Abstract
Intracellular Smad proteins mediate signal transduction of the transforming growth factor-β (TGF-β) superfamily that play pleiotropic roles in hematopoietic development, suggesting that intracellular Smad proteins may play key roles in hematopoietic regulation. Although inhibitory Smad7, which negatively regulates TGF-β signaling, has been implicated in the development of mature hematopoietic cells, a role for Smad7 in regulating more primitive hematopoietic cells has yet to be examined. Here, Smad7 was overexpressed in primary human severe combined immunodeficient (SCID) repopulating cells (SRCs), representing a common myeloid/lymphoid precursor cell with the functional capacity to repopulate the bone marrow of nonobese diabetic (NOD)/SCID recipient mice. Retroviral transduction of Smad7 into human umbilical cord blood (CB)-SRCs caused a shift from lymphoid dominant engraftment toward increased myeloid contribution, and increased the myeloid-committed clonogenic progenitor frequency in reconstituted mice. Neither myeloid nor B-lymphoid lineage developmental stages were compromised by Smad7 overexpression, suggesting Smad7 regulates cell fate commitment decisions of myeloid/lymphoid precursors by augmenting myeloid differentiation at the expense of lymphoid commitment. In addition, global gene expression analysis using microarray was used to identify potential target genes regulated by Smad7 in primitive hematopoietic cells that may control this process. Our study demonstrates a novel and unexpected role for Smad7 in modulating the cell fate decisions of primary multipotent human repopulating cells and establishes a role for Smad7 in the development of primitive human hematopoietic cells.
Introduction
The transforming growth factor-β (TGF-β) superfamily of extracellular ligands, including the TGF-βs, activins, and bone morphogenetic proteins (BMPs), are responsible for mediating a wide array of cellular responses ranging from proliferation and differentiation to embryonic patterning.1-3 Multiple TGF-β family ligands are involved in embryonic and adult hematopoietic development. TGF-β is best characterized as negative regulator of primitive hematopoietic cells,4,5 while BMP-4 is involved in the induction of hematopoietic tissue from embryonic mesoderm,6,7 and has also been shown to enhance the survival and proliferation of human hematopoietic stem cells following ex vivo culture.8,9
Ligands of the TGF-β superfamily interact with their cognate type I and type II serine-threonine kinase receptors at the cell surface, and transmit signals through members of the Smad family of intracellular signal transducers. Receptor-regulated Smads (R-Smads) signal downstream of TGF-β and activin (Smad2 and Smad3), or BMP (Smad1, Smad5, and Smad8), through receptor-mediated R-Smad phosphorylation, association with Smad4, and nuclear translocation followed by modulation of TGF-β responsive gene expression.1,10-12 In contrast, the inhibitory Smads (I-Smads; Smad6 and Smad7) antagonize TGF-β/R-Smad signal transduction by preventing R-Smad activation at the receptor level and also antagonizing BMPs.13-16
Given the importance of multiple TGF-β ligands in hematopoiesis, it is likely that their intracellular mediators, the Smads, also play key roles in hematopoietic regulation. Smad7 primarily functions as an intracellular antagonist of TGF-β–mediated signaling,14,15 wherein signaling through TGF-β family receptors causes up-regulation of Smad715,17,18 and through a stable association with activated type I TGF-β (TβRI), BMP and activin receptors14,15,19,20 blocks activation of R-Smads.
Smad7 transcription is also induced in response to signaling by various ligands upstream of nuclear factor-κB (NF-κB)21-23 and Jak1-Stat124 pathways, and in response to various physiological stimuli.17,21,22 A few lines of evidence converge to suggest that Smad7 may be an important hematopoietic regulator. Smad7 is capable of antagonizing activin-induced erythroid differentiation in a murine erythroleukemia cell line,23 and inhibits TGF-β–mediated growth arrest in murine B lymphocytes18,25 and primary activated murine T lymphocytes.26 During early hematopoietic development of Xenopus embryos, Smad7 mRNA expression, similar to that of the globin transcripts, is progressively restricted to ventral mesoderm and embryonic blood islands, suggesting that Smad7 is important in early hematopoiesis.27 Very recently, Smad7 was identified as a gene enriched in populations of murine embryonic stem cells (ESCs) and murine and human hematopoietic stem cells (HSCs) through screening of oligonucleotide microarrays.28,29 Taken together, this evidence suggests that in addition to its role in mature hematopoietic cells, Smad7 might play a role in primitive hematopoietic cells with multipotent potential.
