Abstract
Dendritic cells (DCs) are specialized antigen-presenting cells that monitor the antigenic environment and activate naive T cells. The role of DCs is not only to sense danger but also to tolerize the immune system to antigens encountered in the absence of maturation/inflammatory stimuli. Indeed, if a naive T cell encounters its antigen on immature DCs (iDCs), it may differentiate into a T-regulatory (Tr) rather than a T-effector cell. However, little is known about the mechanisms by which iDCs differentiate Tr cells. We developed a standardized and highly reproducible protocol to differentiate Tr cells by repetitive exposure of naive peripheral blood CD4+ T cells to allogeneic iDCs. The resultant Tr cells are phenotypically and functionally identical to type 1 Tr (Tr1) cells because their generation requires production of IL-10 by iDCs, and they suppress T-cell responses through an interleukin-10 (IL-10)– and a transforming growth factor β (TGF-β)–dependent mechanism. In addition, Tr1 cells induced by iDCs do not require the presence of CD4+CD25+ Tr cells for their generation, nor do they express high constitutive levels of CD25 or the transcription factor FoxP3. Thus, iDCs can drive the differentiation of Tr1 cells and can be used to generate large numbers of alloantigen-specific Tr1 cells for clinical use as a cellular therapy to restore peripheral tolerance.
Introduction
Dendritic cells (DCs) are specialized antigen-presenting cells (APCs) that initiate immunity on encountering antigens associated with infection and inflammation.1 This process requires the terminal maturation of DCs, typically induced by agents associated with microbial infection such as through the activation of Toll-like receptor (TLR) and tumor necrosis factor receptor (TNF-R) family members. The resultant fully mature DCs are capable of priming naive T cells to become effector T cells. More recently, the capacity of DCs to influence T-cell differentiation in the absence of inflammation has been explored. These studies have led to the notion that immature or semimature DCs have a distinct role in regulating immune responses because when they present antigens, they promote tolerance rather than immunity.2,3
Peripheral T-cell tolerance can be induced and maintained by a variety of mechanisms, including deletion, induction of T-cell hyporesponsiveness, and differentiation of T-regulatory (Tr) cells. Tr cells include a wide variety of cells with a unique capacity to inhibit effector T-cell responses. Although T cells with suppressive activity exist in essentially all T-cell subsets, CD4+ Tr cells are the best defined. Among these, CD4+CD25+ Tr cells typically arise in the thymus, though some evidence indicates that they can also arise de novo in the periphery4 and can regulate T-cell responses through a cell contact–dependent mechanism yet to be defined.5 In contrast, CD4+ type 1 T-regulatory (Tr1) cells differentiate in the periphery from naive precursors, typically in the presence of interleukin-10 (IL-10), and ultimately regulate T-cell responses through their ability to produce IL-10 and transforming growth factor-β (TGF-β).6,7 Several studies have reported that CD4+CD25+ Tr cells may also suppress responses through the production of IL-10, TGF-β, or both,8,9 leading to some confusion regarding the relationship between CD4+CD25+ Tr and Tr1 cells.5 However, recent data suggest that CD4+CD25+ Tr and Tr1 cells are truly distinct subsets.10 Moreover, the observation that CD4+CD25+ Tr cells may actually contribute to the differentiation of Tr1 cells in vitro11,12 and in vivo13 may call for reinterpretation of studies that found CD4+CD25+ Tr cells mediated cytokine-dependent suppressive effects.
A great deal of interest has surrounded the hypothesis that when DCs are in an immature or a “tolerogenic” state, they drive the differentiation of these Tr cells. Indeed, targeting antigens to immature DCs in vivo through antibody-mediated delivery or inducible gene expression results in antigen-specific tolerance in CD4+ and CD8+ T cells.14-18 This immature DC-induced tolerance appears to be caused by a combination of antigen-specific deletion14,16,17 and induction of Tr cells.15,18 In addition, repetitive stimulation of cord blood CD4+ T cells with immature DCs results in the differentiation of Tr cells in vitro.19 Interestingly, though the resultant Tr cells produce high levels of IL-10, their high levels of expression of CD25 and CTLA-4 and their cell contact–dependent suppression lead them to be classified as CD4+CD25+ Tr cells rather than as Tr1 cells.
To define whether immature DCs induce the differentiation of Tr1 cells or CD4+CD25+ Tr cells, we investigated the effects of repetitive priming of naive peripheral blood CD4+ T cells with immature monocyte-derived DCs.
Materials and methods
Differentiation of DCs
Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by centrifugation over Ficoll-Hypaque gradients (Nycomed Amersham, Uppsala, Sweden). Approval was obtained from the San Raffaele Scientific Institute Institutional Review Board for these studies. In addition, informed consent was provided for the use of blood samples according to the Declaration of Helsinki. CD14+ monocytes were isolated as the adherent fraction after incubation for 1 hour in RPMI 1640 (BioWhittaker, Verviers, Belgium) supplemented with 10% fetal calf serum (FCS) (BioWhittaker), 100 U/mL penicillin/streptomycin (Bristol-Myers Squibb, Sermoneta, Italy), and 50 μM 2 mercaptoethanol (Bio-Rad, Segrate, Italy) (DC medium) at 37°C. After extensive washing, adherent monocytes were differentiated into DCs by culture in 10 ng/mL recombinant human IL-4 (rhIL-4) (R&D Systems, Minneapolis, MN) and 100 ng/mL recombinant human granulocyte macrophage–colony-stimulating factor (rhGM-CSF) (Schering-Plough, Kenilworth, NJ) in DC medium. After 5 days, DCs were left unstimulated or were transferred to wells containing irradiated (100 Gy) 3T3 fibroblasts expressing human CD40L (a kind gift from Vincenzo Russo) to induce maturation. After another 2 days, immature and mature DCs were collected and irradiated at 60 Gy. DCs were either used directly or frozen until needed for restimulation. Purity and maturation state of DCs were routinely checked by flow cytometric analysis to determine the expression of CD1a, CD14, CD83, and HLA-DR. Typically, the cultures contained more than 90% CD1a+CD14– cells. In some experiments, immature and mature DCs were also tested for levels of expression of PD-L1, SLAM, and ILT-4 (kind gifts from Gregorio Aversa), ILT-3 (Immunotech, Marseilles, France), and CD45RB and CD45RO (BD Biosciences, San Jose, CA).
