Abstract
Mast cells are the sentinels of immune systems and, like other immuno-competent cells, they are produced by hematopoietic stem cells. We analyzed the expression of signal transducer and activator of transcription 4 (Stat4), and investigated its role in mast cells. Murine mast cells are usually divided into 2 distinct populations by their distribution and contents of their granules: mucosal mast cells (MMCs) and connective tissue–type mast cells (CTMCs). Stat4 protein was detected in CTMCs but not in MMCs. The absence of Stat4 expression in cultured mast cells was due to the presence of Stat6. In T-helper (Th) cells, Stat4 plays an important role in Th1 shift by inducing a set of genes, such as interferon gamma (IFN-γ) and interleukin-18 receptor α subunit (IL-18Rα). As in Th1 shift, we found that Stat4 trans-activated these genes in the Stat4-expressing cultured mast cells, namely, microphthalmia transcription factor (MITF)–deficient cultured MMCs, Stat6-deficient cultured MMCs, and cultured CTMCs. Stat4 also enhanced expression of nitric oxide synthase 2 (NOS2) in CTMCs, which brought about increased levels of NO-dependent cytotoxic activity. These data indicate that expression of Stat4 in CTMCs plays an important role on Th1 immune responses.
Introduction
Mast cells are immuno-competent cells distributed in almost all tissues.1 They are derived from hematopoietic stem cells2 and achieve their mature phenotypes while residing tissues.3 In mice, mature mast cells are usually classified as mucosal mast cells (MMCs) or connective tissue–type mast cells (CTMCs).1 This distinction is based on their tissue distribution and the contents of their granules.1 MMCs are mainly located in gastrointestinal mucosa and lung bronchi. They have chondroitin sulfate-containing granules, which are stained by alcian blue but not by safranin. CTMCs are widespread in all connective tissue, including skin and peritoneum. The granules of CTMCs are rich in heparin and stained by both alcian blue and safranin.
Proliferation of MMCs is dependent on T-cell–derived cytokines, such as interleukin 3 (IL-3) and IL-4.4,5 IL-3–dependent cultured mast cells show immature phenotypes, and are almost identical to MMCs (hereafter, called cultured MMCs).4,6 Cultured MMCs can be easily obtained and have therefore been studied intensively as representative MMCs. These cells produce large amounts of the type 2 T-helper (Th2) cytokines, IL-4 and IL-13, to which they are able to respond.1 However, cultured MMCs secrete only subdetectable levels of interferon gamma (IFN-γ) and IL-12.7,8 These findings emphasize the functional importance of mast cells in Th2 responses, such as atopic and allergic diseases, rather than Th1 immune responses. Meanwhile, survival of CTMCs is supported by Kit ligand (Kit L)/stem cell factor/Steel factor/mast cell growth factor,9 and Kit L–dependent cultured mast cells have CTMC-like phenotypes10 (hereafter, called cultured CTMCs).
Cytokine signaling is critical for differentiation of the Th cell population into Th1- or Th2-type cells. IL-12 signaling promotes Th1 development, whereas IL-4 stimulation induces Th2 differentiation. Stat4 and Stat6 are involved in IL-12 and IL-4 signal transduction, respectively.11,12 Stat4-deficient mice show impaired Th1 responses11 and Th2 responses are reduced in Stat6-deficient mice.12 However, Stat4 seems to be dispensable for Th1 development, because Th cells derived from Stat4/Stat6 double-deficient mice decrease Th2 responses but show Th1-dominant phenotypes.13 Thus, the absence of Stat6, but not the presence of Stat4, is necessary for a Th1 shift.