To understand the biological role of Smad7 in primitive human hematopoiesis, we have used a retroviral gene transfer approach to examine the effect of Smad7 on candidate human HSCs capable of repopulating the bone marrow (BM) of nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice.30,31 In comparison with control vector–transduced SCID repopulating cells (SRCs), Smad7 SRCs exhibited a shift from a B-lymphoid dominant graft toward a graft with a higher frequency of myeloid progeny within the murine bone marrow (BM). This phenotypic increase in myeloid progeny was coupled with a higher frequency of functional clonogenic myeloid progenitors isolated from the mouse BM. Our data suggest that Smad7 is capable of altering the balance of cell fate commitment decisions within human SRCs, which represent a common lymphoid/myeloid precursor cell with in vivo repopulating function. This study identifies a novel role for Smad7 in cell fate commitment decisions in primary human multipotent cells.
Materials and methods
Reverse transcription–polymerase chain reaction analysis
mRNA was extracted from purified cells using an mRNA extraction kit, and was reverse-transcribed into cDNA using a first-strand cDNA synthesis kit, according to the manufacturer's instructions (Amersham Biosciences, Baie d'Urfé, QC, Canada). Reverse transcription–polymerase chain reaction (RT-PCR) reactions were used to detect Smad7 and β-glucuronidase transcripts using a GeneAmp 9700 (PE Applied Biosystems, Foster City, CA) under the following PCR conditions: an initial 2-minute denaturation at 96°C, followed by 40 cycles of 45-second denaturation at 94°C, 45-second annealing at 60°C, and 2-minute extension at 72°C, followed by a final extension at 72°C for 10 minutes. Primer sequences used for amplification of 307–base pair (bp) Smad7 transcripts were: forward 5′-CCA TCA CCT TAG CCG ACT CT-3′; and reverse 5′-GCA AAA GCC ATT CCC CTG AG-3′. Primer sequences used for amplification of β-glucuronidase transcripts (960 bp, 1.0 kilobase [kb], 1.1 kb) were forward 5′-ACT ATC GCC ATC AAC AAC ACA CTC ACC-3′, and reverse 5′-GCT CTG AAT AAT GGG CTT CTG-3′.
Retroviral vectors
A BamHI linker was subcloned into the HpaI-SnaBI sites of the MIEV vector32 (a kind gift from Robert Hawley, George Washington University Medical Center, Washington, DC). A 2.9-kb EcoRI-BamHI fragment coding for the full-length human Smad7 gene14 (from pCMV5-Smad7, a kind gift from Jeffrey Wrana, Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, ON, Canada), was subcloned into the EcoRI-BamHI sites of the modified MIEV vector, upstream of an internal ribosomal entry site (IRES) and an enhanced green fluorescent protein (GFP) reporter gene, as previously described (K.C., K. Jay, B. Murdoch, M.B., manuscript submitted).
Retroviral packaging lines
The bicistronic Smad7 vector and control MIEV vector were transfected into the amphotrophic PA317 packaging line33 using Fugene6 (Roche, Laval, QC, Canada). Transient retroviral supernatant was harvested, supplemented with 8 μg/mL polybrene (Sigma, St Louis, MO), and used to infect the gibbon ape leukemia virus (GaLV)–pseudotyped PG13 packaging line34 to create stable producer lines releasing Smad7 or control vector retroviral particles. Fluorescence-activated cell-sorting (FACS) was used to isolate stable producer lines expressing the GFP transgene through 2 sequential sorts for GFP+ cells. Bulk cultures of selected PG13-Smad7 and PG13-vector cells were confirmed to produce retroviruses capable of transducing human cells by titration on HeLa cells with titers of 8 × 105 to 9 × 105 infectious particles/mL (K.C., K. Jay, B. Murdoch, M.B., manuscript submitted).
