or GM-CSF + IL-4. Double stainings with anti-CD11c and anti-IL-2 or isotype control antibodies were performed 6 hours after LPS, CD40L, or LPS + CD40L treatment in the presence of IL-6, TNF-α, and IL-1β. This experiment represents 1 of 4 independent experiments with comparable results. (C) Comparison of CD40 and Langerin expression on MoDCs. MoDCs + IL-4 (dotted lines), MoDCs + IL-15 (thin lines), and MoDCs + IL-13 (thick lines). (D) IL-12p70 production by MoDCs was measured in the supernatant after 24 hours of stimulation with LPS or CD40L with enzyme-linked immunosorbent assay (ELISA). The range of the standard is 3 pg/mL to 200 pg/mL. (E) IL-2 production of MoDCs + IL-15 activated by activated CD4+ T cells. Double stainings with anti-CD11c and anti-IL-2 or isotype control antibodies were performed 6 hours after CD4+ T-cell activation. Panels A and B are representative of 1 of 4 experiments each. Panels C, D, and E are representative of 1 of 2 experiments each.
IL-2 production by human MoDCs derived with GM-CSF + IL-15, GM-CSF + IL-13, or GM-CSF + IL-4 after stimulation. (A) Expression of maturation markers (CD80, CD86, and CD83) by MoDCs after stimulation with LPS + CD40L. Unstimulated DCs (solid lines) or DCs stimulated for 24 hours with LPS + CD40L (dotted lines). (B) IL-2 production by MoDCs derived with GM-CSF + IL-15, GM-CSF + IL-13, or GM-CSF + IL-4. Double stainings with anti-CD11c and anti-IL-2 or isotype control antibodies were performed 6 hours after LPS, CD40L, or LPS + CD40L treatment in the presence of IL-6, TNF-α, and IL-1β. This experiment represents 1 of 4 independent experiments with comparable results. (C) Comparison of CD40 and Langerin expression on MoDCs. MoDCs + IL-4 (dotted lines), MoDCs + IL-15 (thin lines), and MoDCs + IL-13 (thick lines). (D) IL-12p70 production by MoDCs was measured in the supernatant after 24 hours of stimulation with LPS or CD40L with enzyme-linked immunosorbent assay (ELISA). The range of the standard is 3 pg/mL to 200 pg/mL. (E) IL-2 production of MoDCs + IL-15 activated by activated CD4+ T cells. Double stainings with anti-CD11c and anti-IL-2 or isotype control antibodies were performed 6 hours after CD4+ T-cell activation. Panels A and B are representative of 1 of 4 experiments each. Panels C, D, and E are representative of 1 of 2 experiments each.
The difference in IL-2 production was not caused by a differential expression of CD40. In fact, the expression of CD40 on MoDCs generated in the various culture conditions was comparable (Figure 1C). In contrast, Langerin was weakly expressed (up to 30% of MoDCs only when generated in the presence of IL-15) (Figure 1C). The lack of ability to produce IL-2 was not attributable to a general impairment. Indeed MoDCs generated with IL-13 and IL-4 produced high amounts of IL-12p70 after LPS or CD40L stimulation (Figure 1D). Interestingly, MoDCs generated with IL-15 did not produce IL-12p70 after maturation with LPS or with CD40L (Figure 1D).
To test a more physiologic stimulation, we used activated CD4 T cells, which express CD40L (Figure 1E), to induce IL-2 production by MoDCs derived in the presence of IL-15. As expected, DCs were able to produce IL-2 (Figure 1E) with an even higher percentage of MoDC IL-2+ compared with stimulation with the J558 CD40L-expressing cells (Figure 1B). In conclusion, only MoDCs generated with IL-15 acquired the ability to produce IL-2 after CD40L stimulation.