Stat proteins are important not only for the Th shift but also in the development and function of MCs. Both IL-3 and Kit L have been reported to activate Stat5a/Stat5b.14,15 Because the signals of these cytokines are indispensable for mouse mast cell development,4,9,10 mast cells cannot develop in Stat5a/Stat5b double-deficient mice.16 IL-4 is also important for mast cell function5 and Stat6 is involved in the signaling of this cytokine.12 Stat6-dependent IL-4 signaling in cultured MMCs suppresses expression of IL-4,17 high-affinity immunoglobulin E (IgE) receptor (FcϵRI),18 and Kit.19 In addition, mast cells have a cell lineage–specific truncated form of Stat6 that is produced through cleavage by Stat6-protease at the carboxyl terminus.20
In this study, we found that CTMCs expressed Stat4 protein but MMCs did not. Expression of Stat4 appeared to be suppressed by activation of Stat6 in cultured MMCs. Stat4 induced IFN-γ production and increased nitric oxide (NO)–dependent tumor cell–lytic activity in CTMCs. Our data demonstrate the involvement of CTMCs in Th1 immune responses via Stat4 activation.
Materials and methods
Mice
C57BL/6 (B6)+/+ and Balb/c+/+ mice were purchased from Japan SLC (Hamamatsu, Japan). These mice were maintained by repeated backcrosses to our own inbred colonies.
The original stock of VGA-9-tg/+ mice, in which the mouse vasopressin–Escherichia coli–galactosidase transgene is integrated at the 5′-flanking region of the mi gene, was kindly provided by Dr H. Arnheiter (National Institutes of Health, Bethesda, MD). The integrated transgene was maintained by repeated backcrosses to our own inbred B6 colony.21 B6VGA-9-tg/VGA-9-tg mice were obtained by mating among the B6VGA-9-tg/+ mice. Mice of the VGA-9-tg/VGA-9-tg genotype were identified by their white coat color. B6tg/tg mice do not express microphthalmia transcription factor (MITF), which is encoded by the mi locus; these mice were termed “MITF-KD mice.”
B6-Stat6–deficient mice were provided by Dr S. Akira (Osaka University, Osaka, Japan). This knockout mouse line was generated by target disruption of the Stat6 gene in an embryonic stem (ES) cell line of C57BL/6 origin.22 Balb/c-Stat6–deficient and Balb/c-Stat4–deficient mice were purchased from the Jackson Laboratory (Bar Harbor, ME).11,12
Cells
The P815 cell line and the L929 cell line were purchased from the American Type Culture Collection (Bethesda, MD). P815 cells were maintained in α-minimal essential medium (α-MEM; ICN Biomedicals, Costa Mesa, CA) supplemented with 10% fetal calf serum (FCS; Nippon Bio-supp Center, Tokyo, Japan). L929 cells were maintained in Dulbecco modified Eagle medium (DMEM; ICN Biomedicals) supplemented with 10% FCS. Ψ2 helper virus-free packaging cells were obtained from Dr A. Ito (Kobe University, Kobe, Japan) and maintained in DMEM (ICN Biomedicals) supplemented with 10% FCS.
Mice of 4 to 6 weeks of age were used to obtain cultured MCs. Mice were killed by decapitation after ether anesthesia, and their spleens were removed. To prepare spleen cell suspensions, spleens were passed through a mesh. Spleen cells were cultured in α-MEM supplemented with 10% FCS and 10 ng/mL recombinant mouse IL-3 (rmIL-3; R&D Systems, Minneapolis, MN) for 6 weeks.4 Half of the medium was replaced every 5 days. More than 99% of nonadherent cells contained alcian blue–positive/safranin-negative granules, and were therefore considered to be cultured MMCs. In some experiments, spleen cells were cultured in α-MEM supplemented with 10% FCS and 50 ng/mL rmKit L (R&D Systems) for 8 weeks.10 Half of the medium was replaced every 5 days. More than 99% of nonadherent cells contained alcian blue–positive/safranin-positive granules, and were therefore considered to be cultured CTMCs.
Antibodies
The anti-Stat4 antibody (Ab; C-20), anti-Stat6 (M-20) Ab, and anti-NOS2 Ab were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The anti–mouse IL-18Rα Ab was purchased from R&D Systems. The anti–α-tubulin Ab (DM1A) was purchased from Sigma Chemical (St Louis, MO). The secondary Abs were peroxidase-labeled anti–rabbit or anti–mouse Ig G antibodies (MBL, Nagoya, Japan).