Purification of T cells
CD4+ T cells were purified from PBMCs by negative selection using the untouched CD4+ T cell isolation kit (Miltenyi Biotech, Auburn, CA), according to the manufacture's instructions. A portion of the resultant CD4+ T cells was cryopreserved for later use, and the remainder was depleted of CD45RO+ cells using anti-CD45RO–coupled magnetic beads and LD negative-selection columns (Miltenyi Biotech). Resultant cells were routinely more than 90% CD4+CD45RO–CD45RA+. For some experiments, the CD4+CD45RO– T cells were subsequently depleted of CD25+ cells using anti-CD25–coupled magnetic beads (Miltenyi Biotech).
T-cell differentiation
DCs (1 × 105) were cultured with 1 × 106 allogeneic CD4+CD45RO– T cells in 1 mL X-vivo 15 medium (BioWhittaker), supplemented with 5% pooled AB human serum (BioWhittaker) and 100 U/mL penicillin/streptomycin (Bristol-Myers Squibb). After 6 or 7 days, rhIL-2 (40 U/mL) (Chiron, Amsterdam, Holland) was added, and cells were expanded for another 7 to 8 days. Fourteen days after initiation of the culture, T cells were collected, washed, and restimulated with immature or mature DCs from the same allogeneic donor used in the primary culture. After 3 days, rhIL-2 was added. One week after initiation of the second stimulation, T cells were collected, and a portion was tested for its proliferative and suppressive capacity, whereas the remainder was restimulated a third time. In some experiments, neutralizing anti–IL-10R (3F9, 30 μg/mL; BD PharMingen, San Diego, CA) monoclonal antibodies (mAbs) were added at the initiation of each round of stimulation, and each time the cells were split. T cells stimulated repeatedly with immature DCs are referred to as T(imm), and those stimulated repeatedly with mature DCs are referred to as T(mat). Cultures with immature DCs typically resulted in a 10-fold reduction in T-cell expansion compared with cultures stimulated with mature DCs. This reduced proliferation was not caused by increased apoptosis (data not shown).
Transduction with lentiviral vector
Lentiviral vector was produced by calcium-phosphate transient transfection of 293T cells using the pCCLsin.cPPT.hPGKΔNGFR.Wpre plasmid to confer expression of ΔLNGFR as a cell surface marker gene. CD4+CD45RO– T cells were primed with immature or mature DCs, as described, and, after 5 days, the T cell/DC coculture was transduced with lentiviral vector at a multiplicity of infection of 20:1, in the presence of 8 μg/mL polybrene (Sigma, St Louis, MO) and IL-2 (40 U/mL). The next day, cells were collected, washed, and cultured for another 8 days. The percentage of transduced T cells was routinely approximately 20%. At the end of the 14-day primary stimulation period, the ΔLNGFR+ T cells were purified by incubation with biotinylated anti–nerve growth factor receptor (anti-NGFR) mAbs followed by streptavidin-coupled microbeads and separation over magnetic columns (Miltenyi Biotech). After purification, T cells, which were more than 90% ΔLNGFR positive, were restimulated with immature DCs, as described.
Proliferation and suppression of T cells
To analyze the proliferative capacity of T(imm) or T(mat) in response to polyclonal activation, 96-well round-bottom plates (Costar, Cambridge, MA) were coated overnight at 4°C with anti-CD3 (OKT3; Jansen-Cilag, Raritan, NJ) mAbs (1 μg/mL) in 0.1 M Tris, pH 9.5, and were washed 3 times with phosphate-buffered saline (PBS). T cells were plated at an initial density of 2.5 × 105 cells/mL (50 000 cells/well) in a final volume of 200 μL medium in the absence or presence of IL-2 (100 U/mL) and soluble anti-CD28 mAbs (1 μg/mL) (BD PharMingen). To test for the capacity of T(imm) or T(mat) cells to suppress proliferation or cytokine production, autologous CD4+ T cells were thawed and stimulated with allogeneic mature DCs (10:1, T cells/DCs) or monocytes (CD3-depleted PBMCs, irradiated 60 Gy) (1:1, T cells/monocytes). Naive CD4+ T cells were stimulated alone or in the presence of T(imm) or T(mat) cells (1:1 ratio) in a final volume of 200 μL complete medium in 96-well round-bottom plates. In some cultures, anti–IL-10R (30 μg/mL, 3F9) or anti–TGF-β (50 μg/mL, 1D11; R&D Systems) mAbs, or both, were added. After the indicated time, either wells were pulsed for 16 hours with 1 μCi (0.037 MBq)/well [3H]-thymidine or supernatants were collected for analysis of interferon-γ (IFN-γ) production.
To test for the suppressive capacity of T(imm) cells by flow cytometry, naive CD4+ T cells were labeled with 5-(and-6)–carboxy fluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR) and were stimulated with mature DCs at a 10:1 ratio in the presence of T(imm) or T(mat) cells. After 5 days, proliferation of the CFSE-labeled naive T cells was determined by flow cytometric analysis. In some experiments, naive CD4+ T cells labeled with CFSE were stimulated with mature DCs at a 10:1 ratio in the presence of absence of T(imm) that had been transduced with lentivirus, as described. After 5 days, cocultures were stained for ΔNGFR to distinguish the T(imm) from the naive CD4+ T cells, and proliferation of the CFSE-labeled naive T cells was analyzed by flow cytometric.
ELISAs
T(imm) and T(mat) were stimulated with mature allogeneic DCs at a 10:1 ratio (T cells/DCs). Supernatants were collected after 24 hours for IL-2 and IL-4, 48 hours for IL-10 and IFN-γ, and 72 hours for TGF-β. To assess the amount of IL-10 produced by immature DCs, DCs were cultured alone or with allogeneic CD4+ T cells at a 10:1 ratio (T cells/DCs) in the presence or absence of anti–IL-10R mAbs (30 μg/mL). Supernatants were harvested after 48 hours. Levels of IL-2, IL-4, IL-10, and IFN-γ were determined by capture enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (BD Biosciences). Levels of TGF-β in acidified supernatants were determined by capture ELISA according to the manufacture's instructions (R&D Systems). The limits of detection were as follows: IL-2, 20 pg/mL; IL-4, 20 pg/mL; IL-10, 20 pg/mL; IFN-γ, 60 pg/mL; and TGF-β, 60 pg/mL.