DCs from IL-15-deficient mice produce a reduced level of IL-2 but are still functional
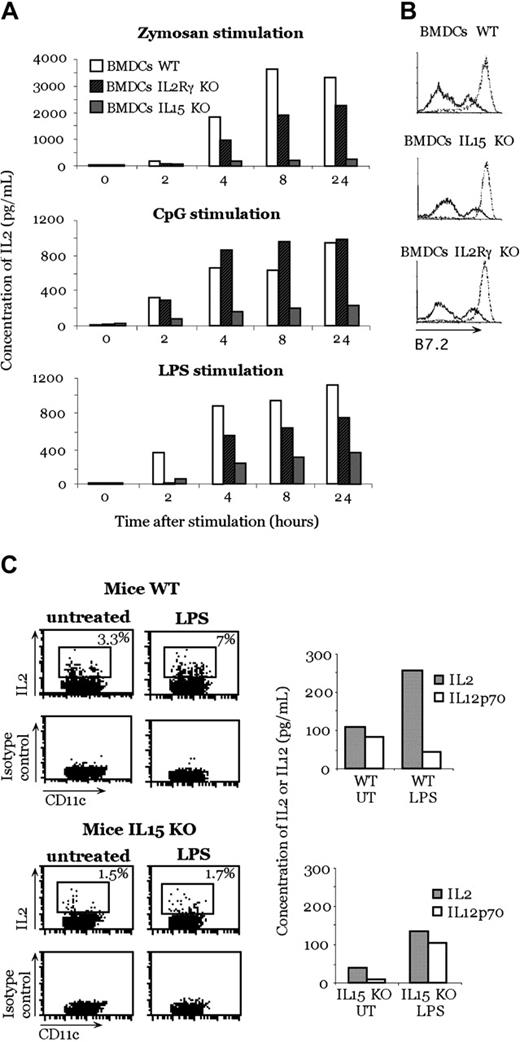
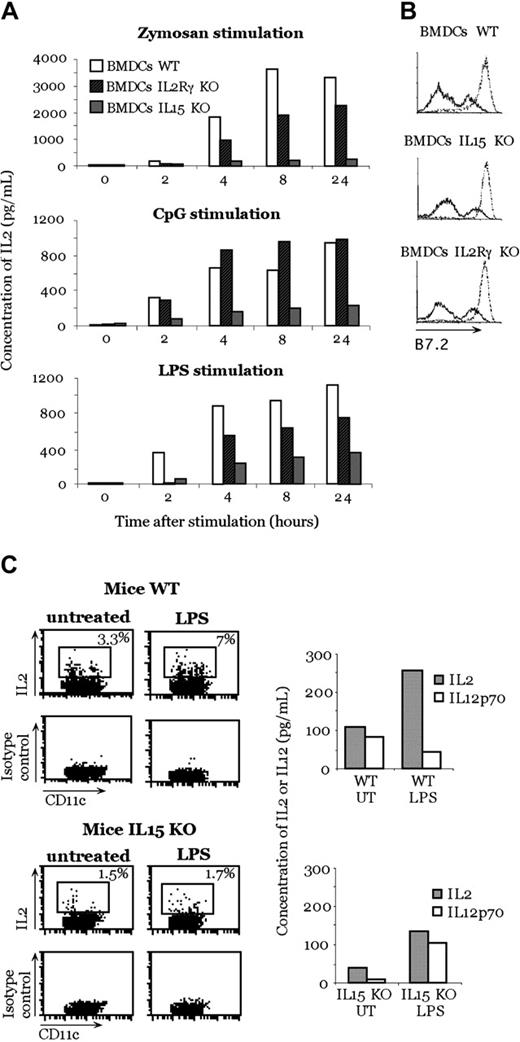
Because mouse DCs produce IL-15 early after activation with inflammatory stimuli,14 we investigated whether the presence of IL-15 is required in the mouse system to render DCs capable of producing IL-2 after activation. We generated BMDCs from IL-15- or IL-2Rγ-deficient and WT mice and stimulated them with zymosan A, CpG, and LPS. We used IL-2Rγ-deficient DCs because this receptor transduces signals of many different cytokines, such as IL-2, IL-15, IL-4, IL-7, and IL-9. After stimulation with zymosan A, CpG, and LPS, we observed a strong reduction in IL-2 production by BMDCs generated from IL-15-deficient mice in comparison with BMDCs generated from WT mice (Figure 2A). As did zymosan A, LPS stimulation produced a reduced IL-2 secretion by IL-2Rγ-deficient mice BMDCs compared with WT BMDCs (Figure 2A). When BMDCs from IL-2Rγ-deficient mice were stimulated with CpG, we did not see a difference in IL-2 production compared with WT BMDCs.