Transfection of cultured MCs
The pCX4bsr vector (a modified pCXbsr vector) was kindly provided by Dr T. Akagi (Osaka Bioscience Institute, Osaka, Japan).23 A clone containing full-length MITF was isolated from B6+/+ cultured MCs cDNA library.24 A clone containing full-length Stat6 or truncated Stat6 was kindly provided by Dr H. Nakajima (Chiba University, Chiba, Japan).25 The pCX4bsr full-length MITF, full-length Stat6, truncated Stat6, activated form Stat6, or empty pCX4bsr vector was transfected into the packaging cell line Ψ2, and blasticidin-resistant Ψ2 cell clones were selected by culturing in α-MEM supplemented with 10% FCS, 10 ng/mL rmIL-3, and 3 μg/mL blasticidin (Invitrogen, Carsbad, CA). To obtain infected cultured MCs, a subconfluent monolayer of transfected Ψ2 cell clones was irradiated with a single dose of 30 Gy γ radiation. Freshly prepared spleen cells were added to the above-mentioned monolayer, and cultured in α-MEM supplemented with 10% FCS and 10 ng/mL rmIL-3. Blasticidin-resistant cultured MCs were selected by continuous culture in α-MEM supplemented with 10% FCS, 10 ng/mL rmIL-3, and 1.5 μg/mL blasticidin for 6 weeks.26
Immunoblotting
Whole-cell extracts of cultured MCs were obtained, as previously described.27 The extracts were suspended in loading buffer, boiled, and analyzed by immunoblot with anti-Stat4, anti-Stat6, anti–mouse IL-18Rα, anti-NOS2, and anti-α-tubulin Abs.
Immunohistochemistry
Tissues derived from B6+/+ mice were fixed in freshly prepared 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 12 hours at 4°C. Tissues were then dehydrated and embedded in paraffin. Serial sections (4-μm thick) were cut. One section was stained with alcian blue and nuclear fast red to identify MCs, while the others were used for detection of Stat4 expression. Immuno-reacted cells to anti-Stat4 Ab were visualized with peroxidase and 0.05% diaminobenzidine–0.02% H2O2 solution, according to the manufacturer's instructions.
Cytokine measurements
Cultured MCs were recultured for 8 hours at 1 × 105 cells/mL in 24-well plates, together with rmIL-12 (20 ng/mL) and rmIL-18 (20 ng/mL). The culture supernatants were assayed for IFN-γ (R&D Systems) using an enzyme-linked immunosorbent assay (ELISA) kit, according to the manufacturer's instructions.
Cytotoxicity assay
Cultured MCs were recultured for 16 hours at 1 × 105 cells/mL in α-MEM supplemented with 10% FCS and 10 ng/mL rmIL-3, in the presence of P815 or L929 cells. P815 or L929 cells were morphologically distinct from cultured mast cells. After reculture, the viability of P815 or L929 cells was assessed by their ability to eject trypan-blue.
Results
Expression of Stat4 protein in mast cells
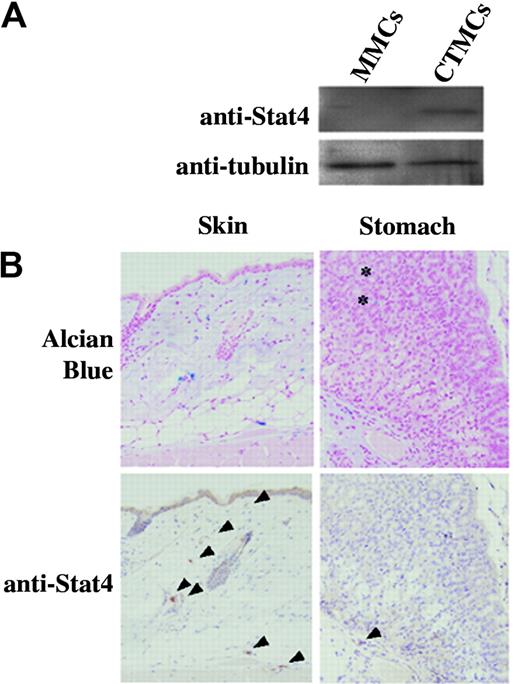
First, expression of Stat4 protein in mucosal-type and connective tissue–type cultured mast cells was examined by immunoblotting. Although Stat4 protein was not detectable in cultured MMCs, significant expression was detected in cultured CTMCs (Figure 1A). Immunohistochemical analyses of mature MMCs and CTMCs in connective tissue and mucosa were carried out to confirm expression of Stat4. Alcian blue staining was used to detect both types of mast cells. All alcian blue–positive cells in the skin and in the submucosal layer of the stomach were mature CTMCs and these cells expressed Stat4 (Figure 1B). Alcian blue–positive cells in the gastric mucosa were mature MMCs and these cells were not stained by anti-Stat4 Ab (Figure 1B). The data of Figure 1B were obtained from B6+/+ mice and essentially similar results were obtained from Balb/c+/+ mice.
