Abstract
Allogeneic bone marrow transplantation (BMT) is a potentially curative treatment for both inherited and acquired diseases of the hematopoietic compartment; however, its wider use is limited by the frequent and severe outcome of graft-versus-host disease (GVHD). Unfortunately, efforts to reduce GVHD by removing donor T cells have resulted in poor engraftment and elevated disease recurrence. Alternative cell populations capable of supporting allogeneic hematopoietic stem/progenitor cell engraftment without inducing GVHD could increase numbers of potential recipients while broadening the pool of acceptable donors. Although unfractionated CD4+ T cells have not been shown to be an efficient facilitating population, CD4+CD25+ regulatory cells (T-reg's) were examined for their capacity to support allogeneic hematopoietic engraftment. In a murine fully major histocompatibility complex (MHC)-mismatched BMT model, cotransplantation of donor B6 T-reg's into sublethally conditioned BALB/c recipients supported significantly greater lineage-committed and multipotential donor progenitors in recipient spleens 1 week after transplantation and significantly increased long-term multilineage donor chimerism. Donor engraftment occurred without GVHD-related weight loss or lethality and was associated with tolerance to donor and host antigens by in vitro and in vivo analyses. Donor CD4+CD25+ T cells may therefore represent a potential alternative to unfractionated T cells for promotion of allogeneic engraftment in clinical hematopoietic cell transplantation. (Blood. 2005;105:1828-1836)
Introduction
Bone marrow transplantation (BMT) is a potentially curative treatment for both hematologic and nonhematologic diseases, but the wider use of allogeneic BMT is limited by the frequent and severe outcome of graft-versus-host disease (GVHD).1 Unfortunately, efforts to reduce GVHD by removing donor T cells from the graft have resulted in poor engraftment and elevated disease recurrence, indicating a pivotal role of donor T cells in promoting engraftment of the donor hematopoietic compartment.2,3 CD8 T cells have been found to be much more potent facilitators of both initial and long-term engraftment than CD4 T cells in murine transplantation models.4,5 However, both CD8 and CD4 T cells are capable of mediating GVHD. Moreover, attempting to reduce GVHD by limiting perforin and FasL function has not prevented GVHD, and CD8 T cells would likely have detrimental effects on engraftment and the capacity for graft-versus-leukemia (GVL) responses.5-9 Therefore, identifying cell populations capable of supporting allogeneic hematopoietic stem/progenitor cell (HSPC) engraftment without inducing GVHD could circumvent the hazards of conventional T cells, thereby broadening the pool of acceptable donors.
Although unfractionated CD4 T cells have not been shown to possess efficient facilitating potential, the CD25+ regulatory fraction, representing approximately 10% of peripheral CD4 T cells, represents a potentially attractive alternative to CD8 T cells for promotion of engraftment.4,5 Identified by their role in suppressing autoimmunity, CD4+CD25+ T cells have demonstrated a capacity to inhibit T-cell activation in vitro and T-cell-mediated autoimmune disease in vivo.10,11 This T-cell suppression has been employed to inhibit alloreactive responses and prevent GVHD in murine models of hematopoietic transplantation.12-15 Although suppression has been demonstrated in vitro to require signaling via their antigen receptor, CD4+CD25+ suppressor cells and responder T cells can have distinct T-cell-receptor (TCR) specificities.16,17 Little is presently understood regarding CD4+CD25+ antigen recognition in vivo. With regard to their application to transplantations, it is interesting to consider the possibility that donor CD4+CD25+ T cells could be activated by transplanted self-antigens and/or recipient-derived alloantigens.16,18-22 We therefore hypothesized that appropriate TCR signaling of donor CD4+CD25+ T cells would occur after transplantation, and this population could assist donor HSPCs in overcoming the host resistance independent of the specter of GVHD.
The present studies have assessed the capacity of donor CD4+CD25+ T cells to support engraftment of donor bone marrow grafts in a complete major histocompatibility complex (MHC)-mismatched (B6 → BALB/c) BMT model. The transplantation of CD4+CD25+ T cells into sublethally conditioned recipients resulted in decreased rejection of both lineage-committed and multipotential donor hematopoietic progenitors within the first week of the transplantation. Although CD25-CD4 T cells also supported donor progenitors early after BMT, recipients of marrow grafts supplemented with CD4+CD25- T cells ultimately developed GVHD-associated weight loss and mortality. In contrast to donor progenitor support by CD4+CD25- T cells, mice receiving CD4+CD25+ T cells did not develop GVHD-associated symptoms and demonstrated long-term (6 months after BMT) multilineage donor chimerism. Notably, chimerism was associated with unresponsiveness to donor and host antigens by in vitro assay and by the failure to reject B6 and BALB/c skin grafts. The rejection of “third-party” skin grafts demonstrated maintenance of immune responsiveness in these animals. We conclude that CD4+CD25+ T cells may therefore provide a new approach for promotion of engraftment during allogeneic BMT.
Materials and methods
Mice
Seven to 8-week-old female BALB/c and C57BL/6 (B6) mice were purchased from Jackson Laboratories (Bar Harbor, ME). C57BL/6 TgN(act-EGFP)OsbC15-001-FJ001 green fluorescence protein (GFP) transgenic breeder mice (B6-gfp) were originally provided by Dr Timothy Ley (Washington University, St Louis), and B6 CD8-/- breeders were originally purchased from Jackson Laboratories. Mice were maintained in pathogen-free conditions in the Department of Microbiology and Immunology at the University of Miami Miller School of Medicine.
BM transplantation
An MHC class I/II-mismatched model was used in which BALB/c mice (H-2d) were recipients. One day before transplantation, BALB/c recipients were conditioned with a sublethal 7.0-Gy dose of total body irradiation (TBI; cobalt 60 source; dose rate, 44.5 cGy/min). BM cells obtained by flushing of donor B6-GFP femora and tibiae were resuspended at a concentration of 2.5 × 107/mL in RPMI-1640 medium and incubated with anti-Thy1.2 murine antibodies (mAbs; HO-13-4) at 4°C for 30 minutes. The cells were then washed and incubated at 37°C for 30 minutes in the presence of complement (Rabbit Low Tox-M; Cedarlane Laboratories, Hornby, ON, Canada). After being washed with RPMI-1640 medium, T-cell-depleted (TCD) BM cells were collected, and the viable cells were counted with trypan blue staining. TCD BM cells (0.5 × 106 to 2 × 106) were injected intravenously into recipients alone (control) or together with selected numbers of B6 T cells (see “Preparation of donor T cells”). A minimum of 3 BM transplant recipients per time point was used in each experiment. Recipients not killed for colony-forming unit (CFU) analysis were monitored for GVHD induction by changes in total body weight and overall survival.