Quantitative PCR
Total RNA was extracted with Eurozol (Euroclone, Celbio, Milan, Italy), and cDNA was synthesized using the high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). Levels of FoxP3 and HPRT mRNA were quantitated using Assay on Demand real-time polymerase chain reaction (PCR) kits (Applied Biosystems) with TaqMan Master Mix (Applied Biosystems). Samples were run in duplicate, and relative expression of FoxP3 was determined by normalizing to HPRT expression in each set of samples to calculate fold-change in value. mRNAs from freshly isolated CD4+CD25+ Tr or CD4+CD25– T cells were used as positive and negative controls, respectively.
Statistical analysis
All analyses for statistically significant differences were performed with the Student paired t test. P < .05 was considered significant. All cultures were performed in triplicate, and error bars represent the standard deviation.
Results
Cell surface phenotype of immature and mature DCs
We first performed extensive phenotypic analysis to confirm the differentiation and maturation status of DCs. DCs were differentiated from CD14+ monocytes in the presence of IL-4 and GM-CSF for 5 days and were left unstimulated or were activated by coculture with murine fibroblasts expressing CD40L for 48 hours. As expected, cultures of immature and mature DCs were routinely more than 90% CD1a+CD14– (Figure 1A). Immature DCs were CD83– and HLA-DRlow; after activation for 48 hours by CD40 ligation, CD83 and HLA-DR were strongly up-regulated (Figure 1A). We next determined whether molecules previously associated with tolerogenic DCs were expressed by immature or mature DCs. As previously reported, immature DCs expressed significantly lower levels of PD-L1 compared with mature DCs (Figure 1B).20 The mean fluorescence intensity (MFI) of PD-L1 on immature DCs was 27.4 ± 11.6 compared with 43.5 ± 10.9 for mature DCs (n = 4; P ≤ .008). Similarly, immature DCs expressed low levels of SLAM, with MFIs of 9.6 ± 3.9 on immature DCs compared with 18.7 ± 6.2 on mature DCs (n = 6; P ≤ .02). In contrast, expression of CD45RO, CD45RB, ILT-3, and ILT-4 was significantly higher on immature DCs (Figure 1B). MFI of CD45RB on immature DCs was 29.7 ± 15.5 compared with 13.7 ± 6.5 on mature DCs (n = 4; P ≤ .03), expression of CD45RO was 17.2 ± 5.7 on immature DCs compared with 9.3 ± 3.3 on mature DCs (n = 4; P ≤ .01), expression of ILT-3 was 60 ± 22 on immature DCs compared with 41 ± 20 on mature DCs (n = 4; P ≤ .04), and expression of ILT-4 was 12.1 ± 4.0 on immature DCs compared with 5.5 ± 3.9 on mature DCs (n = 5; P ≤ .028).
Phenotype of immature and mature DCs. After 5 days of differentiation in IL-4 and GM-CSF, monocyte-derived DCs were left immature or were matured for 48 hours by the activation of CD40. DCs were then analyzed by flow cytometry to determine levels of expression of CD1a, CD14, CD83, and HLA-DR. (A) Percentages of positive cells, set according to the isotype-matched controls (not shown), are shown in each quadrant. (B) Expression of PD-L1, SLAM, CD45RO, CD45RB, ILT-4, and ILT-3 was also determined. Results are representative of (A) 17 and (B) 6 independent experiments.
Phenotype of immature and mature DCs. After 5 days of differentiation in IL-4 and GM-CSF, monocyte-derived DCs were left immature or were matured for 48 hours by the activation of CD40. DCs were then analyzed by flow cytometry to determine levels of expression of CD1a, CD14, CD83, and HLA-DR. (A) Percentages of positive cells, set according to the isotype-matched controls (not shown), are shown in each quadrant. (B) Expression of PD-L1, SLAM, CD45RO, CD45RB, ILT-4, and ILT-3 was also determined. Results are representative of (A) 17 and (B) 6 independent experiments.
Immature DCs induce T-cell hyporesponsiveness
We next standardized the protocol originally described by Jonuleit et al19 to determine the effects of repetitive stimulation of peripheral blood CD4+CD45RO– T cells with immature or mature DCs. CD4+CD45RO– T cells were cocultured with DCs at a 10:1 ratio, as described in “Materials and methods,” and subsequently tested for their ability to proliferate in response to mature DCs after 1, 2, or 3 rounds of stimulation. As shown in Figure 2A, with subsequent rounds of activation, T cells primed with allogeneic immature DCs became increasingly hyporesponsive to reactivation with mature DCs. After 3 rounds of stimulation, an average reduction of 71% ± 5% (n = 17) in antigen-induced proliferation was observed compared with T cells repetitively primed with mature DCs. Similar results were obtained in response to polyclonal activation (Figure 2B), with an average reduction in proliferation of 75% ± 5% (n = 17) after 3 rounds of activation with immature DCs. This hyporesponsiveness could be rescued by the addition of anti-CD28 mAbs and exogenous IL-2 (Figure 2B).
Induction of T-cell hyporesponsiveness by immature DCs. Peripheral blood CD4+CD45RO– T cells were stimulated with immature or mature allogeneic DCs 1, 2, or 3 times. (A) At the end of each stimulation period, T cells were tested for their ability to proliferate in response to mature allogeneic DCs. (B) In addition, after the third round of activation, their proliferative response to polyclonal activation was tested by stimulation with immobilized anti-CD3 mAb (1 μg/mL), in the absence or presence of soluble anti-CD28 mAb (1 μg/mL) and IL-2 (100 U/mL). After 48 hours of culture, [3H]-thymidine was added for an additional 16 hours. Results are representative of 17 independent experiments.
Induction of T-cell hyporesponsiveness by immature DCs. Peripheral blood CD4+CD45RO– T cells were stimulated with immature or mature allogeneic DCs 1, 2, or 3 times. (A) At the end of each stimulation period, T cells were tested for their ability to proliferate in response to mature allogeneic DCs. (B) In addition, after the third round of activation, their proliferative response to polyclonal activation was tested by stimulation with immobilized anti-CD3 mAb (1 μg/mL), in the absence or presence of soluble anti-CD28 mAb (1 μg/mL) and IL-2 (100 U/mL). After 48 hours of culture, [3H]-thymidine was added for an additional 16 hours. Results are representative of 17 independent experiments.