Retroviral transduction of human hematopoietic cell lines
Human megakaryocytic leukemia lines MBA.135 and M-O7e36 were grown in Iscove modified Dulbecco medium (IMDM) with 10% fetal bovine serum (FBS) and 100 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (M-O7e only). For transduction with Smad7 or vector retroviruses, cells were resuspended once in 0.45 μm filtered virus-containing supernatants, collected in IMDM with 10% FBS and 300 U/mL GM-CSF, and supplemented with 8 ng/mL polybrene. Two days after retroviral transduction, cultured cells were washed with phosphate-buffered saline (PBS), and GFP+ cells were selected by fluorescence-activated cell sorting for subsequent expansion.
Assessment of Smad7 overexpression in retrovirally transduced cells
PG13 packaging lines and MBA.1 cells transduced with vector or Smad7 retrovirus, sorted for GFP+ expression, were analyzed by Western blotting for detection of Smad7 protein. Cell lysates were prepared using lysis buffer containing 10% Triton, 1M Tris (tris(hydroxymethyl)aminomethane), 0.5M EDTA (ethylenediaminetetraacetic acid) and protease inhibitors leupeptin and aprotinin at 10 mg/mL, and proteins were separated using 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membrane. Equal amounts of protein were loaded per lane in all experiments as determined by Coomassie staining of parallel gels used for subsequent Western blotting. Smad7 protein was detected using the Smad7 antibody H-79 (Santa Cruz Biotechnologies, Santa Cruz, CA), a goat anti-rabbit–horseradish peroxidase (HRP) 2° antibody (Santa Cruz Biotechnologies) and enhanced chemiluminescence system (ECL; Amersham Pharmacia), and Kodak X-omat film (Kodak, Eastman, NY).
TGF-β1 growth inhibition assay
M-O7e cells were cultured in IMDM with 10% FBS and 300 U/mL GM-CSF (Amgen, Thousand Oaks, CA) in 96-well suspension plates (BD Biosciences, Mississauga, ON, Canada). Smad7–M-O7e or vector–M-O7e GFP+ cells (20 000 each) were seeded in triplicate for each experiment. Cells were treated with 1 ng/mL TGF-β1 (R&D Systems, Minneapolis, MN) or nothing every other day. Total viable cells were enumerated using a hemacytometer and trypan blue staining on days 2, 4, and 6. Results presented are from 2 independent experiments.
Human cell purification
Samples of full-term human umbilical cord blood (CB) were obtained in conjunction with local ethical and biohazard authorities of the University of Western Ontario and London Heath Sciences Centre. CB samples were diluted 1:3 in PBS, and mononuclear cells (MNCs) were collected by separation on Ficoll-paque (Amersham Biosciences). Contaminating red blood cells were lysed with a 0.8% ammonium chloride solution. Lineage depleted (Lin–) cells were purified by negative selection using a StemSep device (StemCell Technologies, Vancouver, BC, Canada) as previously described.37 Cells expressing lineage commitment markers (Lin+) were separately eluted from the column for use as accessory cells during transplantation.38 CB Lin– and Lin+ populations were cryopreserved in fetal bovine serum (FBS) and 10% DMSO (dimethyl sulfoxide), and stored in liquid nitrogen.
Production of retrovirus-conditioned supernatants
PG13 packaging lines stably transduced with either the control or Smad7 retroviral vector were grown in α–minimal essential medium (MEM) with 7% FBS. For collection of virus-conditioned supernatant (VCS), cells at near confluence were grown in IMDM with 10% FBS (serum-rich VCS) or 2.5% FBS (serum-deficient VCS) for 24 hours. Fresh VCS was harvested and filtered with a 0.45-μm syringe filter before transduction.