Expression of Stat4 protein in mast cells. (A) Western blot analysis of cultured MMCs and cultured CTMCs. Cell lysates were blotted with anti-Stat4 (C-20) and anti–α-tubulin Abs. (B) Immunohistochemical analysis of tissue mast cells derived from the skin and stomach of B6+/+ mice. Mast cells are identified as alcian blue–positive cells in the tissue. Anti–Stat4-reacted cells are indicated by arrowheads. Mucosal mast cells (asterisk) were not stained with anti-Stat4 Ab.
Expression of Stat4 protein in mast cells. (A) Western blot analysis of cultured MMCs and cultured CTMCs. Cell lysates were blotted with anti-Stat4 (C-20) and anti–α-tubulin Abs. (B) Immunohistochemical analysis of tissue mast cells derived from the skin and stomach of B6+/+ mice. Mast cells are identified as alcian blue–positive cells in the tissue. Anti–Stat4-reacted cells are indicated by arrowheads. Mucosal mast cells (asterisk) were not stained with anti-Stat4 Ab.
Relationship of Stat4, Stat6, and MITF in mast cells
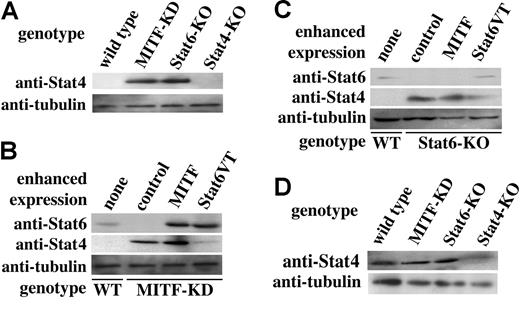
We examined the expression of Stat4 in cultured MMCs derived from the mutant mice that lack Stat6 or MITF (Stat6-deficient and MITF-KD mice, respectively). As shown in Figure 2A, cultured MMCs of MITF-KD mice expressed Stat4 protein and, in addition, Stat6 was not detectable (Figure 2B). Similarly, cultured MMCs derived from Stat6-deficient mice expressed Stat4. It is conceivable that 2 transcription factors, MITF and Stat6, are involved in the expression of Stat4 in cultured MMCs.
Western blot analysis of expression of Stat6 or Stat4 proteins in cultured MMCs. Indicated cell lysates were blotted with anti-Stat6 (M-20), anti-Stat4 (C-20), and anti–α-tubulin Abs. (A) Western blot analysis of cultured MMCs derived from control, MITF-KD, Stat6-deficient, and Stat4-deficient mice. (B) Western blot analysis of cultured MITF-KD MMCs after enforced expression of control vector, MITF, or Stat6VT (activated form Stat6). Lysates of the indicated cultured MMCs were blotted with anti-Stat6, and anti-Stat4 and anti–α-tubulin Abs. (C) Western blot analysis of cultured Stat6-deficient MMCs after the enforced expression of control vector, MITF, or Stat 6VT. Lysates of the indicated cultured MMCs were blotted with anti-Stat6, and anti-Stat4 and anti–α-tubulin Abs. (D) Western blot analysis of expression of Stat4 proteins in cultured CTMCs. Cell lysates were blotted with anti-Stat 4 and anti–α-tubulin Abs.