Preparation of donor T cells
Highly enriched CD4+CD25+ and CD4+CD25- T cells were obtained by positive selection with the Miltenyi magnetic cell sorting system (MACS; Miltenyi, Auburn, CA). B6 CD8-/- T cells were harvested from spleen and lymph node cells and enriched for T cells by panning with goat antimouse immunoglobulin (Ig; Chemicon, Temecula, CA) to deplete B cells. Nonadherent cells were washed and resuspended in MACS buffer (1 × phosphate-buffered saline [PBS] with 0.5% bovine serum albumin [BSA] and 0.02% NaN3) and incubated with phycoerythrin (PE)-conjugated anti-CD25 (PharMingen, San Diego, CA) for 15 minutes on ice. Cells were then washed twice and resuspended in MACS buffer and incubated with anti-PE microbeads (Miltenyi) for 15 minutes at 4°C. Cells were washed again and loaded onto an LS column. The cells retained on the column were eluted and the resulting population was routinely greater than 90% CD4+CD25+, with greater than 98% of the CD4 T cells expressing CD25. The flow-through population was incubated with anti-CD4 microbeads (Miltenyi) and positively selected on an LS column. The resulting eluent was routinely greater than 95% CD4+CD25-. CD8 knockout donors were used in some experiments to eliminate the possibility that effects on allogeneic engraftment were due to contaminating CD8 T cells. CD25-enriched cell populations were stained with Cy-Chrome anti-CD8, PE anti-CD25, and fluorescein isothiocyanate (FITC) anti-CD4 (PharMingen) mAbs and examined for purity by flow cytometric analysis with a FACScan system (Becton Dickinson, Franklin Lakes, NJ).
Colony-forming assays
The granulocyte/macrophage CFU (CFU-GM) and high proliferative potential CFU (CFU-HPP) assays were modified from a previously described method.5 Briefly, recipient spleens were collected on day 7 after BMT, and the nucleated cells were counted. The spleen cells (1.5 × 105 to 2.0 × 105) were cultured in 1-mL mixture containing Iscoves medium, 0.86% methylcellulose (Methocult; StemCell Technologies, Vancouver, BC, Canada), 30% fetal calf serum, 250 μM 2-mercaptoethanol, 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin. GM colonies were generated from cultures of pooled splenocytes from each group supplemented with 50 U/mL recombinant murine interleukin 3 (IL-3; R&D Systems, Minneapolis, MN) for 5 days, whereas HPP colonies were generated by culturing with 100 ng/mL stem cell factor (PEPROTECH, Rocky Hill, NJ), 50 U/mL IL-3, and 2700 U/mL IL-1 (PEPROTECH) for 12 days. Cultures were established in triplicate for each concentration and maintained at 37°C in humidified air with 5% CO2. Results are presented as average total CFUs/spleen ± SEM, after multiplying the CFU frequency times the total number of splenocytes.
BM CFUs were assessed 2 months after BMT by aspirating BM from recipient tibiaes and culturing 5 × 104 cells from individual BM transplant recipients in triplicate wells. Culture conditions were as described in the previous paragraph, and donor derivation was determined by GFP fluorescence. Results are presented as percentage of individual recipients' CFUs that were donor derived ± SEM.
Chimerism analysis
Blood and tissue samples of recipients were collected at various time points after BMT. Red blood cells were removed by ammonium chloride potassium treatment. Mononuclear cells were assessed directly for GFP expression or stained as described in “Preparation of donor T cells” with anti-H-2Kb and anti-H-2Kd mAbs, or with anti-CD4, anti-CD8, anti-B220, and anti-Mac-1 mAbs (PharMingen). Percentage of donor BM-derived (GFP+) cells was determined by flow cytometry (FACScan). Forward and side-angle light scatter was used to establish analytic gates containing predominantly lymphoid and nonlymphoid (monocyte/granulocyte) populations.
Immunologic analyses
Polyclonal proliferation assays were performed using spleen and lymph node cells from mice that received a transplant 3 to 4 months after BMT and from controls that did not receive a transplant. Cells (2 × 105 cells/well) were cultured alone or stimulated with anti-CD3 mAb (145-2C11) supernatant at 15% vol/vol or 10 μg/mL LPS for 2.5 days. 3Tdr (1.0 μCi/well [0.037 MBq/well]) was added 24 hours prior to harvest using a multiple automated sample harvester (MASH) and measurement of thymidine uptake was done by liquid scintillation counting. Results are presented as average counts per minute (CPM) ± SEM of triplicate wells.
Mixed lymphocyte responses were performed similarly to the polyclonal proliferation cultures described in “Chimerism analysis.” Responding cells were stimulated with 1 × 105 irradiated stimulated B6 (donor) or BALB/c (host) splenocytes, cultured for 3 days prior to pulsing with thymidine, and harvested the following day as described in “Chimerism analysis.”
Cell-mediated lympholysis (CML) assays were performed by obtaining spleen and lymph node cells from mice that received a transplant 3 to 4 months after BMT and from controls that did not receive a transplant and stimulating 4 × 106 cells/well with or without 2 × 106 irradiated splenocytes from B6 or BALB/c mice. Following 3 days of culture, effector cells were collected from experimental (stimulated) and control (unstimulated) cultures, counted, and incubated for 4 hours with 104 Cr51-labeled target cells expressing H-2b (EL4) or H-2d (P815) MHC. Specific lysis was defined as 100 × (experimental release - spontaneous release)/(detergent release - spontaneous release). Maximum spontaneous release values were less than 10% of the total detergent release values in all experiments.