Retroviral transduction of CB Lin– cells
CB Lin– cells were thawed and placed into serum-free (SF) liquid culture (SF BIT media) consisting of IMDM supplemented with 20% bovine serum albumin, insulin, and transferrin (BIT) 9500 (StemCell Technologies), 10-4 M β-mercaptoethanol, 2 mM l-glutamine, and the following recombinant human hematopoietic cytokines: 100 ng/mL stem cell factor (SCF; Amgen), 100 ng/mL flt-3 ligand (FL), 20 ng/mL interleukin (IL)-3, 20 ng/mL IL-6 (R&D Systems), and 20 ng/mL granulocyte-colony stimulating factor (G-CSF; Amgen) for prestimulation prior to retroviral transduction. CB Lin– cells were prestimulated in 2 mL SF BIT media in 35-mm dishes (Sarstedt, Montreal, QC, Canada) at a density of 2 × 105 cells/mL for 48 hours. In some experiments, Lin– cells from multiple CB samples were pooled prior to retroviral transduction.
For transduction of Lin– cells, 35-mm dishes were precoated with fibronectin (Sigma) at 5 μg/cm2 for 4 to 12 hours. Fibronectin-coated dishes were preloaded twice with 1 mL serum-rich VCS for 30 minutes at room temperature. Prestimulated Lin– cells were harvested, resuspended in serum-depleted VCS supplemented with hematopoietic cytokines as listed for SF BIT media, and placed onto fibronectin-coated dishes preloaded with viral supernatant. Cells were transduced in this manner once every 24 hours for 3 days. Less than 12 hours after the final transduction, cells were harvested for transplantation into NOD/SCID mice, and analysis of gene transfer efficiency.
Analysis of gene transfer efficiency into CB Lin– cells
An aliquot of 2 to 5 × 104 cells was removed from each well prior to transplantation, and were cultured for an additional 1.5 days to allow for GFP expression, for analysis of gene transfer efficiency into the bulk CB Lin– population. Cells were analyzed by flow cytometry for GFP expression in combination with CD34, on a FACSCalibur flow cytometer (BD Biosciences). The gene transfer efficiency was determined as the percentage of cells expressing GFP 2 days after the final VCS exposure.
Transplantation of human cells into NOD/SCID mice
After 4.5 days of retroviral transduction, the entire contents of wells initially seeded with 4 × 105 CB Lin– cells were transplanted into sublethally irradiated (3.5 Gy [350 rad] cesium 137 [137Cs]) NOD/LtSz-Prkdcscid/Prkdcscid (NOD/SCID) or β2 microglobulin-null NOD/LtSz-SCID (NOD/SCID/β2m–/–) mice,39,40 along with 1 × 105 irradiated (15 Gy [1500 rad] 137Cs) CB Lin+ accessory cells, according to our standard protocol.41 The mice were killed 5 to 8 weeks after transplantation. Murine bone marrow (BM) cells were collected from femurs, tibiae, and iliac crests for analysis of human engraftment.
Flow cytometric analysis of human cell engraftment and gene marking
Cells harvested from the mouse BM were prepared for flow cytometry by lysing contaminating red blood cells with a 0.8% ammonium chloride solution, and subsequent washing with PBS with 3% FBS. Cells were resuspended at 106 cells/mL in PBS + 3% FBS for staining with fluorochrome conjugated antibodies at 5 μg/mL (all purchased from BD Biosciences or Beckman Coulter, Mississauga, ON, Canada). Cells were stained for 30 minutes at 4°C, and washed 3 times with PBS + 3% FBS before being analyzed on a FACSCalibur or FACSVantage SE using Cell Quest software (BD Biosciences). At least 20 000 CD45+ events were acquired for each analysis. For analysis of human cell engraftment, cells were stained with a human-specific pan-leukocyte marker CD45, or the corresponding mouse immunoglobulin (Ig) G1 isotype control. Mice engrafted with human (CD45+) cells were further analyzed for expression of the retroviral transgene by detection of the GFP reporter gene in FL1. In pairs of mice engrafted with CB Lin–-derived SCID repopulating cells (SRCs) transduced with control and Smad7 vectors, and expressing GFP+ cells at sufficient levels, the human graft was further analyzed for myeloid/lymphoid hematopoietic reconstitution. Transduced human cells gated on CD45 expression were analyzed for the presence of primitive hematopoietic cells (CD34), committed myeloid cells (CD33, CD13, CD14, CD15), and committed B-lymphoid cells (CD19, CD10), in combination with GFP expression.
