Abstract
Anaplastic large cell lymphoma (ALCL) is a highly proliferative neoplasm that frequently carries the t(2;5)(p23;q35) and aberrantly expresses nucleophosmin–anaplastic lymphoma kinase (NPM-ALK). Previously, NPM-ALK had been shown to activate the phosphatidylinositol 3 kinase (PI3K)/Akt pathway. As the cyclin-dependent kinase (CDK) inhibitor p27Kip1 (p27) is usually not expressed in ALCL, we hypothesized that activated Akt (pAkt) phosphorylates p27 resulting in increased p27 proteolysis and cell cycle progression. Here we demonstrate that inhibition of pAkt activity in ALCL decreases p27 phosphorylation and degradation, resulting in increased p27 levels and cell cycle arrest. Using immunohistochemistry, pAkt was detected in 24 (57%) of 42 ALCL tumors, including 8 (44%) of 18 ALK-positive tumors and 16 (67%) of 24 ALK-negative tumors, and was inversely correlated with p27 levels. The mean percentage of p27-positive tumor cells was 5% in the pAkt-positive group compared with 26% in the pAkt-negative group (P = .0076). These findings implicate that Akt activation promotes cell cycle progression through inactivation of p27 in ALCL.
Introduction
The biologic significance of the serine/threonine kinase Akt in lymphomagenesis has been recently established in a mouse model.1 In that study, forced Akt expression in hematopoietic stem cells of Eμ-Myc mice markedly accelerated lymphomagenesis, and the murine lymphomas were characterized by rapid cell proliferation and low apoptotic rate. Anaplastic large cell lymphoma (ALCL) is an aggressive form of malignant lymphoma of T/null immunophenotype.2 A subset of ALCL tumors carries variant chromosomal aberrations involving the ALK (anaplastic lymphoma kinase) gene on chromosome 2p23.3 The most common of these aberrations is the t(2;5)(p23;q35), which results in the expression of the chimeric protein nucleophosmin (NPM)–ALK.4 Recent studies have shown that, at least in part, NPM-ALK mediates oncogenesis through phosphorylation/activation of Akt (pAkt).5,6 We have previously shown that the cyclin-dependent kinase (CDK) inhibitor p27, a negative regulator of G1-S cell cycle transition,7,8 is absent in the majority of ALCL tumors,9 implying an important role for p27 in the pathogenesis of ALCL. Recently, it was demonstrated in breast cancer that pAkt is capable of phosphorylating p27, causing its cytoplasmic retention and subsequent degradation through the ubiquitin-proteasome pathway.10-12
We hypothesized that phosphorylation of p27 is mediated by pAkt in ALCL. In this report, we have demonstrated that inhibition of pAkt significantly decreases threonine p27 phosphorylation and increases total p27 levels, resulting in cell cycle arrest. We have also shown that Akt is activated in a substantial subset of ALCL tumors and that Akt activation is associated with significantly lower levels of p27 and higher tumor cell proliferation. These findings provide evidence that pAkt may promote cell cycle progression through inactivation of p27 in ALCL.
Study design
Cell lines and reagents
Two ALK-positive ALCL cell lines, Karpas 299 and SU-DHL1, were used. The cell lines were maintained at 37°C in RPMI-1640 medium supplemented with 10% fetal calf serum in a humidified atmosphere containing 5% CO2. Akt inhibitor (Akt-II) was purchased from Calbiochem (San Diego, CA). Two proteasome inhibitors were also used, N-acetyl-L-leucyl-L-leucyl-L-norleucinal (LLnL; Sigma, St Louis, MO) and MG132 (carbobenzoxy-L-leucyl-L-leucyl-L-leucinal; Calbiochem).
Western blot analysis
Western blot analysis was performed using standard methods.9 Subcellular fractionation was performed using the NE-PERTM kit (Pierce, Rockford, IL). The antibodies used were Akt, pAkt (both from Cell Signaling Technology, Beverly, MA), p27 (BD Biosciences Pharmingen, San Diego, CA), retinoblastoma protein (Rb; Dakocytomation, Carpinteria, CA), phosphorylated threonine (Santa Cruz Biotechnology, Santa Cruz, CA), and β-actin (Sigma).
Immunoprecipitation
Cell lysates were incubated with anti-p27 antibody overnight at 4°C and subsequently with protein A/G sepharose (Santa Cruz Biotechnology) for 4 hours at 4°C. Four washes with cold phosphate-buffered saline (PBS) and one wash with lysis buffer were then performed, followed by boiling in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)–loading buffer for 5 minutes. Immunoblotting was then performed using anti-p27 antibody or anti–phosphorylated threonine (pThr) antibodies.