Inhibition of IL-2 production in IL-15-deficient mice. (A) IL-2 production by IL-15-deficient, IL-2Rγ-deficient, and WT BMDCs after zymosan A, CpG, and LPS stimulation. (B) Activation profile of BMDCs after stimulation with zymosan. B7-2 molecule expression of unstimulated DCs (solid lines) and after 24-hour zymosan activation (dotted lines). (C) In vivo IL-2 production by DCs after LPS treatment. LPS was injected into WT or IL-15-deficient mice. Three hours later, CD11c+ cells were purified from the spleen and double stained with anti-CD11c and anti-IL-2 or isotype control antibodies. Some sorted cells were put in culture; after 24 hours, the production of IL-2 and IL-12p70 in the supernatant was measured. Panels A, B, and C are representative of 1 experiment each of 3 independent experiments with comparable results.
Inhibition of IL-2 production in IL-15-deficient mice. (A) IL-2 production by IL-15-deficient, IL-2Rγ-deficient, and WT BMDCs after zymosan A, CpG, and LPS stimulation. (B) Activation profile of BMDCs after stimulation with zymosan. B7-2 molecule expression of unstimulated DCs (solid lines) and after 24-hour zymosan activation (dotted lines). (C) In vivo IL-2 production by DCs after LPS treatment. LPS was injected into WT or IL-15-deficient mice. Three hours later, CD11c+ cells were purified from the spleen and double stained with anti-CD11c and anti-IL-2 or isotype control antibodies. Some sorted cells were put in culture; after 24 hours, the production of IL-2 and IL-12p70 in the supernatant was measured. Panels A, B, and C are representative of 1 experiment each of 3 independent experiments with comparable results.
The observed difference in IL-2 production was not caused by a defect in mutant DC maturation because no difference in the expression of B7.2 was observed after zymosan A exposure (Figure 2B). Thus, this phenomenon is not attributed to an inability to undergo maturation.
The relevance of IL-15 in DC production of IL-2 was also tested in vivo. IL-15-deficient or WT mice were injected with LPS, and, 3 hours later, CD11c+ DCs were sorted from spleens to obtain a pure population (98%) of DCs. LPS was used because it was previously shown to be the best stimulus to induce IL-2 production in vivo.4 Less than 2% of DCs from IL-15-deficient mice produced IL-2, whereas up to 7% of DCs from WT mice were IL-2 positive (Figure 2C), as