Analysis of clonogenic progenitors from NOD/SCID mice
In mice with sufficient levels of engraftment and retroviral marking, clonogenic progenitors arising from transduced SRCs were assessed in semisolid methylcellulose culture. Mouse BM cells were sorted on a FACSVantage SE, and primitive human cells expressing the transgene were isolated based on the phenotype CD45+CD34+GFP+. As a control, CD45+CD34+GFP– cells were also isolated from the same mice. In some instances, cells were also selected based on exclusion of the 7-aminoactinomycin D (7-AAD) viability dye. Five thousand CD45+CD34+GFP+ or GFP– cells were placed into Methocult H4320 (StemCell Technologies) supplemented with the following recombinant human cytokines: 50 ng/mL SCF, 10 ng/mL GM-CSF, 10 ng/mL IL-3, and 3 U/mL erythropoietin (epo), and maintained at 37°C with 5% CO2 in a humidified atmosphere. Differential colony counts were performed at 10 to 14 days, according to standard protocols, on an Axiovert 25 microscope (Zeiss, North York, ON, Canada) using a 20 ×/0.40 Ph2 objective. Expression of the GFP transgene was assessed after 7 days with a mercury burner and GFP filter on the Axiovert 25. Light and fluorescent micrographs were acquired with a Sony DXC-950 3CCD color video camera (Sony of Canada, Toronto, ON, Canada) and Northern Eclipse Image Editing Software v2.1 (Empix Imaging, Mississauga, ON, Canada).
Quantitative polymerase chain reaction
Expression of Smad7 (forward primer, 5′AGAAGGTGCGGAGCAAAAT3′ and reverse primer, 5′GTGTGGCGGACTTGATGA3′) was quantified by quantitative polymerase chain reaction (Q-PCR; MX4000; Stratagene, La Jolla, CA) using SYBRGREEN (Stratagene) DNA binding dye. The Q-PCR condition was 2 mM MgCl, 0.4 mM dNTP (deoxyribonucleoside triphosphate), 8% glycerol, 3% DMSO, 150 nM of each primer, 0.375 μL of 1:500 dilution of reference dye, and 2.5 μL of 1:2000 dilution of SYBRGREEN. The Q-PCR reaction conditions were primary denaturation at 95°C for 1 minute and 40 cycles of PCR consisting of 95°C for 10 seconds, 60°C for 1 minute, and 72°C for 30 seconds, followed by analyzing the amplified products using dissociation curve. The signal intensities were normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH; forward primer, 5′TGCACCA CCAACTGCTTAGC3′, and reverse primer, 5′GGCATGGACTGTGGTCA TGAC 3′) and the 2–ΔΔct equation42 was used to calculate the relative expression of Smad7.
Amplification of RNA for microarray analysis
The MessageAmp aRNA kit (Ambion, Austin, TX) was used for RNA amplification as described elsewhere.43 Briefly, total RNA, extracted from transgenic Smad7 and vector cells, was used as the starting material for generating aRNA. First-strand cDNAs were synthesized using poly deoxythymidine (dT) primers followed by second-strand cDNA synthesis. The double-stranded cDNA was used as a template for in vitro transcription (IVT) reaction to generate antisense RNA (aRNA). Biotin-16–uridine-5′-triphosphate (UTP) (Roche) and Biotin-11–cytidine triphosphate (CTP) (Perkin Elmer) nucleotides were incorporated to the newly synthesized RNA during IVT reaction. The quality and quantity of aRNA were evaluated with a spectrophotometer (Fisher Scientific, Hampton, NH) and with an Agilent bioanalyzer (Agilent Technologies, Mississauga, ON, Canada) at the London Regional Genomic Center (LRGC; Robarts Research Institute, London, ON, Canada).