Cell cycle analysis
Cell cycle analysis was performed using a bromodeoxyuridine (BrdU) kit (BD Biosciences Pharmingen) according to the manufacturer's recommended protocol and analyzed by flow cytometry (Becton Dickinson, San Jose, CA).
Immunohistochemistry
Expression of pAkt and p27 were assessed immunohistochemically in 42 ALCL tumors (18 ALK-positive, 24 ALK-negative). The diagnosis was based on the criteria of the World Health Organization classification.2 Full tissue sections, a tissue microarray, and an immunohistochemical method were used as previously described.13,14 The monoclonal antibodies were specific for pAkt (Ser473; Cell Signaling Technology), p27 (Dakocytomation), and Ki-67 (MIB-1; Immunotech, Westbrook, ME).
Statistical analysis
Chi-square and Fisher exact tests were used to compare pAkt expression with various clinicopathologic parameters. The Mann-Whitney U test was chosen for nonparametric correlation of pAkt expression with the percentage of p27-positive tumor cells and the proliferation index (PI). Progression-free survival (PFS)9 was calculated separately for ALK-positive and ALK-negative ALCL groups using the Kaplan-Meier method and log-rank test. Statistical calculations were performed using StatView (Abacus Concepts, Berkeley, CA).
Results and discussion
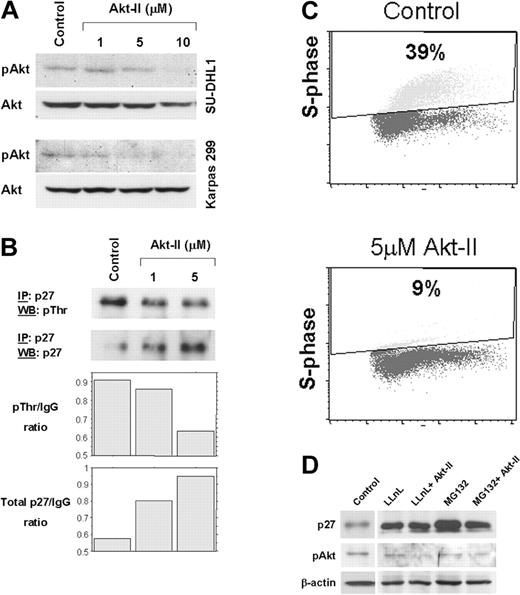
ALCL is a high-grade lymphoma type that frequently lacks p27 expression.9 In this study, we hypothesized that Akt mediates down-regulation of p27 in ALCL. We tested the Akt-II inhibitor used in the present study and found that it substantially decreases Akt kinase activity (data not shown). Western blot analysis revealed a concentration-dependent decrease of pAkt levels compared with Akt in Karpas 299 and SU-DHL1 cells treated with Akt-II (Figure 1A). Immunoprecipitation showed that threonine-phosphorylated p27 decreased, whereas total p27 increased after treatment of ALCL cells with increasing concentrations of Akt-II (Figure 1B). To test the effect on cell cycle progression, BrdU incorporation and flow cytometry showed, at 24 hours after treatment with 5 μM of Akt-II, that the fraction of Karpas 299 cells in S phase decreased from 39% to 9%, indicating the occurrence of cell cycle arrest at the G1-S phase (Figure 1C). Treatment of ALCL cells with two 26S proteasome inhibitors, LLnL and MG132, resulted in increased total p27 levels (Figure 1D), suggesting that p27 is primarily regulated through ubiquitin-proteasome–mediated degradation in our in vitro system, as shown in other cell types.15 Treatment of ALCL cells with Akt-II in the presence of the proteasome inhibitors at a concentration known to completely inhibit proteasome-mediated protein degradation resulted in no additional increase of total p27 protein level (Figure 1D). These results demonstrate that in ALCL, Akt inhibition causes cell cycle arrest that can be attributed to a significant decrease of threonine-phosphorylation and inactivation of p27.