assessed by intracellular staining. This result was further confirmed by testing cytokine production from the same sorted cells. In contrast, the absence of IL-15 in the KO mice did not affect the ability to produce other cytokines, such as IL-12p70, in response to the same stimulus (LPS) (Figure 2C). As a whole, these results indicate a relevant role of IL-15 in mouse DCs.
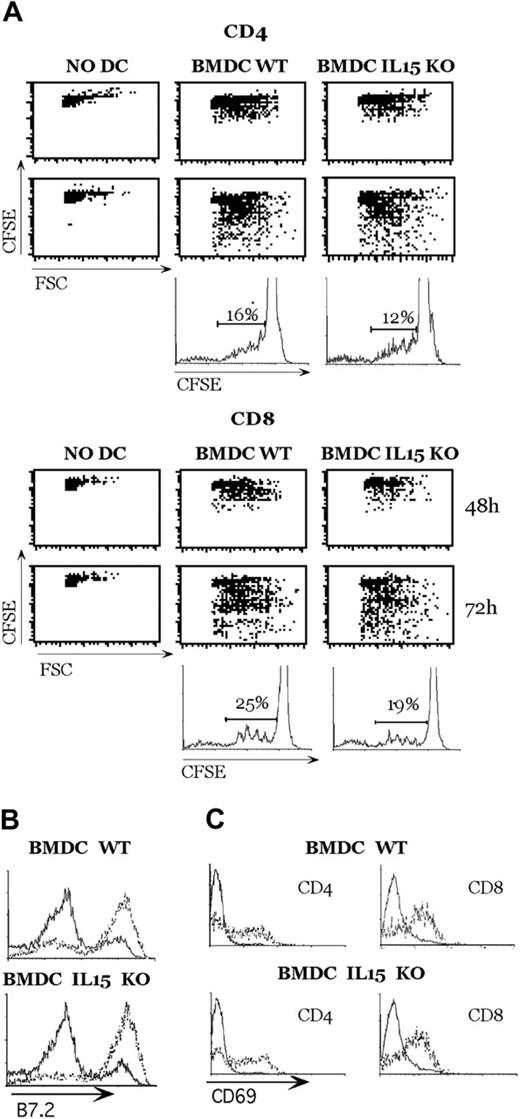
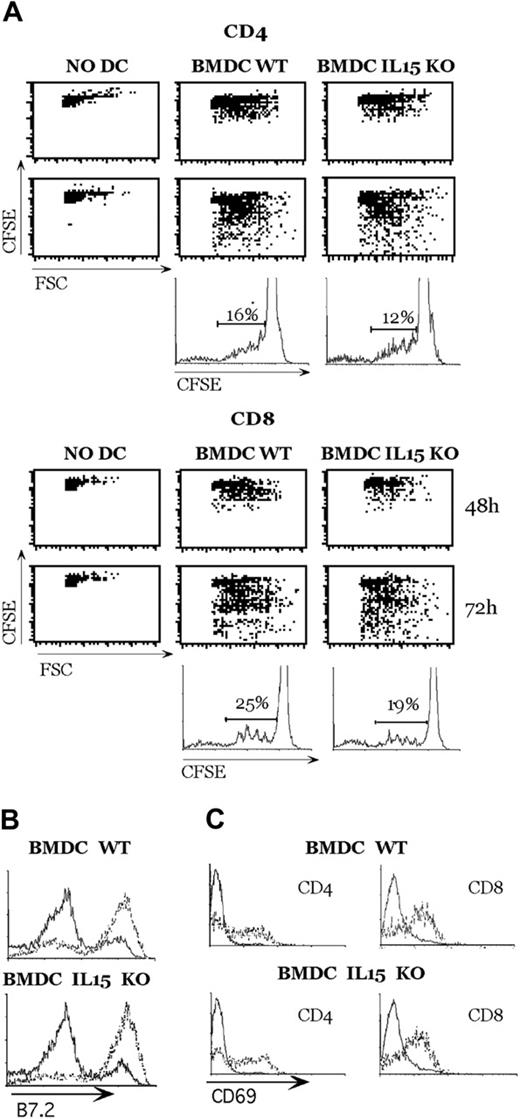
To evaluate whether DCs from IL-15-deficient mice were functionally responsive, we performed an allogeneic mixed lymphocyte reaction. As shown in Figure 3A, BMDCs from WT and IL-15 KO mice could induce CD4+ and CD8+ T-cell proliferation. The lateness of IL-15-deficient mice in inducing T-cell proliferation was not caused by the difference in BMDC maturation (Figure 3B) or in T-cell activation (Figure 3C), but it could have been caused by an absence of IL-15 and a reduced production of IL-2 by DCs.
Mouse DCs from IL-15-deficient mice induce alloreactive T-cell activation. (A) DCs (5 × 105) were stimulated with zymosan A in 24-well plates, and 1 × 106 CSFE-labeled CD4+ or CD8+ allogeneic T cells were added to the culture. Cycling cells were analyzed at the indicated time points by FACS analysis. (B) Expression of the indicated molecules by unstimulated DCs (solid lines) or DCs that had been activated by zymosan A for 18 hours (dotted lines) were analyzed by flow cytometry. (C) After 48 hours of culture, CD69 expression was evaluated on small noncycling T lymphocytes (solid lines) and large T-cell blasts (dotted lines). This experiment represents 1 of 2 independent experiments with comparable results.
Mouse DCs from IL-15-deficient mice induce alloreactive T-cell activation. (A) DCs (5 × 105) were stimulated with zymosan A in 24-well plates, and 1 × 106 CSFE-labeled CD4+ or CD8+ allogeneic T cells were added to the culture. Cycling cells were analyzed at the indicated time points by FACS analysis. (B) Expression of the indicated molecules by unstimulated DCs (solid lines) or DCs that had been activated by zymosan A for 18 hours (dotted lines) were analyzed by flow cytometry. (C) After 48 hours of culture, CD69 expression was evaluated on small noncycling T lymphocytes (solid lines) and large T-cell blasts (dotted lines). This experiment represents 1 of 2 independent experiments with comparable results.