Skin graft rejection was measured by isolating tail skin from B6, BALB/c, and C3H/HeJ (third-party) mice and placing the grafts on the tails of BM transplant recipients and controls that did not receive a transplant 3 to 4 months after BMT. Grafts were prepared after donor mice were anesthetized and swabbed with 70% ethanol. Briefly, rectangular sections of skin were removed from the dorsal side of the tail and replaced in reverse orientation onto the dorsal surface of recipient mouse tails where 3 distinct graft beds were made by removing slightly larger rectangular sections of skin down the length of the tail. Casts of plastic tubing were placed on recipient tails for 2 days and then removed for initial scoring of the grafts. Only grafts that appeared vascularized and healthy were followed and scored daily. Grafts were scored as rejected following complete absence of hair and epithelial breakdown (100% necrotic).
Statistical analyses
Colony, chimerism, and proliferation data were analyzed with a Student 2-tailed unpaired t test or one-way analysis of variance (ANOVA). Weight losses were compared by linear regression, and rejection was analyzed with the Fisher test. Survival of BM transplant recipients and skin grafts was compared by log-rank analysis. The confidence interval was 95%, and a P value less than .05 was considered significant.
Results
CD4+CD25+ T cells support day-7 splenic donor progenitors following allogeneic BMT
To assess the capacity of CD4+CD25+ T cells to support engraftment during allogeneic hematopoietic cell transplantation (HCT), a complete MHC-mismatched H-2b → H-2d transplantation model was employed. A sublethal conditioning dose of 7.0 Gy TBI was selected to leave intact a significant myeloid compartment and host resistance capacity (data not shown). Varying numbers of TCD BM were then examined to determine doses that failed to engraft when transplanted alone.
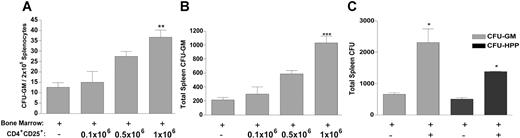
Examination for donor progenitor cells (PCs) after BMT can be used as an early assessment for the presence of immunologic resistance in the host. BALB/c mice were conditioned (7.0 Gy) and received a transplant 1 day later with B6-GFP TCD BM with and without enriched CD4+CD25+ T cells from B6 CD8-/- mice. Recipients were killed 7 days after BMT and splenocytes were harvested to assess for presence of donor CFU-GMs and CFU-HPPs. The use of GFP transgenic donor mice allowed for identification of donor-derived CFUs in recipient spleens. Titration of CD4+CD25+ cell numbers demonstrated support of donor CFU-GMs, and the highest dose (1 × 106) of CD4+CD25+ T cells mediated the greatest supportive effect, with regard to both frequency (Figure 1A) and total numbers (Figure 1B). This T-cell dose was therefore selected for all subsequent studies. Mice receiving CD4+CD25+ T cells at the time of transplantation demonstrated significantly greater numbers of donor CFU-GMs and CFU-HPPs, demonstrating increases in both committed hematopoietic lineage as well as more primitive multipotential progenitor populations within the first week of the transplantation (Figure 1C).
Donor CD4+CD25+ T cells increase frequency and total number of allogeneic CFUs in recipient spleens 7 days after BMT. B6-GFP BM-TCD (2 × 106) was transplanted alone or with 0.11 × 106 to 1 × 106 B6 CD8-/-CD4+CD25+ T cells into 7.0-Gy irradiated BALB/c recipients (n = 3/group). Seven days later, recipient spleens were analyzed for frequency of donor CFU-GMs (A) and total splenic donor CFU-GMs (B). Using this same model together with 1 × 106 CD4+CD25+ T cells from B6 CD8-/- donors, recipient spleens were analyzed 7 days later for total donor CFU-GMs (▦) and CFU-HPPs (▪) (C). Data represent mean CFU/spleen ± SEM of pooled splenocytes. Panel A indicates frequency; panels B and C, total. Results presented are from an individual experiment (representative of 3 independent experiments that simultaneously examined both CFU progenitors, and more than 20 experiments that examined CFU-IL-3 splenic activity). *P < .05; **P < .01; and ***P < .001, respectively, compared with BM transplanted alone.
Donor CD4+CD25+ T cells increase frequency and total number of allogeneic CFUs in recipient spleens 7 days after BMT. B6-GFP BM-TCD (2 × 106) was transplanted alone or with 0.11 × 106 to 1 × 106 B6 CD8-/-CD4+CD25+ T cells into 7.0-Gy irradiated BALB/c recipients (n = 3/group). Seven days later, recipient spleens were analyzed for frequency of donor CFU-GMs (A) and total splenic donor CFU-GMs (B). Using this same model together with 1 × 106 CD4+CD25+ T cells from B6 CD8-/- donors, recipient spleens were analyzed 7 days later for total donor CFU-GMs (▦) and CFU-HPPs (▪) (C). Data represent mean CFU/spleen ± SEM of pooled splenocytes. Panel A indicates frequency; panels B and C, total. Results presented are from an individual experiment (representative of 3 independent experiments that simultaneously examined both CFU progenitors, and more than 20 experiments that examined CFU-IL-3 splenic activity). *P < .05; **P < .01; and ***P < .001, respectively, compared with BM transplanted alone.
CD4+CD25- T cells also support donor-derived day-7 splenic CFU-GMs, but CD4+CD25+ T cells augment donor CFUs without induction of GVHD
Experiments were performed comparing the support of donorderived day-7 splenic colonies by equivalent numbers of distinct donor T-cell populations. CD25+ and CD25-CD4 T cells were prepared from B6 CD8-/- mice. Results indicated that CD25+ and CD25-CD4 T cells exhibited a comparable capacity to support donor CFU-GMs when cotransplanted with the B6 bone marrow inoculum into BALB/c mice (Figure 2A). Notably, prolonged assessment of BM transplant recipient mice for signs of GVHD demonstrated divergent consequences dependent on the CD4 T-cell subset that received transplants. Mice receiving TCD BM plus CD25-CD4 T cells exhibited GVHD-associated weight loss and mortality (Figure 2B). In contrast, recipients of TCD BM and CD4+CD25+ T cells did not exhibit weight loss, and all mice survived more than 100 days after BMT. No differences in weight or lethality were detected between this latter group and control recipients receiving TCD BM alone. Experiments were also performed with mice that received transplants of TCD BM and CD25-depleted T cells (containing both CD4 and CD8 T cells). Similar to the findings described earlier in this paragraph, after CD25-CD4 T-cell transplantations, these recipients experienced weight loss and mortality characteristic of GVHD, contrasting mice receiving CD4+CD25+ T cells in this independent experiment. The latter mice did not demonstrate these changes and possessed a normal CD4/CD8 ratio in peripheral blood (data not shown).