Inhibition of Akt increases total p27 levels and induces cell-cycle arrest in ALCL cells. (A) Akt-II inhibitor induced gradual decrease of pAkt (serine 473) levels. At a concentration of 10 μM, Akt-II induced almost complete absence of pAkt at 12 hours. Total Akt was also probed using the same membrane. No substantial changes were noticed in Akt levels. Top panel, SU-DHL1; bottom panel, Karpas 299. (B) Immunoprecipitation studies revealed a decrease in threonine phosphorylation of p27 (top panel) and an increase in total p27 levels in Karpas 299 cells treated with Akt-II inhibitor at 12 hours. WB indicates Western blot; and IP, immunoprecipitation. Densitometry of the immunoblot bands showed a substantial decrease in the threonine-phosphorylated p27/immunoglobulin G (IgG) ratio that was associated with increased total p27/IgG ratio. (C) Cell cycle analysis using BrdU uptake and flow cytometry in Karpas 299 cells 24 hours after treatment with Akt-II inhibitor. The S-phase fraction was 9% in cells treated with 5 μM of the Akt-II inhibitor compared with 39% in untreated (control) cells. Similar results were obtained for SU-DHL1 cells. (D) Total p27 levels after proteasome inhibition in ALCL cells. Treatment of Karpas 299 cells with LLnL and MG132 proteasome inhibitors for 16 hours resulted in a significant increase of total p27 levels (lanes 2 and 4 compared with lane 1), due to decreased p27 degradation through the ubiquitin-proteasome system. LLnL and MG132 were used at a concentration of 35 μM each and were previously shown to adequately block proteasome activity (data not shown). Pretreatment of ALCL cells with proteasome inhibitors for 4 hours followed by treatment of cells with both proteasome inhibitors and Akt-II for 12 hours resulted in no additional increase of total p27 levels (lanes 3 and 5), which demonstrates complete blockade of proteasome-mediated degradation.
Inhibition of Akt increases total p27 levels and induces cell-cycle arrest in ALCL cells. (A) Akt-II inhibitor induced gradual decrease of pAkt (serine 473) levels. At a concentration of 10 μM, Akt-II induced almost complete absence of pAkt at 12 hours. Total Akt was also probed using the same membrane. No substantial changes were noticed in Akt levels. Top panel, SU-DHL1; bottom panel, Karpas 299. (B) Immunoprecipitation studies revealed a decrease in threonine phosphorylation of p27 (top panel) and an increase in total p27 levels in Karpas 299 cells treated with Akt-II inhibitor at 12 hours. WB indicates Western blot; and IP, immunoprecipitation. Densitometry of the immunoblot bands showed a substantial decrease in the threonine-phosphorylated p27/immunoglobulin G (IgG) ratio that was associated with increased total p27/IgG ratio. (C) Cell cycle analysis using BrdU uptake and flow cytometry in Karpas 299 cells 24 hours after treatment with Akt-II inhibitor. The S-phase fraction was 9% in cells treated with 5 μM of the Akt-II inhibitor compared with 39% in untreated (control) cells. Similar results were obtained for SU-DHL1 cells. (D) Total p27 levels after proteasome inhibition in ALCL cells. Treatment of Karpas 299 cells with LLnL and MG132 proteasome inhibitors for 16 hours resulted in a significant increase of total p27 levels (lanes 2 and 4 compared with lane 1), due to decreased p27 degradation through the ubiquitin-proteasome system. LLnL and MG132 were used at a concentration of 35 μM each and were previously shown to adequately block proteasome activity (data not shown). Pretreatment of ALCL cells with proteasome inhibitors for 4 hours followed by treatment of cells with both proteasome inhibitors and Akt-II for 12 hours resulted in no additional increase of total p27 levels (lanes 3 and 5), which demonstrates complete blockade of proteasome-mediated degradation.