Human pDCs transcribe high levels of IL-2 mRNA after stimulation
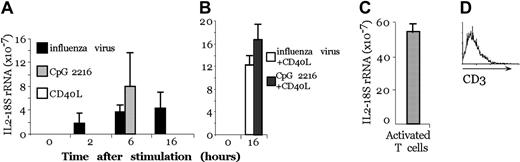
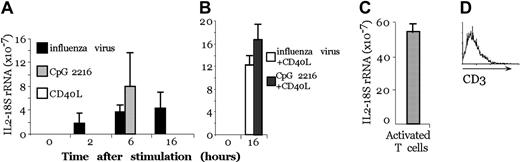
We then tested whether human pDCs were able to produce IL-2 after stimulation. The interferon-producing cells (IPCs), renamed pDCs, exerted potent antiviral activity because of the large amounts of type 1 IFN produced in response to viruses and microbial stimuli, including bacterial DNA.15 pDCs express different cytokines and different toll-like receptors (TLRs) than MoDCs. In particular, MoDCs express TLR 2, 3, 4, and 5, whereas pDCs express TLR 7, 8, and 9.16 We purified pDCs from human blood using a double-selection procedure, first by magnetic bead purification and second by FACS sorting, to obtain a pure population (98%) of pDCs. In these populations, no T-cell contamination was present, as indicated by anti-CD3 FACS analysis (Figure 4D).
Activated pDCs transcribe the IL-2 gene. (A) Real-time PCR on pDCs after 2, 4, 6, and 16 hours of treatment with CpG, CD40L, or influenza virus. (B) Real-time PCR on pDCs after 4-hour pretreatment with CpG or influenza virus and 12-hour treatment with CD40L. (C) Real-time PCR on activated T cells with anti-CD3 and PMA. (A-C) IL-2 transcripts and 18S rRNA were quantified with real-time florigenic RT-PCR. Transcript abundance is represented as the ratio of IL-2 to 18S rRNA. Error bars represent the standard deviation of 3 different experiments. (D) CD3 profile of the sorted population.
Activated pDCs transcribe the IL-2 gene. (A) Real-time PCR on pDCs after 2, 4, 6, and 16 hours of treatment with CpG, CD40L, or influenza virus. (B) Real-time PCR on pDCs after 4-hour pretreatment with CpG or influenza virus and 12-hour treatment with CD40L. (C) Real-time PCR on activated T cells with anti-CD3 and PMA. (A-C) IL-2 transcripts and 18S rRNA were quantified with real-time florigenic RT-PCR. Transcript abundance is represented as the ratio of IL-2 to 18S rRNA. Error bars represent the standard deviation of 3 different experiments. (D) CD3 profile of the sorted population.
By real-time PCR, we observed that human pDCs, after 2-hour stimulation with influenza virus, transcribed IL-2 mRNA and that the transcript level increased with time (after 6 and 16 hours of stimulation), whereas using CpG, IL-2 mRNA expression could be detected only after 6 hours (Figure 4A). In contrast, pDC stimulation with CD40L did not induce IL-2 transcription unless the cells were previously activated with CpG or influenza virus for 12 hours and then were challenged with CD40L for another 4 hours. Thus, reactivation with CD40L induced a second wave of IL-2 transcription in cells pretreated with CpG (Figure 4B), though there was no modification of the quantity of IL-2 transcript if the cells were pretreated with influenza virus, probably because of saturation of the signal. Figure 4C shows that activated T cells that produce the IL-2 protein have a major quantity of IL-2 mRNA compared with pDCs.
Discussion
In this study we have investigated whether, as in mouse DCs, human MoDCs can produce IL-2 and what signals are required for such activation. We found that MoDCs generated in the presence of IL-15, but not MoDCs generated in the presence of IL-4 and IL-13, can express IL-2 on activation with CD40L. In the mouse system, only immature DCs acquire the ability to produce IL-2 after activation, and, according to this observation, Langerhans cells efficiently produce IL-2 on LPS encounter.4 MoDCs generated with IL-15 show Langerhans cell-like characteristics, such as Langerin expression12 (Figure 1C). Moreover, they show a lower expression of CD83 after activation with LPS plus CD40L compared with MoDCs derived with IL-4 and IL-13, suggesting the acquisition of a less-mature phenotype, which could also explain their lack of IL-12 production.