T(imm) cells have suppressive capacity
The finding that repetitive in vitro stimulation of peripheral blood CD4+CD45RO– T cells with immature DCs resulted in profoundly hyporesponsive T cells suggested that these cells might also have acquired suppressive capacity. We therefore tested the ability of T(imm) to suppress the responses of naive autologous CD4+ T cells on challenge with allogeneic mature DCs. Naive CD4+ T cells were stimulated with mature DCs alone or in the presence of T(imm) or T(mat) cells (1:1 ratio), and proliferation was assessed 1, 2, 3, or 4 days after initiation of the culture. Naive CD4+ T cells stimulated with mature DCs displayed the kinetics of a primary response, with proliferation peaking after 4 days of culture (Figure 3A). As expected, T(mat) cells generated in vitro using allogeneic mature DCs displayed the kinetics of a secondary response when rechallenged with DCs from the same donor, with proliferation peaking at day 2. T(imm) cells remained hyporesponsive throughout the time course. Adding T(mat) cells to the primary mixed lymphocyte reaction (MLR) resulted in increased proliferation at day 2. Importantly, adding T(imm) cells suppressed the proliferation of naive CD4+ T cells in response to mature DCs. An average reduction of 69% ± 19% (n = 13) in the proliferation of naive CD4+ T cells was observed at 4 days after initiation of the culture. These data were mirrored when we examined the production of IFN-γ: adding T(mat) cells to the primary MLR resulted in an additive effect, whereas adding T(imm) cells resulted in an almost complete suppression of IFN-γ production (Figure 3B).
Induction of Tr cells by immature DCs. Peripheral blood CD4+CD45RO– T cells were stimulated 3 times with immature or mature allogeneic DCs. T cells were collected and tested for their ability to suppress responses of autologous CD4+ T cells. (A) Thawed CD4+ T cells were stimulated with mature DCs alone (MLR) or in the presence of T(imm) or T(mat) cell lines at a 1:1 ratio. [3H]-Thymidine was added at the indicated time for an additional 16 hours. (B) In parallel, supernatants were collected after 72 hours and analyzed by ELISA to determine levels of IFN-γ. (A-B) Results are representative of 17 independent experiments.
Induction of Tr cells by immature DCs. Peripheral blood CD4+CD45RO– T cells were stimulated 3 times with immature or mature allogeneic DCs. T cells were collected and tested for their ability to suppress responses of autologous CD4+ T cells. (A) Thawed CD4+ T cells were stimulated with mature DCs alone (MLR) or in the presence of T(imm) or T(mat) cell lines at a 1:1 ratio. [3H]-Thymidine was added at the indicated time for an additional 16 hours. (B) In parallel, supernatants were collected after 72 hours and analyzed by ELISA to determine levels of IFN-γ. (A-B) Results are representative of 17 independent experiments.
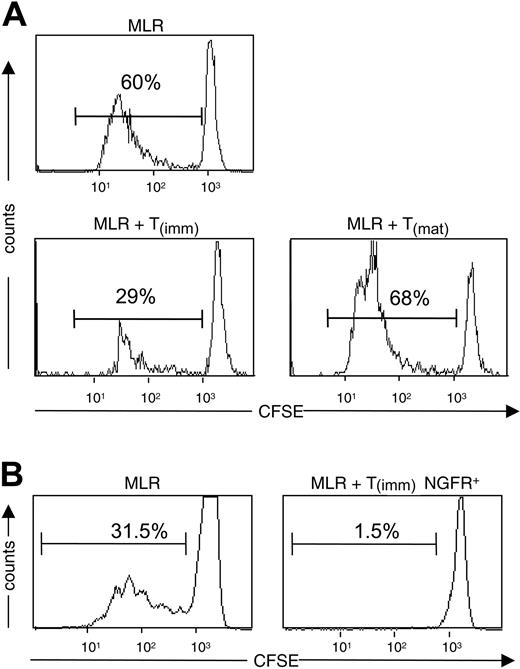
Because of the inability to independently assess the proliferation of T-cell lines and naive CD4+ T cells in thymidine assays, we also investigated the suppressive capacity of T(imm) cells using CFSE-labeling and flow cytometric analysis. Adding T(imm) cells to primary MLRs performed with CSFE-labeled naive CD4+ T cells demonstrated that T(imm) cells suppressed the proliferation of the naive cells, whereas adding T(mat) cells resulted in an increase in the percentage of divided cells (Figure 4A). However, in these experiments, the inability to specifically gate on the naive CD4+ T cells made statistical analysis imprecise. We therefore performed similar experiments with T(imm) cells, which were genetically marked with the truncated nerve growth factor (NGF) R cell surface maker (ΔLNFGR) by lentiviral (LV)–mediated gene transfer and thus could be excluded from the CSFE analysis. Five days after the initial stimulation with immature DCs, T cells were transduced with LV-ΔLNFGR. These experiments were performed with lentiviral vector rather than retroviral vector to ensure that nondividing and dividing cells were transduced. 9 days later, ΔLNGFR+ T(imm) cells were purified using magnetic beads, and cells were cultured and restimulated as described. At the end of the third round of activation, ΔLNGFR+ T(imm) cells were tested for their ability to suppress proliferation of autologous CD4+ T cells, which were labeled with CFSE. As expected, adding ΔLNGFR+ T(imm) cells resulted in the profound suppression of proliferation of the primary MLR (Figure 4B).
Suppressive activity of Tr cells by immature DCs. Naive CD4+ T cells labeled with CFSE were stimulated with mature DCs in the presence of T(imm) or T(mat) cells at a 1:1 ratio. (A) After 5 days of culture, the amount of proliferation was determined by flow cytometric analysis. (B) Naive CD4+ T cells were stimulated with immature DCs and infected with lentivirus encoding ΔLNGFR 5 days after initiation of the culture. ΔLNGFR+ cells were purified and restimulated with immature DCs twice. Autologous CD4+ T cells were labeled with CFSE and were stimulated with mature DCs in the absence or presence of a 1:1 ratio of ΔLNGFR+ T(imm) cells. After 5 days of coculture, the amount of proliferation of the naive CD4+ T cells was determined by flow cytometric analysis. Shown are histograms gated on NGFR-negative cells. Results are representative of 2 independent experiments.
Suppressive activity of Tr cells by immature DCs. Naive CD4+ T cells labeled with CFSE were stimulated with mature DCs in the presence of T(imm) or T(mat) cells at a 1:1 ratio. (A) After 5 days of culture, the amount of proliferation was determined by flow cytometric analysis. (B) Naive CD4+ T cells were stimulated with immature DCs and infected with lentivirus encoding ΔLNGFR 5 days after initiation of the culture. ΔLNGFR+ cells were purified and restimulated with immature DCs twice. Autologous CD4+ T cells were labeled with CFSE and were stimulated with mature DCs in the absence or presence of a 1:1 ratio of ΔLNGFR+ T(imm) cells. After 5 days of coculture, the amount of proliferation of the naive CD4+ T cells was determined by flow cytometric analysis. Shown are histograms gated on NGFR-negative cells. Results are representative of 2 independent experiments.