Microarray analysis
Human HG-U133A chips (Affymetrix, Santa Clara, CA) were used for hybridization reactions. Probe preparations, hybridization, washing, and data acquisition were performed at the LRGC according to standard protocol provided by Affymetrix. The quality of array experiments was primarily evaluated by looking at the number of present and absent calls and the ratio of signal intensity of 3′ to 5′ end of housekeeping genes such as GAPDH and β-actin. The GeneSpring 6.0 software (Silicon Genetics, Redwood City, CA) was used for data analysis such as normalization and clustering. The data were normalized per chip and per gene using algorithms in GeneSpring 6.0. For per chip normalization, data in each chip was normalized to the 50th percentile of the measurements taken from that chip. Per gene normalization was done by dividing the mean signal intensity from each gene in the Smad7 transgenic cell sample to the mean of the same gene in the vector control. Genes that were significantly (P < .05) differentially (more than 2 times) expressed and called as present in at least one of the replicates were considered to be differentially expressed in Smad7 transgenic cells.
Statistical analysis
Values shown represent the mean ± SEM for “n” number of experiments. Statistical analyses were performed using a 2-tailed, paired Student t test. Results were reported as significant if P was less than or equal to .05.
Results
De novo expression of Smad7 in human hematopoietic tissues, construction and evaluation of Smad7 retroviral vector
To determine whether Smad7 might regulate TGF-β/BMP signaling pathways in primitive human hematopoietic cells, we have examined various human fetal blood (FB) and CB populations by reverse transcriptase–polymerase chain reaction (RT-PCR) analysis for Smad7 expression (Figure 1A). Smad7 mRNA was expressed in the FB and CB Lin– CD34+CD38– cell populations known to be enriched for primitive hematopoietic cells with multilineage repopulating function.30 Additionally, Smad7 mRNA was detected in mature lineage-committed myeloid (CD33+), B-lymphoid (CD19+), and T-lymphoid (CD3+) lineages. To compare the level of Smad7 expression among cells comprising the human hematopoietic hierarchy, real-time quantitative PCR (Q-PCR) for Smad7 transcript relative to GAPDH was used. A representative curve generated for Smad7 and GAPDH Q-PCR is shown, using Lin–CD34+CD38– cells and the more mature Lin–CD34+CD38+ cells, which are devoid of repopulating cells but enriched for progenitors (Figure 1B). In Lin–CD34+CD38– CB cells, Smad7 was expressed at more than 2-fold the level in Lin–CD34+CD38+ cells (Figure 1B) suggesting the Smad7 may play in role in primitive repopulating stem cells. Furthermore, expression of Smad7 was regulated among differentiated hematopoietic cells, and was preferentially expressed among the myeloid subsets indicated, with low levels of expression in cells within the B-cell lineage (Figure 1C). These data indicate that expression of Smad7 is tightly regulated during human hematopoiesis, and suggests that Smad7 may be involved in regulating the process of human hematopoietic differentiation.