Western blot analysis of expression of Stat6 or Stat4 proteins in cultured MMCs. Indicated cell lysates were blotted with anti-Stat6 (M-20), anti-Stat4 (C-20), and anti–α-tubulin Abs. (A) Western blot analysis of cultured MMCs derived from control, MITF-KD, Stat6-deficient, and Stat4-deficient mice. (B) Western blot analysis of cultured MITF-KD MMCs after enforced expression of control vector, MITF, or Stat6VT (activated form Stat6). Lysates of the indicated cultured MMCs were blotted with anti-Stat6, and anti-Stat4 and anti–α-tubulin Abs. (C) Western blot analysis of cultured Stat6-deficient MMCs after the enforced expression of control vector, MITF, or Stat 6VT. Lysates of the indicated cultured MMCs were blotted with anti-Stat6, and anti-Stat4 and anti–α-tubulin Abs. (D) Western blot analysis of expression of Stat4 proteins in cultured CTMCs. Cell lysates were blotted with anti-Stat 4 and anti–α-tubulin Abs.
We expressed MITF in cultured MITF-KD MMCs to investigate the relationships among MITF, Stat6, and Stat4. MITF recovered expression of Stat6 in these mutant cultured MMCs. However, Stat4 protein expression levels were comparable with those of control MITF-KD MMCs, after enforced expression of MITF (Figure 2B). We also examined the effect of Stat6 overexpression on expression levels of Stat4 in cultured MITF-KD MMCs. Stat4 expression levels were dramatically reduced in these cells (relative to control MMCs) by expression of the activated form of Stat6 (Figure 2B). Overexpression of the normal or truncated form of Stat6 in cultured MITF-KD MMCs significantly up-regulated Stat6 expression, but did not influence Stat4 expression (data not shown). Next, we assessed the influence of MITF and Stat6 overexpression on Stat4 expression in cultured Stat6-deficient MMCs. Overexpression of the activated form of Stat6 decreased Stat4 expression in cultured Stat6-deficient MMCs (Figure 2C), but overexpression of MITF, normal Stat6, or the truncated form of Stat6 did not decrease Stat4 expression (Figure 2C and data not shown).
Expression levels of Stat4 in cultured CTMCs from wild-type, MITF-KD, Stat6-deficient, and Stat4-deficient mice were examined. As shown in Figure 2D, CTMCs of MITF or Stat6 mutated mice had Stat4 expression patterns, comparable to those found in wild-type mice.
Stat4 induced Th1-related molecules in mast cells
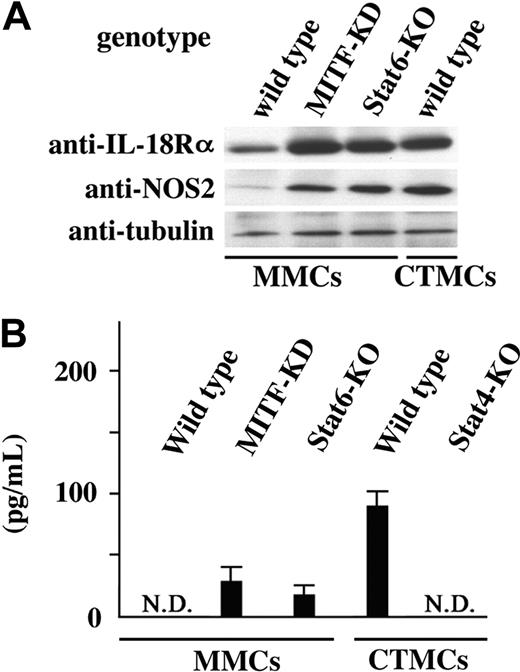
A set of genes including IL-18Rα has been identified as the transcriptional target of Stat4 in T cells.28 Cultured MMCs express IL-18Rα, and secrete IL-13 and IL-4 in response to IL-18.29 To determine whether the IL-18Rα gene was a target of Stat4 in mast cells, we examined expression levels of IL-18Rα in cultured mast cells expressing Stat4, namely, cultured MMCs of MITF-KD mice, cultured MMCs of Stat6-deficient mice, and cultured wild-type CTMCs. Expression levels of IL-18Rα were significantly higher in Stat4-positive cultured mast cells than in Stat4-negative wild-type cultured MMCs (Figure 3A). IL-18 stimulation induces IFN-γ production by T and natural killer (NK) cells in synergy with IL-12,30 but not in cultured MMCs. We then examined IFN-γ production by Stat4-positive cultured MCs, using IL-18 in combination with IL-12. IFN-γ production was not detectable in Stat4-negative cultured mast cells (Figure 3B). In contrast, Stat4-positive cultured mast cells produced IFN-γ by IL-18 stimulation in combination with IL-12 (Figure 3B).