Differentiation of Tr with immature DCs does not require naturally occurring CD4+CD25+ Tr cells
We tested whether there was a role for CD4+CD25+ Tr cells in the induction of Tr cells from peripheral blood T cells. The highly purified CD4+CD45RO– cells contained a small fraction (approximately 3%-5%) of CD25+ cells (Figure 5A). Thus we depleted CD25+ cells from CD4+CD45RO– cells, and the 2 populations were stimulated with immature DCs in parallel. Cultures initiated with both populations contained T cells that differentiated into Tr cells able to suppress the proliferation of naive autologous CD4+ T cells in response to mature DCs (Figure 5B). Furthermore, we could not detect a significant increase in expression of CD25 on T(imm) cells compared with control T(mat) cells (data not shown). To further exclude the possibility that T(imm) cells were similar to CD4+CD25+ Tr cells, we analyzed the levels of expression of the transcription factor FoxP3. In these experiments, levels of FoxP3 mRNA were expressed relative to those found in highly purified CD4+CD25– T cells and CD4+CD25+ Tr cells, which provided a negative and a positive control, respectively. As shown in Figure 5C, FoxP3 mRNA is expressed at levels that were similar in T(imm) and T(mat) cells but that were significantly lower than those in freshly isolated CD4+CD25+ Tr cells. However, T(imm) and T(mat) cell lines did express more FoxP3 than freshly isolated CD4+CD25– Tr cells, a finding related to the fact that FoxP3 is up-regulated on activation in human T cells (Rosa Bacchetta, Laura Passerini, and M.G.R., unpublished data, May 2004). Together, these data indicate that peripheral blood CD4+ Tr cells primed with immature DCs do not derive from, and are not equivalent to, CD4+CD25+ Tr cells.
CD25+ cells are dispensable for the differentiation of Tr cells by immature DCs. (A) Levels of CD25 expression on peripheral blood CD4+CD45RO– or CD4+CD45RO–CD25– T cells were analyzed by flow cytometry. (B) Subsequently, CD4+CD45RO– or CD4+CD45RO–CD25– T cells were stimulated with immature allogeneic DCs. At the end of 3 rounds of activation, T cells were collected and tested for their ability to suppress responses of autologous CD4+ T cells. Thawed CD4+ T cells were stimulated with mature DCs alone (MLR) or in the presence of a 1:1 ratio of T(imm) or CD25– T(imm) cells. [3H]-Thymidine was added at the indicated time for an additional 16 hours. (C) T(imm) cell lines were compared with T(mat) cell lines for the expression of mRNA for FoxP3. Relative levels of FoxP3 expression were determined by quantitative real-time PCR. The amounts of FoxP3 mRNA are expressed as relative to CD4+CD25– T cells (which were given an arbitrary value of 1). mRNA from freshly isolated CD4+CD25+ Tr cells was used as positive control. Results are representative of 3 independent experiments.
CD25+ cells are dispensable for the differentiation of Tr cells by immature DCs. (A) Levels of CD25 expression on peripheral blood CD4+CD45RO– or CD4+CD45RO–CD25– T cells were analyzed by flow cytometry. (B) Subsequently, CD4+CD45RO– or CD4+CD45RO–CD25– T cells were stimulated with immature allogeneic DCs. At the end of 3 rounds of activation, T cells were collected and tested for their ability to suppress responses of autologous CD4+ T cells. Thawed CD4+ T cells were stimulated with mature DCs alone (MLR) or in the presence of a 1:1 ratio of T(imm) or CD25– T(imm) cells. [3H]-Thymidine was added at the indicated time for an additional 16 hours. (C) T(imm) cell lines were compared with T(mat) cell lines for the expression of mRNA for FoxP3. Relative levels of FoxP3 expression were determined by quantitative real-time PCR. The amounts of FoxP3 mRNA are expressed as relative to CD4+CD25– T cells (which were given an arbitrary value of 1). mRNA from freshly isolated CD4+CD25+ Tr cells was used as positive control. Results are representative of 3 independent experiments.
T(imm) cells are phenotypically and functionally equivalent to Tr1 cells
Given that T cells primed by immature DCs did not appear to be equivalent to CD4+CD25+ Tr cells, we next examined whether they had characteristics of IL-10–producing Tr1 cells. We first determined the cytokine production profile of T(imm) and T(mat) cells after activation with mature DCs. As shown in Table 1, T(mat) cells produced all cytokines tested. In contrast, although T(imm) cells clearly produced IL-10, IFN-γ, and TGF-β, they consistently failed to produce significant levels of IL-2 or IL-4. T(imm) cells produced slightly lower amounts of IL-10 compared with T(mat) cells, whereas levels of TGF-β were not significantly different. Although T(imm) cells did produce IFN-γ, levels were at least 10-fold lower than those produced by T(mat) cells. Thus, T(imm) cells display a profile of cytokine production similar to that of Tr1 cells: IL-10+IFN-γ+TGF-β+IL-2–IL-4–.
We next investigated whether the suppression of proliferation and cytokine production by T(imm) cells was mediated through the production of IL-10, TGF-β, or both. We first performed suppression experiments as described in Figure 3A, in which naive CD4+ T cells were activated with mature DCs in the absence or presence of T(imm) cells. Adding neutralizing anti–IL-10R and anti–TGF-β mAbs had little effect on the suppression of proliferation by T(imm) cells (Figure 6A) but, importantly, completely reversed the suppression of IFN-γ production on activation with mature DCs (Figure 6B). Adding isotype control antibodies had no effect (data not shown). Because it is well known that fully mature DCs are not susceptible to the inhibitory effects of IL-10 or TGF-β,21 we subsequently performed parallel experiments using allogeneic monocytes rather than mature DCs to induce the proliferation of naive T cells. Under these conditions, adding neutralizing anti–IL-10R and anti–TGF-β mAbs completely reversed the suppression of proliferation (Figure 6C) and IFN-γ production (data not shown). Together, these data indicate that Tr cells generated by repetitive stimulation with immature DCs are phenotypically and functionally equivalent to Tr1 cells.