CD25+ and CD25-CD4+ T cells support donor splenic CFU-GMs 7 days after BMT, but CD4+CD25+ T cells do not lead to GVHD. B6-GFP BM-TCD (2 × 106, A; 1 × 106, B) was transplanted alone or with 1 × 106 CD25+ or CD25-B6 CD4+ T cells into 7.0-Gy irradiated BALB/c recipients. Recipients were killed 7 days after BMT for total splenic donor CFU-GM analysis. Data presented are mean CFU/spleen ± SEM of pooled splenocytes and 3 independent experiments (A). Error bars in B represent mean and SEM of individual mouse weights. Groups of BM transplant recipients (n = 6-7/group) were monitored to assess the development of GVHD-associated weight loss (B) and mortality (C). □ indicates BM only; ♦, BM + CD4+CD25+; ▿, BM + CD4+CD25-. *** indicates significance of P < .001 compared with BM transplanted alone.
CD25+ and CD25-CD4+ T cells support donor splenic CFU-GMs 7 days after BMT, but CD4+CD25+ T cells do not lead to GVHD. B6-GFP BM-TCD (2 × 106, A; 1 × 106, B) was transplanted alone or with 1 × 106 CD25+ or CD25-B6 CD4+ T cells into 7.0-Gy irradiated BALB/c recipients. Recipients were killed 7 days after BMT for total splenic donor CFU-GM analysis. Data presented are mean CFU/spleen ± SEM of pooled splenocytes and 3 independent experiments (A). Error bars in B represent mean and SEM of individual mouse weights. Groups of BM transplant recipients (n = 6-7/group) were monitored to assess the development of GVHD-associated weight loss (B) and mortality (C). □ indicates BM only; ♦, BM + CD4+CD25+; ▿, BM + CD4+CD25-. *** indicates significance of P < .001 compared with BM transplanted alone.
Donor CD4+CD25+ T cells prevent rejection and support increased long-term donor chimerism
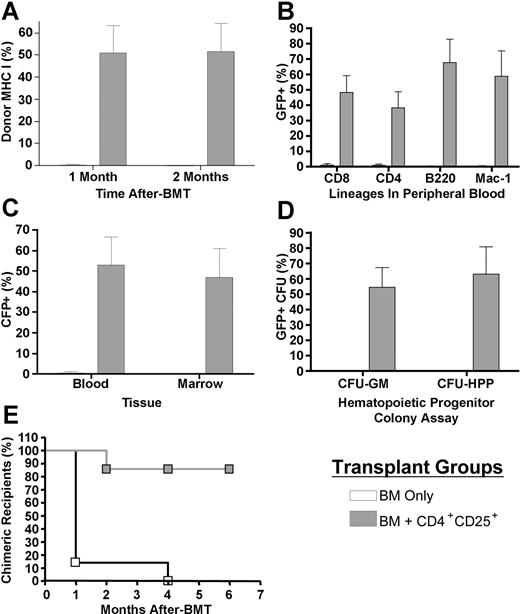
To rigorously assess the ability of CD4+CD25+ T cells to support chimerism following transplantation, B6-GFP TCD BM (5 × 105) was transplanted with and without B6 CD8-/-CD4+ CD25+ T cells into 7.0-Gy irradiated BALB/c recipients. One and 2 months later, peripheral blood and bone marrow were harvested to assess the presence of donor marrow-derived cells by fluorescence-activated cell sorter (FACS) analysis. Expression of MHC class I, serving as a marker present on both donor-derived (H2-Kb) and host-derived (H2-Kd) populations, was assessed in addition to GFP expression (restricted to donor) to rigorously determine chimerism levels. Only 1 of 7 BALB/c recipients of B6 TCD BM alone contained detectable levels of donor cells (2.3%). However, all of the recipients (7/7) of BM plus CD4+CD25+ T cells were found to contain donor cells (mean, 51.0% donor; range, 0.5%-78.2%) and 5 of 7 contained greater than 60%. Continued assessment of these recipients indicated that the mixed donor chimerism was clearly present 2 months after BMT, and this chimerism included both lymphoid (CD4+, CD8+, B220+) and myeloid (Mac-1+) lineages (Figure 3A-B). Notably, a similar overall level of GFP+ donor marrow-derived cells (average, 46.8%) was observed in the bone marrow of mice that received CD4+CD25+ transplants. To directly assess for levels of donor progenitor cells in the marrow compartment, lineage-committed GM and multipotential HPP progenitor colonies were determined (Figure 3C-D). Both CFU progenitor populations were identified, and the levels of chimerism (ie, ∼ 50%) were comparable to those observed in the peripheral blood and overall marrow compartment. By 4 months after BMT, donor-derived cells were not detectable in the peripheral blood of any BM transplant recipients who did not receive CD4+CD25+ T cells, whereas chimerism remained (2%-97% donor) in all but one CD4+CD25+ recipient (Figure 3E). In summary, transplants containing CD4+CD25+ T cells were associated with decreased rejection of the donor marrow inoculum (P < .01).
Hematopoietic chimerism following cotransplantation of donor CD4+CD25+ T cells. B6-GFP TCD BM (5 × 105) was transplanted alone or together with 1 × 106 CD4+CD25+ T cells into 7.0-Gy irradiated BALB/c recipients (n = 7/group). One and 2 months after BMT, peripheral blood was harvested and individual samples were analyzed by flow cytometry to determine chimerism by expression of GFP and MHC class I (A). Lymphoid and myeloid lineages were assessed in peripheral blood 2 months after BMT (B). Donor marrow-derived GFP expression was also compared between peripheral blood and bone marrow (C). CFU-GM and CFU-HPP assays were performed on cells harvested from recipient marrow (D). Rejection of the donor inoculum was assessed by the presence of donor chimerism in BM transplant recipients up to 6 months after BMT (E). □ indicates BM only; ▦, BM + CD4+CD25+. Data presented are the mean and SEM from blood or marrow (A-C) or marrow CFU chimerism (D) from results of an individual experiment. Peripheral chimerism was confirmed in this transplantation model in 2 independent experiments.