Th1-related molecules in mast cells. (A) Western blot analysis of expression of IL-18Rα and NOS2 proteins in cultured MMCs derived from control, MITF-KD, and Stat6-deficient mice or cultured CTMCs derived from control mice. (B) IFN-γ production by cultured MMCs and cultured CTMCs. Culture supernatants of the indicated cultured mast cells with stimulation of IL-12 and IL-18 were analyzed with ELISA.
Th1-related molecules in mast cells. (A) Western blot analysis of expression of IL-18Rα and NOS2 proteins in cultured MMCs derived from control, MITF-KD, and Stat6-deficient mice or cultured CTMCs derived from control mice. (B) IFN-γ production by cultured MMCs and cultured CTMCs. Culture supernatants of the indicated cultured mast cells with stimulation of IL-12 and IL-18 were analyzed with ELISA.
The expression of NOS2 (nitric oxide synthase 2) is induced in M-1 macrophages that produce larger amounts of Th1 cytokines.31 NOS2 expression was detectable in Stat4-positive cultured mast cells, whereas Stat4-negative cultured mast cells only faintly expressed NOS2 (Figure 3A). NO mediates cytotoxic activity to the P815 mastocytoma cell line, which is NO-sensitive but tumor necrosis factor α (TNF-α)–resistant.32 Cultured CTMCs showed increased cytotoxicity to P815 cells, relative to cultured MMCs (Table 1). In contrast, cytotoxic activity of cultured Stat4-deficient CTMCs was comparable to that of cultured Stat4-deficient MMCs (Table 1). TNF-α is also reported to mediate cytotoxicity of rat peritoneal mast cells.33 We estimated cytotoxic activity of cultured mast cells to an NO-resistant/TNF-α–sensitive L929 cell line.32 The cytotoxicity of cultured CTMCs was increased relative to that of cultured MMCs, in both Balb/c+/+ and Stat4-deficient mouse genetic background mice (Table 1).
Discussion
Seven members of the Stat protein family have been identified in mammalian cells. They play important roles in cytokine signaling in immunocompetent cells, including mast cells.11-19,25 Stat4 is involved in IL-12 signaling, and is expressed in T cells, B cells, NK cells, dendritic cells (DCs), and macrophages.11,34 Stat4 transactivates some genes related to Th1 immune responses in these cell lineages.28 In the present study, we clearly demonstrate that Stat4 protein is also expressed in mast cells.
Stat4 influences the cytokine secretion profiles of mast cells. IL-18 is a unique cytokine that stimulates both Th1 and Th2 cytokine production, depending on its cytokine milieu.29,30 In NK cells, IL-18 stimulation induces IFN-γ production in synergy with IL-12,30 but induces IL-13 when in combination with IL-2.35 It is also known that cultured MMCs produce the Th2 cytokines IL-4 and IL-13 after IL-18 stimulation.29 In the present study, IL-18 administration induced Th1 cytokine IFN-γ production of cultured CTMCs in synergy with IL-12. Both Stat4 and T-bet play important roles in IFN-γ production of Th and NK cells.36,37 The production of IFN-γ in mast cells was dependent on Stat4 rather than on T-bet, because the expression of T-bet was not detectable in cultured CTMCs by reverse transcriptase–polymerase chain reaction (RT-PCR; T.R.K., unpublished observation). Similar to NK cells, mast cells are able to produce both Th1 and Th2 cytokines.