Role of IL-10 and TGF-β in suppression mediated by T(imm) cells. (A) After 3 rounds of activation with immature DCs, T(imm) cells were tested for their ability to suppress the proliferation of CD4+ T cells in response to mature DCs, in the absence or presence of anti–IL-10R (30 μg/mL) and anti–TGF-β (50 μg/mL) mAbs. (B) Similar experiments were performed to assess the suppression of IFN-γ production by CD4+ T cells in response to mature DCs. (C) In parallel, proliferation and suppression in response to allogeneic monocytes was tested. [3H]-Thymidine was added at the indicated time for an additional 16 hours. Results are representative of 3 independent experiments.
Role of IL-10 and TGF-β in suppression mediated by T(imm) cells. (A) After 3 rounds of activation with immature DCs, T(imm) cells were tested for their ability to suppress the proliferation of CD4+ T cells in response to mature DCs, in the absence or presence of anti–IL-10R (30 μg/mL) and anti–TGF-β (50 μg/mL) mAbs. (B) Similar experiments were performed to assess the suppression of IFN-γ production by CD4+ T cells in response to mature DCs. (C) In parallel, proliferation and suppression in response to allogeneic monocytes was tested. [3H]-Thymidine was added at the indicated time for an additional 16 hours. Results are representative of 3 independent experiments.
Differentiation of Tr1 cells by immature DCs requires IL-10
IL-10 is a key differentiation factor for Tr1 cells.7 We investigated whether IL-10 was required for the generation of Tr1 cells induced by immature DCs. CD4+CD45RO– T cells were stimulated repetitively with immature DCs in the absence or presence of neutralizing anti–IL-10R or control immunoglobulin G (IgG) mAbs. As shown in Figure 7A, the differentiation of T cells in the presence of neutralizing anti–IL-10R mAbs completely reversed the hyporesponsive state, in terms of proliferation and IFN-γ production, induced by immature DCs. Moreover, the absence of IL-10 also prevented the induction of Tr cells with suppressive activity (Figure 7B).
Autocrine IL-10 is required for the differentiation of T(imm) cells by immature DCs. Peripheral blood CD4+CD45RO– T cells were stimulated with immature allogeneic DCs in the absence or presence of anti–IL-10R or control IgG mAbs (30 μg/mL). After 3 rounds of stimulation, T cells were collected and tested (A) for their ability to proliferate in response to mature DCs and (B) to suppress the response of autologous CD4+ T cells. [3H]-Thymidine was added after (A) 48 hours and (B) 72 hours for an additional 16 hours. In parallel, supernatants were collected after (A) 48 hours and (B) 72 hours, and IFN-γ secretion was measured by ELISA. Results are representative of 3 independent experiments.
Autocrine IL-10 is required for the differentiation of T(imm) cells by immature DCs. Peripheral blood CD4+CD45RO– T cells were stimulated with immature allogeneic DCs in the absence or presence of anti–IL-10R or control IgG mAbs (30 μg/mL). After 3 rounds of stimulation, T cells were collected and tested (A) for their ability to proliferate in response to mature DCs and (B) to suppress the response of autologous CD4+ T cells. [3H]-Thymidine was added after (A) 48 hours and (B) 72 hours for an additional 16 hours. In parallel, supernatants were collected after (A) 48 hours and (B) 72 hours, and IFN-γ secretion was measured by ELISA. Results are representative of 3 independent experiments.
Finally, we investigated the source of autocrine IL-10 in these cultures. Although higher expression of IL-10 mRNA was detected in immature DCs than in mature DCs (data not shown), we found that, as previously reported,22 significant levels of IL-10 (Table 2) were produced by immature DCs, but only when blocking anti–IL-10R mAbs were added to the cultures. Adding T cells to these cultures did not appreciably alter the amount of detectable IL-10 in the absence of presence of anti–IL-10R mAbs, indicating that most IL-10 is produced by immature DCs.
Discussion
We analyzed the capacity of immature monocyte-derived DCs to induce the differentiation of Tr cells from peripheral blood CD4+ T cells. Together, our data strongly support the conclusion that immature DCs drive the differentiation of IL-10–producing Tr1, and not CD4+CD25+ Tr cells. Immature DCs produce autocrine IL-10, and, in conjunction with a unique combination of cell surface molecules, this cytokine is essential for the differentiation of Tr1 cells. The resultant Tr1 cells produce IL-10, TGF-β, and IFN-γ, but they do not produce IL-4 or IL-2, nor do they express high levels of FoxP3, and they are hyporesponsive to antigen-specific and polyclonal activation. This hyporesponsiveness can be reversed with the addition of exogenous IL-2 and anti-CD28 mAb. Tr1 cells induced by immature DCs suppress proliferation and cytokine production by autologous CD4+ T cells through an IL-10– and a TGF-β–dependent mechanism. The presence of CD4+CD25+ Tr cells was not required to differentiate Tr1 cells from immature DCs, and the resultant Tr population did not express constitutive levels of CD25 or FoxP3.
Our finding that repeated exposure to human immature DCs induces the differentiation of IL-10–producing Tr cells is similar to findings in previous reports.18,19 However, in contrast to results obtained with cord blood CD4+ T cells indicating that when immature DCs were used to prime cord blood CD4+ T cells, the resultant Tr cells shared many characteristics with naturally occurring CD4+CD25+ Tr cells,19 we found that peripheral blood T(imm) cells mediated cytokine-dependent suppression and did not acquire the phenotype of CD4+CD25+ Tr cells. The reason for this difference is unclear, but it is important to note that the phenotypes of naive CD4+ T cells in peripheral and cord blood are distinct. Cord blood CD4+ T cells contain a significantly higher proportion of CD4+CD25+ Tr cells than peripheral blood CD4+ T cells, and most are CD45RA+.23 Furthermore, cord blood T cells have an innate capacity to produce high levels of autocrine IL-10,24 which we have previously shown can have a key role in Tr1 cell differentiation.25 Thus, it is possible that stimulating cord blood CD4+ T cells with immature DCs might have resulted in a combination of expansion of preexisting CD4+CD25+ Tr cells and differentiation of IL-10–producing Tr1 cells. In such a mixed population, though IL-10 would have been detected, any functional effects might have been masked by the presence of cytokine-independent CD4+CD25+ Tr cells.
T(imm) cells did not express constitutive CD25 or high levels of mRNA for FoxP3, a transcription factor highly expressed in mouse and human CD4+CD25+ Tr cells.26-28 Moreover, neither murine nor human IL-10–producing Tr cells express high levels of FoxP3 mRNA29,30 (see also Rosa Bacchetta, Laura Passerini, and M.G.R., unpublished data, May 2004). Thus, our data strongly support the conclusion that Tr cells induced by immature DCs are distinct from naturally occurring CD4+CD25+ Tr cells.