Subcellular fractionation showed that Akt and pAkt are predominantly localized in the cytoplasm in Karpas 22 cells (Figure 2A). Weak nuclear localization of pAkt was also observed and thus we cannot exclude the possibility of limited nuclear translocation. Similar results were obtained using SU-DHL1 cells. Using immunohistochemistry in ALCL tumors, pAkt expression was also predominantly localized in the cytoplasm (Figure 2C-D), with nuclear translocation observed only rarely in tumor cells. pAkt was detected in 24 of 42 ALCL tumors including 8 (44%) of 18 ALK-positive tumors and 16 (67%) of 24 ALK-negative tumors (Figure 2C-D). No association between pAkt and ALK expression was observed (P = .15, Fisher exact test). Although previous in vitro studies have shown that NPM-ALK is capable of phosphatidylinositol 3 kinase (PI3K) activation resulting in Akt phosphorylation at serine 473,5,6 a number of proteins other than NPM-ALK have also been shown to interact and activate Akt in other tumor types.16
Expression of Akt and p27 in ALCL primary tumors. (A) Subcellular fractionation followed by Western blot analysis demonstrated that Akt and pAkt are predominantly localized in the cytoplasm of Karpas 299 and SU-DHL1 cells. Retinoblastoma protein (Rb) was detected exclusively in the nuclear fraction and served as a control for nuclear localization of proteins in these cells. (B) Box-plot showing the significant difference in the percentage of p27-positive tumor cells between pAkt-positive and pAkt-negative ALCL tumors (P = .0076). Only nuclear expression of total p27 was considered positive in this analysis. Error bars indicate 5%-95%. (C-D) Immunohistochemical detection of pAkt in ALCL tumors showed strong cytoplasmic expression of pAkt in a subset of ALK-positive and ALK-negative ALCL tumors, shown in panels C and D, respectively. (Immunohistochemical staining, 600 × original magnification, obtained with a BX51 Olympus microscope [Melville, NY] and a DP12 Olympus camera.) Images were viewed through a universal semi-apochromat objective lens (UPlan Fl, Olympus America Inc, Woodbury, NY); original magnification × 400; numerical aperture 0.75.
Expression of Akt and p27 in ALCL primary tumors. (A) Subcellular fractionation followed by Western blot analysis demonstrated that Akt and pAkt are predominantly localized in the cytoplasm of Karpas 299 and SU-DHL1 cells. Retinoblastoma protein (Rb) was detected exclusively in the nuclear fraction and served as a control for nuclear localization of proteins in these cells. (B) Box-plot showing the significant difference in the percentage of p27-positive tumor cells between pAkt-positive and pAkt-negative ALCL tumors (P = .0076). Only nuclear expression of total p27 was considered positive in this analysis. Error bars indicate 5%-95%. (C-D) Immunohistochemical detection of pAkt in ALCL tumors showed strong cytoplasmic expression of pAkt in a subset of ALK-positive and ALK-negative ALCL tumors, shown in panels C and D, respectively. (Immunohistochemical staining, 600 × original magnification, obtained with a BX51 Olympus microscope [Melville, NY] and a DP12 Olympus camera.) Images were viewed through a universal semi-apochromat objective lens (UPlan Fl, Olympus America Inc, Woodbury, NY); original magnification × 400; numerical aperture 0.75.
Expression of pAkt inversely correlated with total p27 levels. The mean percentage of p27-positive tumor cells was 5% in the pAkt-positive group compared with 26% in the pAkt-negative group (P = .0076, Mann-Whitney u test; Figure 2C). When p27 was analyzed as a categoric variable using a 10% cutoff, pAkt and p27 expression were inversely correlated; only 2 (11%) of 18 pAkt-positive tumors compared with 7 (44%) of 16 pAkt-negative tumors were p27 positive (P = .05, Fisher exact test). In addition, pAkt expression was significantly associated with the PI of ALCL tumors as assessed by Ki-67 marker. The mean PI was 75% in the pAkt-positive group compared with 56% in the pAkt-negative group (P = .011, Mann-Whitney test). A statistical trend toward inferior 5-year PFS for patients with pAkt-positive tumors was observed within the ALK-positive (67% vs 100%; P, not significant, log-rank) but not ALK-negative ALCL groups. However, the small number of patients within the ALK-positive and ALK-negative subgroups precludes definite conclusions regarding the prognostic significance of Akt activation in ALCL.
These findings indicate that the high PI reported in ALCL14 can be partially explained by constitutive activation of Akt leading to threonine phosphorylation at Thr157 or Thr19817 and increased degradation of p27. Low p27 levels, in turn, lead to increased phosphorylation and inactivation of Rb resulting in release of E2F transcription factors and cell cycle progression. This is further corroborated by the frequent loss of Rb expression due to deletion of rb locus recently reported in ALCL.18 Of note, we have shown that the presence of Rb, which is frequently phosphorylated, is associated with low p27 levels and adverse clinical outcome in these tumors.18
Using an in vitro system, a recent study has shown that forced expression of NPM-ALK into the Ba/F3 murine pro-B cells caused down-regulation of p27 via pAkt/FOXO3a.19 Using ALK-positive ALCL cell lines, we demonstrate here that pAkt is involved in p27 down-regulation. However, the absence of a significant correlation between pAkt and ALK status in ALCL tumors indicates that other mechanisms may play a role in pAkt-mediated down-regulation of p27 in vivo.