Hematopoietic chimerism following cotransplantation of donor CD4+CD25+ T cells. B6-GFP TCD BM (5 × 105) was transplanted alone or together with 1 × 106 CD4+CD25+ T cells into 7.0-Gy irradiated BALB/c recipients (n = 7/group). One and 2 months after BMT, peripheral blood was harvested and individual samples were analyzed by flow cytometry to determine chimerism by expression of GFP and MHC class I (A). Lymphoid and myeloid lineages were assessed in peripheral blood 2 months after BMT (B). Donor marrow-derived GFP expression was also compared between peripheral blood and bone marrow (C). CFU-GM and CFU-HPP assays were performed on cells harvested from recipient marrow (D). Rejection of the donor inoculum was assessed by the presence of donor chimerism in BM transplant recipients up to 6 months after BMT (E). □ indicates BM only; ▦, BM + CD4+CD25+. Data presented are the mean and SEM from blood or marrow (A-C) or marrow CFU chimerism (D) from results of an individual experiment. Peripheral chimerism was confirmed in this transplantation model in 2 independent experiments.
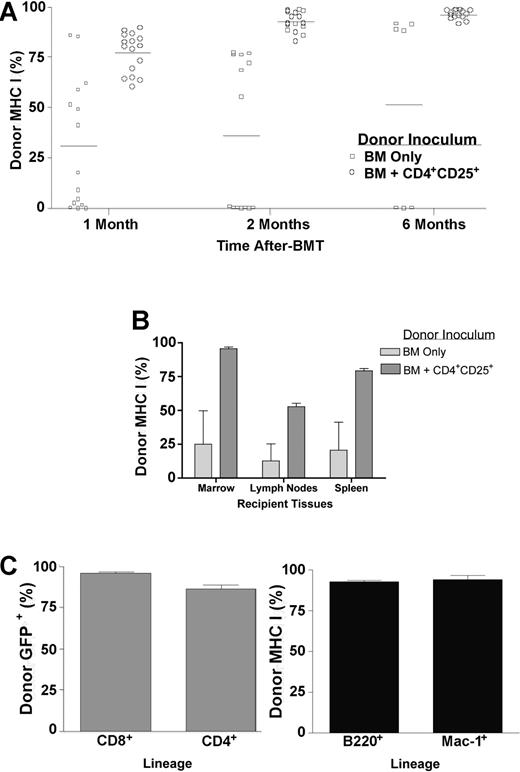
We also assessed transplants in which higher numbers of TCD BM were infused. A marrow dose of 1 × 106 B6-GFP TCD BM alone resulted in some of the BALB/c recipients expressing significant donor chimerism. However, greater levels of donor chimerism were observed in peripheral blood 1 month after BMT in recipients who received cotransplants of CD4+CD25+ T cells. The chimerism observed in recipients of BM only ranged from 0% to 85.6% donor with a mean of 31.2 (n = 15). Chimerism levels of donor marrow-derived cells in recipients of CD4+CD25+ T cells (n = 16) clustered between 60.5% and 89.6% donor, with a mean of 77.2% (Figure 4A). Notably, CD4+CD25+ T-cell recipients exhibited enhanced myeloid lineage donor chimerism, with donor Mac-1+ cells representing a greater proportion of the total peripheral blood cell (PBC) compartment (means, 1.7% for recipients of BM alone and 7.9% for recipients who received cotransplants of CD4+CD25+ T cells) and the ratio of donor to host Mac-1+ cells being 15-fold greater (data not shown). All host compartments examined, including bone marrow, spleen, and lymph node, demonstrated greater levels of donor chimerism in CD4+CD25+ T-cell recipients versus those mice who received marrow alone (Figure 4B). Longer-term assessment in peripheral blood of mice receiving CD4+CD25+ T cells indicated increasing donor chimerism. Those recipients of 1 × 106 TCD BM alone that engrafted also demonstrated increasing chimerism (Figure 4A). However, there was a significantly greater level of chimerism in the recipients of CD4+CD25+ T cells (P < .001 two months after BMT, P < .01 six months after BMT). Six months after transplantation, BM-only recipients who had not rejected their graft now contained an equivalent level of chimerism to recipients of CD4+CD25+ T cells. Donor marrow-derived cells at this time included T cells (CD4+ and CD8+), B cells (B220+), and myeloid-lineage (Mac-1+) cells (Figure 4C). In summary, chimerism analyses using multiple doses of donor bone marrow demonstrated that transplantation with CD4+CD25+ T cells resulted in significantly augmented kinetics and frequency of multilineage donor engraftment.
Donor CD4+CD25+ T cells increase long-term multilineage chimerism levels. B6-GFP TCD BM (1 × 106) was transplanted alone or with 1 × 106 CD4+CD25+ T cells into 7.0-Gy irradiated BALB/c recipients. Peripheral blood was harvested and flow cytometry was performed to determine chimerism 1, 2, and 6 months after BMT by expression of GFP and MHC class I (n = 7-16 recipients; A). □ indicates BM only; ○, BM + CD4+CD25+. Data presented are pooled from 6 experiments. Horizontal bars indicate the average percent of positive staining of donor MHC class I for each group. MHC class I staining was also used to determine chimerism 1 month after BMT in bone marrow, lymph nodes, and spleens of transplant recipients (n = 3/group from one of the individual experiments included in panel A) (B). Light gray bars indicate BM only; dark gray bars, BM + CD4+CD25+. Multilineage donor chimerism was assessed 6 months after BMT in recipients who received transplants of B6-GFP BM and B6 CD4+CD25+ T cells by splenic expression of GFP, CD8, and CD4 and by expression of MHC class I, B220, and Mac-1 in peripheral blood (C). ▦ indicates spleen; ▪, blood. Data represent pooled results from individual mice (n = 3-9/group) obtained from 2 of the experiments included in panel A. Error bars are mean % chimerism and SEM from individual mice (B,C).