We have shown here that the effects of neutralizing anti–IL-10 mAbs on the proliferation of CD4+ T cells should also be tested on stimulation with monocytes because mature DCs are not susceptible to the suppressive effects of IL-10 produced by Tr1 cells.21 Interestingly, though the blockade of IL-10 and TGF-β did not affect the suppression of proliferation by T(imm) cells, it completely restored the production of IFN-γ by CD4+ T cells activated with mature DCs. These data suggest that the mechanisms by which Tr1 cells suppress proliferation and cytokine production may be distinct and that the suppression of cytokine production may be more prominent.31 Neutralizing anti–IL-10 and anti–TGF-β mAbs also reversed the anergic state of Tr1 cells (data not shown). These data are in accordance with previous findings that anti–IL-10 and anti–TGF-β mAbs can enhance the proliferative response of Tr1 cells.25
Interestingly, the anergic state of Tr1 cells induced by immature DCs was completely reversed on polyclonal activation in the presence of anti-CD28 mAb and IL-2 but was maintained when the cells were activated with fully mature DCs. We can speculate that the inability of fully mature DCs to revert the anergic state of Tr1 cells induced by immature DCs is attributable to a lower strength of T-cell receptor (TCR) signaling than that obtained with polyclonal stimulation using anti-CD3 and anti-CD28 mAbs.
The mechanisms by which immature DCs promote the differentiation of Tr1 cells are not completely clear. As shown here, autocrine production of IL-10 is an essential component of the mechanism (Table 2; Figure 6). However, other molecules are clearly involved because IL-10 alone is insufficient to induce the differentiation of Tr1 cells.25 We found that, compared with mature DCs, immature DCs express high levels of ILT-4 (Figure 1B), an inhibitory molecule that binds to HLA-G and that is induced by IL-1032 and by ILT-3, which mediates the tolerogenic effects of DCs exposed to CD8+CD28– T-suppressor cells.33 Interestingly, CD45RB, a key marker for murine DCs that differentiate Tr1 cells,34 was also found to be significantly up-regulated on human immature DCs, which induce Tr1 cells (Figure 1B). Conversely, immature DCs expressed low levels of PD-L1 and SLAM (Figure 1B). Therefore, ILT-4 and ILT-3 or different isoforms of CD45 may play a role in the induction of Tr1 cells by immature DCs.
Myeloid DCs can be made tolerogenic by a variety of means, including treating them with IL-10,35 a combination of IL-10 and TGF-β,36 or immunosuppressive agents such as vitamin D337 ; exposing them to certain types of bacteria38 or certain endogenous proteins such as heavy chain ferritin39 ; or transducing them with retroviral vectors that encode tolerogenic molecules such as IL-10, TGF-β, CTLA-4, or Notch ligands.40 In addition, specialized subsets of DCs, which are strictly dedicated to tolerance induction, even in a mature state, can also induce Tr1 cells. For example, DEC205+B220+CD19– DCs isolated from the liver and activated in vitro with IL-3 and CD40 cross-linking induce Tr1 cells.41 Similarly, human DC2 cells can induce the differentiation of IL-10–producing CD8+ Tr cells.42 Whether the Tr cells that arise after stimulation with different types of tolerogenic DCs are phenotypically or functionally equivalent to the Tr1 cells induced by immature DCs remains to be determined.
The requirement for multiple rounds of stimulation with immature DCs to differentiate Tr1 cells is intriguing. The fact that T cells must encounter tolerogenic DCs multiple times before they differentiate into Tr1 cells suggests that in vivo this may be a safeguard to ensure that tolerance is only induced toward antigens consistently presented in a noninflammatory environment.
In conclusion, immature DCs can efficiently differentiate and expand antigen-specific Tr1 cells in vitro. These findings are consistent with the idea that in the absence of inflammation, DCs function to tolerize the immune system to ingested/inhaled proteins and proteins derived from dying cells derived from normal cell turnover.43 This level of control may also be operational on CD8+ T cells.18 The fact that immature DCs induce IL-10–producing Tr1 cells is consistent with the hypothesis that Tr1 cells are exclusively induced de novo in the periphery. The ability to expand and genetically mark large numbers of alloantigen-specific Tr1 cells is an important step toward the use of these cells as therapy to induce tolerance after transplantation and in autoimmune diseases.
Prepublished online as Blood First Edition Paper, October 12, 2004; DOI 10.1182/blood-2004-03-1211.
Supported by a grant from the Italian Telethon Foundation.