Prepublished online as Blood First Edition Paper, September 16, 2004; DOI 10.1182/blood-2004-06-2125.
H.M.A. is a recipient of the Career Development Award from the National Institutes of Health (NIH) Leukemia Specialized Programs of Research Excellence (SPORE) grant to MD Anderson Cancer Center.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 2. Expression of Akt and p27 in ALCL primary tumors. (A) Subcellular fractionation followed by Western blot analysis demonstrated that Akt and pAkt are predominantly localized in the cytoplasm of Karpas 299 and SU-DHL1 cells. Retinoblastoma protein (Rb) was detected exclusively in the nuclear fraction and served as a control for nuclear localization of proteins in these cells. (B) Box-plot showing the significant difference in the percentage of p27-positive tumor cells between pAkt-positive and pAkt-negative ALCL tumors (P = .0076). Only nuclear expression of total p27 was considered positive in this analysis. Error bars indicate 5%-95%. (C-D) Immunohistochemical detection of pAkt in ALCL tumors showed strong cytoplasmic expression of pAkt in a subset of ALK-positive and ALK-negative ALCL tumors, shown in panels C and D, respectively. (Immunohistochemical staining, 600 × original magnification, obtained with a BX51 Olympus microscope [Melville, NY] and a DP12 Olympus camera.) Images were viewed through a universal semi-apochromat objective lens (UPlan Fl, Olympus America Inc, Woodbury, NY); original magnification × 400; numerical aperture 0.75.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/2/10.1182_blood-2004-06-2125/6/m_zh80020572760002.jpeg?Expires=1765931245&Signature=uZi-JtIV68T0an5Mo4jWghd4pQqVC7LKQeoqsNbjgZUZ7EF1rN5hv9XqoWoGHzdY8-vpuHEt-3fRQMG8ENAxecXUTz2fH1mJjlhNT8-NEpANpFNePcN8slBtQ39gblOi6EcFPpERJ9y~-NX8ruUj7sWc8FfKtlJcgGSRxB8YcDVwJ1LJK~r4zYy9ieOr6pFJXx4DETY2IDSX~iSuDwIib8Byo--AyxxNA0qHtPWuBpqmX5tmgww7civGmEWDbzz6nd1nCICfZaNWLDAYwfIdAf2OQPXiiLOZ92Qij7jYnLV1zSCDFdJwuMXb2Rf7~MZEUzmx6IGVYfQmaRxLgxUbxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 2. Expression of Akt and p27 in ALCL primary tumors. (A) Subcellular fractionation followed by Western blot analysis demonstrated that Akt and pAkt are predominantly localized in the cytoplasm of Karpas 299 and SU-DHL1 cells. Retinoblastoma protein (Rb) was detected exclusively in the nuclear fraction and served as a control for nuclear localization of proteins in these cells. (B) Box-plot showing the significant difference in the percentage of p27-positive tumor cells between pAkt-positive and pAkt-negative ALCL tumors (P = .0076). Only nuclear expression of total p27 was considered positive in this analysis. Error bars indicate 5%-95%. (C-D) Immunohistochemical detection of pAkt in ALCL tumors showed strong cytoplasmic expression of pAkt in a subset of ALK-positive and ALK-negative ALCL tumors, shown in panels C and D, respectively. (Immunohistochemical staining, 600 × original magnification, obtained with a BX51 Olympus microscope [Melville, NY] and a DP12 Olympus camera.) Images were viewed through a universal semi-apochromat objective lens (UPlan Fl, Olympus America Inc, Woodbury, NY); original magnification × 400; numerical aperture 0.75.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/2/10.1182_blood-2004-06-2125/6/m_zh80020572760002.jpeg?Expires=1765931246&Signature=kzDnExCIIqaM3OIoQp33TMDldWQZ8Akxu4pz9wDHL~WF6KJgxGg5nXG3ki56OAiXry9R62pctXw73KzxzoH1aCtPTux3G9UEHKQnYDtX0EbXxHNcNF4Llro6bTl5lwUyNFXW8iz4PEgBen~YyebaTutzj6vvKNL1nAr2-dvTAwyoXlCqQ2lZ1ye19rQKKxazCkVLomtJh2nDu3WHKp72Qn8O0oLfK5ewq6n6zbjZyAmT7~wQcnqGMujJGvLWoT1HgGKlAN3mwftxyczzfmBUwakq0h0jz7Oe9G56avHuJqTMmIWe5x2gZkiJoQVksSz-YeTzUZehlhYILRLPL5TlFA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)