Mast cells are considered to have major roles in Th2 responses, but some reports suggest the additional involvement of mast cells in Th1 responses, such as immunity against tumors. Clinically, mast cell infiltration is related to favorable prognoses in breast cancer38 and soft-tissue tumors.39 Anticarcinogenetic roles of mast cells in the skin have been demonstrated using W/Wv mice, which are deficient in mast cells.40 Rat peritoneal mast cells showed cytotoxic activity to some tumor cell lines through NO and TNF-α production.33 Our conclusion is that CTMCs are able to be involved in the Th1 responses, but not MMCs. Cytotoxic activity of CTMCs was analyzed by tumor cell–lytic activity to P815 and L929 cell lines (Table 1). For both cell lines, cultured CTMCs showed greater cytotoxic activity than did cultured MMCs. Meanwhile, cytotoxicity of Stat4-deficient cultured CTMCs against L929 cells was comparable to that of wild-type cultured CTMCs, but cytotoxicity against P815 cells was significantly lower. This discrepancy is presumably due to the different sensitivity of P815 cells and L929 cells to NO and TNF-α. P815 cells are NO-sensitive/TNF-α–resistant, and L929 cells are NO-resistant/TNF-α–sensitive.32 We subsequently observed that NO-dependent cytotoxic activity increased in CTMCs, Stat4-dependently, and that TNF-α–dependent cytotoxicity also increased in CTMCs, but in a Stat4-independent manner.
Th cells develop into 2 distinct subsets, Th1/Th2, on the basis of cytokine secretion patterns.11,12 DCs and macrophages are also divided into 2 populations, mature/immature DCs and M-1/M-2 cells.31,41 Mature DCs and M-1 cells produce larger amounts of Th1 cytokines than Th2 cytokines.31,41 Mast cells also consist of 2 populations, CTMCs/MMCs.1 From the viewpoint of cytokine secretion, CTMCs appeared to be more similar to mature DCs and M-1 cells, and MMCs to immature DCs and M-2 cells. Stat4 is induced during maturation processes in DCs.42 We studied the induction of Stat4 in cultured MMCs transferred into Kit L–dependent culture, which change the phenotype of cultured MMCs to that of CTMCs.10 Stat4 induction was observed after more than 8 weeks of Kit L–dependent culture (data not shown). This period seems to correspond to the time necessary for trans-differentiation of MMCs to CTMCs after intracutaneous or intraperitoneal transplantation.3 On the other hand, Stat4 induction is only observed overnight in DCs.42 The mechanism of maturation-dependent Stat4 induction in mast cells seems quite different from that of DCs.
In addition to Kit L–Kit signaling, MITF plays a pivotal role in mast cell development.21 Various MITF null mutant mice have abnormal mast cell phenotypes,21 because MITF trans-activates some important genes for mast cell function.24 We showed that Stat6 is one of the transcriptional targets of MITF in cultured MMCs. This is compatible with the importance of Stat6 in MMC function.17-19,25 T cells also express MITF.43 We examined the possibility of the involvement of MITF in the expression of Stat6 in Th cells, like mast cells. Purified CD4+ cells derived from B6-MITF-KD mice expressed Stat6 protein at levels comparable to those of B6+/+ mice (T.R.K., unpublished observations). MITF is involved in Stat6 expression in mast cells, but not in Th cells. Our data also suggest that Stat6 suppresses expression of Stat4 in MMCs, which also occurs in Th cells. In Th cells, ectopic expression of activated Stat6 induces the expression of GATA-3,44 which down-regulates Stat4 expression.45 However, other factor(s) must mediate Stat4 suppression by Stat6 in MMCs, because cultured MMCs do not express GATA-3.46 Regulatory mechanisms of Stat6 and Stat4 in mast cells employ different molecules from those used in Th cells.
Prepublished online as Blood First Edition Paper, September 30, 2004; DOI 10.1182/blood-2004-07-2811.
Supported by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr S. Koyasu for helpful discussion, and Mr M. Kohara and Ms T. Sawamura for technical assistance.