Donor CD4+CD25+ T cells increase long-term multilineage chimerism levels. B6-GFP TCD BM (1 × 106) was transplanted alone or with 1 × 106 CD4+CD25+ T cells into 7.0-Gy irradiated BALB/c recipients. Peripheral blood was harvested and flow cytometry was performed to determine chimerism 1, 2, and 6 months after BMT by expression of GFP and MHC class I (n = 7-16 recipients; A). □ indicates BM only; ○, BM + CD4+CD25+. Data presented are pooled from 6 experiments. Horizontal bars indicate the average percent of positive staining of donor MHC class I for each group. MHC class I staining was also used to determine chimerism 1 month after BMT in bone marrow, lymph nodes, and spleens of transplant recipients (n = 3/group from one of the individual experiments included in panel A) (B). Light gray bars indicate BM only; dark gray bars, BM + CD4+CD25+. Multilineage donor chimerism was assessed 6 months after BMT in recipients who received transplants of B6-GFP BM and B6 CD4+CD25+ T cells by splenic expression of GFP, CD8, and CD4 and by expression of MHC class I, B220, and Mac-1 in peripheral blood (C). ▦ indicates spleen; ▪, blood. Data represent pooled results from individual mice (n = 3-9/group) obtained from 2 of the experiments included in panel A. Error bars are mean % chimerism and SEM from individual mice (B,C).
Recipients of CD4+CD25+ T cells are immunocompetent in vitro and in vivo but do not respond against donor or host antigens
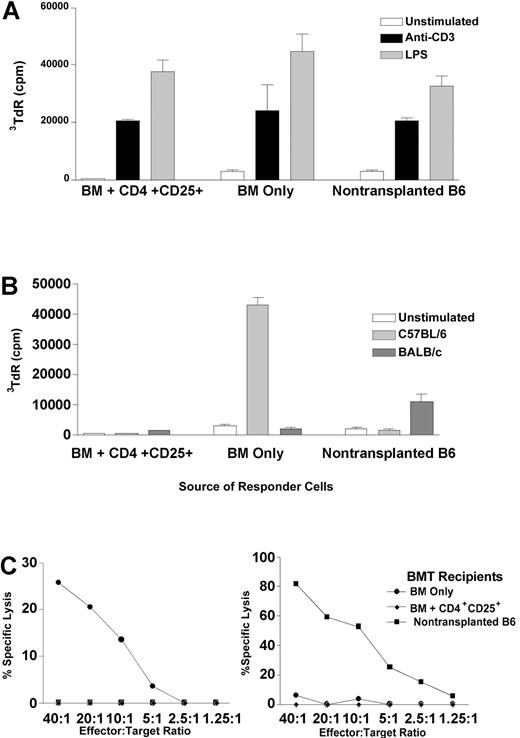
In order to assess the immune competence of mice receiving potentially suppressive CD4+CD25+ T cells in their donor inoculum, experiments were performed with transplant recipients 3 to 4 months after BMT. Splenocytes and lymph node cells from BALB/c recipients of B6 BM and CD4+CD25+ T cells responded to both soluble anti-CD3 mAb and LPS stimulation equivalently to cells from normal B6 mice or recipients of B6 TCD BM alone, indicating the presence of immunocompetent T and B cells (Figure 5A). To examine antigen-specific T-cell reactivity, mixed leukocyte reaction (MLR) and cytotoxic T-lymphocyte (CTL) responses were assessed against B6 (donor) and BALB/c (host) antigens. Although T cells from CD4+CD25+ T-cell BM transplant recipients proliferated to polyclonal stimulation, they did not generate proliferative responses to B6 or BALB/c stimulators in mixed lymphocyte responses. In contrast, BM-only recipients who rejected their grafts demonstrated strong proliferation to donor B6, but not host BALB/c, antigens (Figure 5B). Similarly, responding cells from CD4+CD25+ T-cell BM transplant recipients failed to generate CTLs against targets bearing donor (H2b) or host (H2d) MHC, whereas BM-only recipients who rejected the donor marrow did generate anti-H2b CTLs (Figure 5C).
Recipients that received transplants of marrow and CD4+CD25+ T cells demonstrate proliferative responses to polyclonal stimulators but do not proliferate or generate CTLs to donor or host antigens. Three to 4 months after BMT, chimeric BALB/c recipients of B6 BM and CD4+CD25+ T cells (n = 5-7), BALB/c recipients of B6 BM only who rejected the donor marrow (n = 3-4), and B6 mice that did not receive transplants (n = 4-6) were killed, and spleen and lymph node cells were isolated to assess immune responses. Polyclonal proliferation was measured in response to anti-CD3 mAb (▪) and LPS (▦) (A). Unstimulated cells are represented by □. Generation of proliferative (B) and CTL (C) responses was measured against donor (B6, left) and host (BALB/c, right) background stimulators. In B, light gray bars indicate C57BL/6; dark gray bars, BALB/c. In C, • indicates BM only; ♦, BM + CD4+CD25+; ▪, nontransplanted B6. Each experiment was performed 2 to 3 times. Error bars are mean cpm and SEM of triplicate wells (A,B).
Recipients that received transplants of marrow and CD4+CD25+ T cells demonstrate proliferative responses to polyclonal stimulators but do not proliferate or generate CTLs to donor or host antigens. Three to 4 months after BMT, chimeric BALB/c recipients of B6 BM and CD4+CD25+ T cells (n = 5-7), BALB/c recipients of B6 BM only who rejected the donor marrow (n = 3-4), and B6 mice that did not receive transplants (n = 4-6) were killed, and spleen and lymph node cells were isolated to assess immune responses. Polyclonal proliferation was measured in response to anti-CD3 mAb (▪) and LPS (▦) (A). Unstimulated cells are represented by □. Generation of proliferative (B) and CTL (C) responses was measured against donor (B6, left) and host (BALB/c, right) background stimulators. In B, light gray bars indicate C57BL/6; dark gray bars, BALB/c. In C, • indicates BM only; ♦, BM + CD4+CD25+; ▪, nontransplanted B6. Each experiment was performed 2 to 3 times. Error bars are mean cpm and SEM of triplicate wells (A,B).
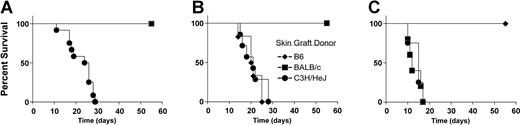
To corroborate and extend these in vitro findings of unresponsiveness to donor and host antigens, homotopic tail skin from donor, host, and third-party C3H/HeJ (H-2k) mice were simultaneously grafted onto CD4+CD25+ T-cell BM transplant recipients. The H-2k grafts were acutely rejected. However, skin grafts from donor (B6) or host (BALB/c) background mice were not rejected by 50 days after grafting (Figure 6). Interestingly, BM-only recipients who rejected the B6 donor marrow graft acutely rejected the B6 (H-2b) skin grafts, whereas chimeric BM-only recipients responded in a similar fashion to CD4+CD25+ T-cell BM transplant recipients (ie, accepting both donor and host grafts while rejecting third-party H-2k skin grafts; data not shown). In summary, all recipients of BM transplants cotransplanted with CD4+CD25+ T cells were immunologically competent as assessed by responses to both third-party antigens and polyclonal B-cell and T-cell stimulation while demonstrating tolerance in vivo to both donor and host antigens.