M.K.L. and S.G. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Figure 2. Induction of T-cell hyporesponsiveness by immature DCs. Peripheral blood CD4+CD45RO– T cells were stimulated with immature or mature allogeneic DCs 1, 2, or 3 times. (A) At the end of each stimulation period, T cells were tested for their ability to proliferate in response to mature allogeneic DCs. (B) In addition, after the third round of activation, their proliferative response to polyclonal activation was tested by stimulation with immobilized anti-CD3 mAb (1 μg/mL), in the absence or presence of soluble anti-CD28 mAb (1 μg/mL) and IL-2 (100 U/mL). After 48 hours of culture, [3H]-thymidine was added for an additional 16 hours. Results are representative of 17 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/3/10.1182_blood-2004-03-1211/6/m_zh80030573190002.jpeg?Expires=1766004179&Signature=PXWDt~iuWYOd7wSAuQLE7qyDIDg3QEOGtg~2O6WPrDvq711XX~~Zbxb4AIu78bc~UFVzkpvwg4RVqjQE20dFRnLaUuqJZvHT6I6-I0bPtNUDT-lMoyfTN9dV6uLrKNbc6~5sUW-6Jzq7pCZNKQ-ALO8uqCS8Luw-A9EDvgQwZUNBf375pnwOXxj5U979VBYvisES9fCaI~J1dCnAC6cRATi8UP7PPPPwfBg2f20CfaGHc~sWmvl1wCH-0MTw2A1GBPMr7B5G7nYXeV6AgpLNn5utPHvjXVZCqXGo0X-8lCqtlXwYdBEf8Vm3wlSN2FDeKw1aD5pnt5dolgtNo3I8pg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Induction of Tr cells by immature DCs. Peripheral blood CD4+CD45RO– T cells were stimulated 3 times with immature or mature allogeneic DCs. T cells were collected and tested for their ability to suppress responses of autologous CD4+ T cells. (A) Thawed CD4+ T cells were stimulated with mature DCs alone (MLR) or in the presence of T(imm) or T(mat) cell lines at a 1:1 ratio. [3H]-Thymidine was added at the indicated time for an additional 16 hours. (B) In parallel, supernatants were collected after 72 hours and analyzed by ELISA to determine levels of IFN-γ. (A-B) Results are representative of 17 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/3/10.1182_blood-2004-03-1211/6/m_zh80030573190003.jpeg?Expires=1766004179&Signature=HLGkmCiztGK4fNxjiBvF-Nl7gIz0wKGgg4xTICssCGfUgf5tAUmB9ZYdG1Z7HaKA3y7BFixnyTmffcbK6lvHHbSxSU98pvhGo0q9Fs1UWUuYOQt-c74fFa2RFBvqR7BD-gaP7ZYTM2-Yvci7QHQQMjiHC7qvva7dNhggTP9Du-hrYOCAzDM649N~h7Y-aO2As2QVGr~LNgRlTiKtI6AUtQdCJ2nedxqRbFW86ME3ZfHrY6ImLf~e1pmT2w~8wEvuGSGGRB5N9BiOdDenF8cnigF2ij1iG6DNW4ch3fos7w~u7Ei-jPRhuz1ogwWB6T6KE9AlKne~k-4Jby1aUNNozQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. CD25+ cells are dispensable for the differentiation of Tr cells by immature DCs. (A) Levels of CD25 expression on peripheral blood CD4+CD45RO– or CD4+CD45RO–CD25– T cells were analyzed by flow cytometry. (B) Subsequently, CD4+CD45RO– or CD4+CD45RO–CD25– T cells were stimulated with immature allogeneic DCs. At the end of 3 rounds of activation, T cells were collected and tested for their ability to suppress responses of autologous CD4+ T cells. Thawed CD4+ T cells were stimulated with mature DCs alone (MLR) or in the presence of a 1:1 ratio of T(imm) or CD25– T(imm) cells. [3H]-Thymidine was added at the indicated time for an additional 16 hours. (C) T(imm) cell lines were compared with T(mat) cell lines for the expression of mRNA for FoxP3. Relative levels of FoxP3 expression were determined by quantitative real-time PCR. The amounts of FoxP3 mRNA are expressed as relative to CD4+CD25– T cells (which were given an arbitrary value of 1). mRNA from freshly isolated CD4+CD25+ Tr cells was used as positive control. Results are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/3/10.1182_blood-2004-03-1211/6/m_zh80030573190005.jpeg?Expires=1766004179&Signature=kL5eyzK0cO0GXf1cApI~SGKS1AkqgshPuGTaq3iiSi4VnNz0DWkY7goZ6up-7lLSdWnnfB9rBlz~TiYPvHs5tBggiHAErRyQiVGy19rqAhHpHRA8a9J27aY6V5hTbIggRMaY1OFEH-FF2NmHmBLRP8VREsS9TC8QfGv8Y4Z63lmI8U8UdkbqbxeIbZOH9-MVWbacr4rzzpgaN6o-tVRJYpRI0Jgwe1cDNS3b4Njyh6N3Lr7TqVh-ajDLKfYYhqEtvAtPx42ZQmYXf8IOfrwNzc6KQpXDLWclhlKKrfo6Mu-khyHTeZIbxYGuHNdtNN0Ovepcm8udUkPJLRgvJeLjyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Role of IL-10 and TGF-β in suppression mediated by T(imm) cells. (A) After 3 rounds of activation with immature DCs, T(imm) cells were tested for their ability to suppress the proliferation of CD4+ T cells in response to mature DCs, in the absence or presence of anti–IL-10R (30 μg/mL) and anti–TGF-β (50 μg/mL) mAbs. (B) Similar experiments were performed to assess the suppression of IFN-γ production by CD4+ T cells in response to mature DCs. (C) In parallel, proliferation and suppression in response to allogeneic monocytes was tested. [3H]-Thymidine was added at the indicated time for an additional 16 hours. Results are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/3/10.1182_blood-2004-03-1211/6/m_zh80030573190006.jpeg?Expires=1766004179&Signature=enTWBrXSLpxB5GdSpmJVWS6cbyl-o58e2r0DcOfQODZb3Y02QKwgmDsSJh7QjEob2tfvi3AZD9~W7s9lKwC2yNnZ~shyUbgudg8aPw5FcFdBdjb-z8K6BPUSFTCePsFGxro9gZh7bjXBg3wGHImTK7SPzmGLQKN9edt4Oo8DN9YiWlIwlVlstX7hCxl3b2ZMYRoCs7ghz8pgja-3aSKznBeryRMxO8x0YzaFrifcVCkR7S8yaRMuH1A9XYw55Y53D0h2bPhWmdKDlbxhNj7yuZ~-uqlridD2djOE5QYIzpJJP5gK8RT~PtIuC8lObTx0eXHfXDViSk7eWei-TvoXUw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Autocrine IL-10 is required for the differentiation of T(imm) cells by immature DCs. Peripheral blood CD4+CD45RO– T cells were stimulated with immature allogeneic DCs in the absence or presence of anti–IL-10R or control IgG mAbs (30 μg/mL). After 3 rounds of stimulation, T cells were collected and tested (A) for their ability to proliferate in response to mature DCs and (B) to suppress the response of autologous CD4+ T cells. [3H]-Thymidine was added after (A) 48 hours and (B) 72 hours for an additional 16 hours. In parallel, supernatants were collected after (A) 48 hours and (B) 72 hours, and IFN-γ secretion was measured by ELISA. Results are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/3/10.1182_blood-2004-03-1211/6/m_zh80030573190007.jpeg?Expires=1766004179&Signature=wHNfWvGaawlSQbu~NkrDY2etdTWXvg7Ifqe4XW8Ynd4Yn4pQiEpYhjQPjhFyON2lK8vSmSt5ruuDOMro6MeGNCZbv11hQXxOC78Rmhj1fNjmf7coPLAvC4WQteNTmvdJq8SkTmH3oBeGuE~0iknZCifMElsDA4bv-iyUz~WhsG7fjIW8VPeirPRShrqYcmyFKLeoUdCUqR7NNs48bQm8qIz~w4Fm-Br6KPvmHq8GTz1oKwF-fqiRb4f8Dmcluor4cM7jlWJCLClWjnpHjIPhZE8dDcRqXR5XirGhhJxWrX3XIT7Y1OSw8mwVg1iFivr7e9-VS5aNXVaOKGxTli11Ng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