In human MoDCs generated with IL-15, IL-2 expression is obtained with T cell-derived signals, such as CD40L. Thus, in human MoDCs, contact with T cells plays a key role, whereas in mouse DCs, we have demonstrated not only that T cell-mediated stimuli but also that microbes alone can induce IL-2 production.4 Thus, IL-2 expression seems to be more tightly regulated by human DCs than by mouse DCs. IL-2 production by human DCs during T-cell contact of activated T cells (the expression of CD40L is fundamental) could have a role in CD8+ T-cell activation or could induce regulation of the immune response because IL-2 is involved in apoptosis.17 In the mouse, DCs produce IL-2 after microbial stimulation, and T-cell contact is not necessary. Thus, IL-2 can activate cells in the infected tissue, such as NK cells, before they migrate to the lymphoid organs. Moreover, it is likely that, in pDCs, the regulation of IL-2 production is not achieved by a rapid turnover of the mRNA, as in T cells, but by post-transcriptional modifications in which IL-15 may play a fundamental role. This also appears to be relevant in mouse DCs because IL-15-deficient cells produce low levels of IL-2. This phenomenon appears not to be caused by an inability to undergo maturation, because we were able to induce full phenotypic and functional maturation of the activation of mutant DCs after encounters with different microbial stimuli. This finding is in contrast to that of a previous work in which DCs derived from IL-15-deficient mice failed to undergo functional maturation or to produce IL-12p70 after activation.18 The IL-15Rα chain is essential for IL-15 ligation but cannot transduce the IL-15 signal19 ; thus, the observed difference in IL-2 production by IL-15-and IL-2Rγ-deficient mice could be explained by a different recruitment pathway of the β chain versus the γ chain: the β chain seems to be essential for the IL-15 signal transduction pathway generated by IL-2 and IL-15, whereas the γ chain tranduces a signal in response to 5 different cytokines. This observation may explain the reduced effect on IL-2 production by the IL-2Rγ-deficient mice.
IL-15 can be produced by human and mouse LPS-activated macrophages, by mouse intestinal epithelial cells on their infection by Listeria monocytogenes,20 in human skin after ultraviolet B irradiation,21 after infection with Mycobacterium leprae, Cryptococcus neoformans, Candida albicans, herpesvirus 6, herpesvirus 7, and hepatitis C.22 Moreover, it has been shown that IL-15/IL-15Rα can be presented in trans to neighboring cells that express intermediate-affinity IL-15R.23 Thus, we can hypothesize that DCs present at, or recruited to, the infection site are in close contact with exogenous IL-15 and produce IL-2 after T-cell contact and activation.
IL-15 and IL-2 are connected to each other by sharing a number of biologic properties: they activate NK, T, and B cells, they induce their proliferation and survival,8,24 and they stimulate cytokine secretion and immunoglobulin synthesis.25,26 For signal transduction, IL-2R and IL-15R share the β and γ components.27 The most relevant functional difference between these 2 cytokines is that IL-2 can induce apoptosis, whereas IL-15 can protect against it.28,29 IL-2 production is restricted to 2 cell types, T cells and DCs, in mice and humans.4,5 In contrast, IL-15 is produced by a variety of cells. A constitutive IL-15 mRNA expression has been shown in many different cell types; regulation occurs at the translational and intracellular trafficking levels,27,30 but IL-2 is regulated at the transcriptional and the mRNA stabilization levels.
Functional synergy between these 2 cytokines has been demonstrated during T-cell activation.31 Indeed, during in vivo activation, T cells are responsive to IL-15, when the cells express the low-affinity IL-2 receptor. Later, when T cells express the high-affinity α chain of the IL-2 receptor, they acquire the ability to respond to IL-2. Our finding that IL-2 production in DCs depends on the presence of IL-15 adds further evidence of a controlled coregulation of these 2 relevant cytokines.
IL-2, IL-15, and their receptors are potential targets for immunotherapy.28,32 IL-2, through its contribution in activation-induced cell death (AICD) for CD4+ cells, and its interference with persistence of CD8+ memory T cells, plays a critical role in the maintenance of peripheral self-tolerance. In contrast, IL-15, through its inhibition of IL-2-mediated AICD29 , and its positive role in the maintenance of CD8+ memory T cells, plays an important role in the preservation of acquired immune responses to foreign pathogens. However, this role of IL-15 carries with it the risk, for the organism, of the survival of self-reactive T cells that could lead to the development of autoimmune diseases. Thus, because the production of IL-15 is critical for the production of IL-2 in mouse and human DCs, this regulation may have a role in modulating the immune response and in reducing the risk for autoimmune diseases.
Prepublished online as Blood First Edition Paper, September 7, 2004; DOI 10.1182/blood-2004-03-1059.
Supported by the Ministry of Education, University and Research (grants FIRB and COFIN); a European Molecular Biology Organization (EMBO) short-term fellowship, and the Italian Association for Cancer Research (AIRC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank J. P. M Van Meerwijk and G. Natoli for helpful discussion and Dr F. Brière (Dardilly, France) for rhIL-13 and anti-Langerin.