Recipients that received transplants of marrow and CD4+CD25+T cells acutely reject third-party skin grafts but accept skin grafts from marrow donor and recipient background mice. Three to 4 months after BMT, chimeric BALB/c recipients of B6 BM and CD4+CD25+ T cells (n = 13, A), BALB/c mice that did not receive transplants (n = 8, B), and B6 mice that did not receive transplants (n = 5, C) received tail skin grafts from donor (B6, ♦), host (BALB/c, ▪), or third-party (C3H/HeJ, •) background mice. Data are pooled from 3 individual experiments.
Recipients that received transplants of marrow and CD4+CD25+T cells acutely reject third-party skin grafts but accept skin grafts from marrow donor and recipient background mice. Three to 4 months after BMT, chimeric BALB/c recipients of B6 BM and CD4+CD25+ T cells (n = 13, A), BALB/c mice that did not receive transplants (n = 8, B), and B6 mice that did not receive transplants (n = 5, C) received tail skin grafts from donor (B6, ♦), host (BALB/c, ▪), or third-party (C3H/HeJ, •) background mice. Data are pooled from 3 individual experiments.
Discussion
Because of the risk of GVHD associated with T cells present in the donor inoculum, we sought to determine if a non-GVHD-inducing donor T-cell population could promote engraftment following allogeneic BMT. Using CD4+CD25+ T cells purified from CD8-/- mice to exclude contaminating CD8 T cells, the findings demonstrated that donor CD4+CD25+ T cells were capable of promoting early and long-term allogeneic hematopoietic engraftment accompanied by tolerance to donor and host antigens. A complete MHC-mismatched H-2b → H-2d sublethally conditioned transplantation model was employed, providing strong antigenic signals for T-cell activation as well as a potent host barrier to donor PC engraftment. B6 CD4+CD25+ donor T cells cotransplanted with syngeneic BM augmented the levels of both lineage-committed and multipotential donor CFUs in allogeneic recipients, an observation extending our previous finding that unfractionated CD4 T cells were capable of generating initial donor chimerism.5 Equivalent numbers of CD25-CD4 T cells were also capable of PC support early after BMT, demonstrating that CD4 support of HSPCs is not limited to the regulatory fraction of this subset. CD8 T-cell HSPC support was more potent versus CD4+CD25+ T cells on a per cell basis (data not shown), consistent with previous results of this and other laboratories.4,5 However, CD8 and CD25-CD4 T cells can mediate vigorous antihost responses resulting in GVHD, as detected in the present studies. Such responses by these cells may be required for their support of donor HSPCs during clinical transplantation, thus preventing the independent regulation of GVH and HSPC support. Therefore, although the early facilitating effect of CD4+CD25+ T cells, with respect to supporting PCs, is not limited to this population, together with the inability to cause GVHD, this cell population represents a unique subset of TCR-expressing lymphoid cells.
Chimerism was analyzed in recipients of marrow supplemented with CD4+CD25+ T cells and recipients of marrow alone. BALB/c BM transplant recipients who received cotransplants of MHC-mismatched B6 marrow and CD4+CD25+ regulatory cells (T-reg's) demonstrated donor HSPC engraftment using a dose of marrow that was rejected within the first 4 months after BMT by 100% of recipients who received BM alone. The long-term (6 months after BMT) persistence of multilineage donor chimerism indicated that donor T-reg's facilitated the engraftment of primitive long-term repopulating HSCs (LTR-HSCs) that are essential for lifelong hematopoietic reconstitution.23 The determination of HSC engraftment is significant because the short-term (day 7 after BMT) CFU assay does not measure engraftment of the most primitive HSCs. Furthermore, although support of highly enriched allogeneic HSCs by donor T cells (CD8) has been observed,24 the present studies provide the first experimental demonstration of long-term T-cell support of allogeneic hematopoietic engraftment 6 months after transplantation, indicating engraftment of LTR-HSCs.
Based on in vitro findings that TCR activation is required for T-reg functions,16,17 T-reg's infused during a BMT must likely be activated in vivo in order to promote engraftment after transplantation. Transplanted T-reg's could recognize autologous (donor-derived, ie, hematopoietic) or allogeneic (host-derived) antigens. Consistent with a role in regulating/suppressing autoimmunity in the periphery, transgenic models have demonstrated autoantigen recognition-based selection of CD4+CD25+ T cells by agonistic ligands in the thymus and recognition of tissue-specific autoantigens in the periphery.25-27 Additionally, CD4+CD25+ T cells from wild-type mice demonstrated greater in vitro proliferation in response to autologous versus allogeneic antigens, although the nature of the autoantigens was not known.18 However, despite such reported skewing toward autoreactivity, murine and human T-reg's have both been shown to demonstrate a broad Vβ repertoire and are therefore likely to possess a wide array of antigen reactivity.16,18,19 Alloreactive T-reg's can be induced following in vitro stimulation20-22 and induced in vivo following exposure to antigen in the presence of anti-CD4 antibody.28 Therefore, it is possible that donor T-reg support of allogeneic engraftment could have been mediated by autoreactive T-reg's recognizing antigens derived from the donor marrow inoculum and/or by alloreactive T-reg's recognizing antigens derived from the BM transplant recipients.
Transgenic TCRs bearing T-reg's have been shown to suppress in vitro proliferation of T cells expressing different transgenic TCRs,16 emphasizing that specificity involving T-reg populations appears to occur at the activation level. Specificity regarding in vivo suppression remains unclear. However, it is possible that their regulation could be directed against T cells recognizing similar (or similarly presented) antigens, perhaps occurring during T-cell interactions with antigenpresenting cells (APCs). If the latter occurs, then autoreactive as well as alloreactive donor T-reg's could suppress antidonor T cells of the host, potentially sparing T-cell responses directed against opportunistic pathogens or, in the setting of malignancy, residual neoplastic cells. Antigen specificity in the donor T-reg population would thus be preferable over globally preactivating all donor T-reg's that might suppress host versus graft (HVG) as well as desirable T-cell responses. In addition to antigen-driven activation of infused T-reg's, homeostatic-driven activation may also be occurring in the lymphopenic environment of conditioned BM transplant recipients. Homeostatic expansion by CD4+CD25+ T cells has been described in TCR transgenic models, although the role of peptide recognition (in addition to MHC class II binding) may vary depending on the particular TCR assessed.29,30 Clearly, the MHC-mismatched transplantation model described here is conducive to potential activation of donor T-reg's by autoantigens and/or alloantigens as well as lymphopenia-induced homeostatic expansion.
Although T-cell-mediated resistance can contribute to rejection of MHC-mismatched allografts within the first week after BMT, natural killer (NK) cells play the predominant role during the first several days after transplantation.31 Therefore, the early support of allogeneic PCs by donor CD4+CD25+ T cells raises several interesting questions. A recent report described suppression of NKT-cell proliferation, cytokine production, and cytotoxicity by human CD4+CD25+ T cells; however, we are unaware of studies demonstrating direct interactions between CD4+CD25+ T cells and NK cells.32 Another study suggested an indirect effect of T-reg cells on NK-cell activation during CD4+CD25+ T-cell-mediated inhibition of innate immunity to bacterial infection.33 Interestingly, IL-10, implicated as a cytokine effector of T-reg-mediated immune suppression in that study and others,28,33-35 has also been reported to suppress dendritic cell production of IL-12, a potent NK stimulator.36,37 Distinguishing between suppressive effects by donor T-reg's on host T versus NK cells is crucial for further elucidating the early PC support described here.
In addition to inhibiting host resistance, T-reg cells could also directly promote engraftment of donor HSPCs. Donor T-reg infusion augmented donor PC levels early (within 1 week) and later (2 months) after transplantation. Such support could be mediated by affecting the seeding, survival, or proliferation of transplanted HSPCs. These effects could be due to trophic cytokine support, as both CD4 and CD8 T cells have been reported to produce growth factors such as IL-3 and GM-colony-stimulating factor (GM-CSF) following allogeneic BMT,38-41 or by altering the hematopoietic environment, such as by decreasing the level of inflammation and rendering the environment more favorable to hematopoiesis. Transforming growth factor β (TGF-β) has frequently been associated with T-regulatory function, and production of IL-3 by CD25 expressing CD4 T cells has been reported.33,42,43 These cytokines have broad hematopoietic effects, including stimulation of progenitors and reported contributions to stem cell expansion.44-46 Additionally, IL-10 has contributed to the expansion of both CFU-GMs and primitive long-term colony-initiating cells (LTC-ICs).47,48 Production of such cytokines could impact the hematopoietic microenvironment. For example, the stem cell niche in the bone marrow may be associated with osteoblasts that are capable of binding and recruiting T cells.49-52 Infused HSPCs and T cells have been reported to cluster together while seeding the hematopoietic niche,53 and we detected the presence of infused T-reg's at 2 weeks and 1 month after BMT in the spleen (0.5 × 105 to 1.7 × 106 and 4.1 × 105 to 9.2 × 105, respectively) and marrow (2.6 × 104 to 1.1 × 105 and 2.2 × 104 to 5.3 × 104, respectively) compartments. Furthermore, the possibility that donor T-reg's could impact HSPCs and their microenvironment is consistent with T-reg suppression of inflammatory pathology, in part to tumor necrosis factor α (TNFα), which can be a negative hematopoietic regulator.33,54,55
The observation that recipients of BM allografts supplemented with CD4+CD25+ T cells were chimeric and absent of clinical signs of GVHD suggested they were tolerant to donor and host antigens. Skin graft rejection, as well as in vitro proliferation and CTL responses, supported this interpretation. Although experiments did not address the mechansism(s) of tolerance induction/maintenance in this study or the possible role of host CD4+CD25+ T cells after BMT in these responses, several observations support the notion that central tolerance is important. First, the multilineage chimerism and donor PCs observed in recipient marrow could have contributed to donor thymic follicular dendritic cells, important for negative selection.56 Although, donor T-reg's were detected as mentioned above in recipient spleens, lymph nodes (LNs), and BM up to 1 month after BMT, they could not be identified 6 months after BMT (data not shown). While we cannot exclude the possibility that low numbers of antigen-specific T-reg's may have persisted, detectable numbers of donor T-reg's were not necessary for maintaining long-term tolerance in the periphery.
Finally, T-reg specificity may be critical for clinical utility in generating hematopoietic allograft tolerance. While the T-reg population in this study was activated in vivo, it has recently been reported that host CD4+CD25+ T cells preactivated in vitro against donor APCs could preferentially augment donor chimerism compared with third-party APCs as assessed 2 weeks after BMT, whereas host or donor CD4+CD25+ T cells globally preactivated ex vivo could augment donor chimerism assessed 3 months after BMT.57,58 Since T-reg's are known to be suppressive and have been implicated in certain neoplastic states,43,59,60 the risk of limiting GVL effects is a relevant concern for transplantations performed as a therapy for malignancy. Recent work showed that donor T-reg's can suppress GVHD while allowing GVL when administered at the time of transplantation or as a delayed infusion.22,61,62 Cotransplantation with T-reg's as described here and subsequent infusion both represent possible modes for promoting engraftment, as we have observed early increases in donor CFUs when delaying T-reg administration until 2 days after BMT (A.H. and R.L., unpublished observation, December 2003). Further investigation into the activating signals and supportive mechanisms used by donor T-reg's for promotion of allogeneic hematopoietic engraftment is currently under investigation.
Prepublished online as Blood First Edition Paper, October 19, 2004; DOI 10.1182/blood-2004-08-3213.
Supported by National Institutes of Health (NIH) grants RR11576 and AI46689 (R.B.L.).
The authors declare that they do not have competing financial interests.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Pantelis Tsoulfas and Shyam Gajavelli (Department of Neurosurgery, University of Miami Miller School of Medicine) for the use of and help with the fluorescence microscopy. We would like to thank Yurong Bu and Dr Rebecca Adkins for their technical assistance with the skin grafting experiments. We also would like to acknowledge the Sylvester Cancer Center for support of the flow cytometry facility.