Smad7 expression, retroviral constructs, and packaging lines. (A) Expression of Smad7 in human fetal blood (FB) and cord blood (CB) hematopoietic cell populations, including the primitive CD34+CD38– cell fraction devoid of lineage commitment markers (Lin–), mature CD33+ myeloid cells, CD19+ B lymphocytes, and CD3+ T lymphocytes, as determined by RT-PCR analysis. An RT-PCR reaction was performed on human fetal head cDNA as a positive control, and on a sample containing no cDNA (H2O) as a negative control. RT-PCR for β-glucuronidase (β-gluc), a housekeeping gene expressed at a single copy per cell, was used to assess the quality of the cDNA templates. Quantitative PCR was used to measure the expression of Smad7 in (B) highly purified Lin–CD34+CD38– and Lin–CD34+CD38+ subsets from human cord blood (summarized as relative expression in Lin–CD34+CD38– [▪] compared with Lin–CD34+CD38+ cells [□], right) and (C) in mature human CB subsets containing the myeloid (▪) and B-cell lineages (□). *Smad7 not detectable by Q-PCR. (D) Control vector and Smad7 retroviral constructs. The gene for Smad7 was subcloned into the control vector backbone using EcoRI and BamHI cloning sites, upstream of an internal ribosomal entry site (IRES). An enhanced green fluorescent protein (EGFP) gene downstream of the IRES acts as a reporter for selection of stable cell lines and tracking of transduced cells. (E) (i) PG13 packaging cell lines transduced with vector or Smad7 retrovirus were isolated by fluorescence-activated cell sorting (FACS) based on GFP expression. (ii) Validation of the PG13 retroviral packaging cell lines by retroviral transduction of the human hematopoietic cell line MBA.1 with vector- or Smad7-containing retrovirus. Transduced cells were selected based on GFP expression and verified by fluorescence microscopy. (F) Western blot analysis of cells transduced with vector or Smad7 retrovirus, and selected based on GFP expression. Equal amounts of protein were loaded per lane in all experiments. PG13 and MBA.1 cell lines transduced with Smad7 retrovirus express a higher level of Smad7 protein than cells transduced with vector retrovirus. Values indicated are protein band intensity relative to vector control. (G) Fold increase in Smad7 mRNA expression in Smad7 compared with vector-transduced HeLa and M-O7e cells, determined by amplified RNA analysis (n = 3). (H) TGF-β growth inhibition assay. 20 000 M-O7e cells, transduced with vector (□) or Smad7 (▪), were grown with 1 ng/mL TGF-β1 or without for 6 days. Total viable cells were enumerated at days 2, 4, and 6 of culture. Shown is the percent difference in growth of Smad7–M-O7e and vector–M-O7e at 1 ng/mL TGF-β1 compared with no TGF-β1 (dashed line), for triplicate samples in 2 independent experiments. Inset shows changes in cell number over time for all treatments.
Smad7 expression, retroviral constructs, and packaging lines. (A) Expression of Smad7 in human fetal blood (FB) and cord blood (CB) hematopoietic cell populations, including the primitive CD34+CD38– cell fraction devoid of lineage commitment markers (Lin–), mature CD33+ myeloid cells, CD19+ B lymphocytes, and CD3+ T lymphocytes, as determined by RT-PCR analysis. An RT-PCR reaction was performed on human fetal head cDNA as a positive control, and on a sample containing no cDNA (H2O) as a negative control. RT-PCR for β-glucuronidase (β-gluc), a housekeeping gene expressed at a single copy per cell, was used to assess the quality of the cDNA templates. Quantitative PCR was used to measure the expression of Smad7 in (B) highly purified Lin–CD34+CD38– and Lin–CD34+CD38+ subsets from human cord blood (summarized as relative expression in Lin–CD34+CD38– [▪] compared with Lin–CD34+CD38+ cells [□], right) and (C) in mature human CB subsets containing the myeloid (▪) and B-cell lineages (□). *Smad7 not detectable by Q-PCR. (D) Control vector and Smad7 retroviral constructs. The gene for Smad7 was subcloned into the control vector backbone using EcoRI and BamHI cloning sites, upstream of an internal ribosomal entry site (IRES). An enhanced green fluorescent protein (EGFP) gene downstream of the IRES acts as a reporter for selection of stable cell lines and tracking of transduced cells. (E) (i) PG13 packaging cell lines transduced with vector or Smad7 retrovirus were isolated by fluorescence-activated cell sorting (FACS) based on GFP expression. (ii) Validation of the PG13 retroviral packaging cell lines by retroviral transduction of the human hematopoietic cell line MBA.1 with vector- or Smad7-containing retrovirus. Transduced cells were selected based on GFP expression and verified by fluorescence microscopy. (F) Western blot analysis of cells transduced with vector or Smad7 retrovirus, and selected based on GFP expression. Equal amounts of protein were loaded per lane in all experiments. PG13 and MBA.1 cell lines transduced with Smad7 retrovirus express a higher level of Smad7 protein than cells transduced with vector retrovirus. Values indicated are protein band intensity relative to vector control. (G) Fold increase in Smad7 mRNA expression in Smad7 compared with vector-transduced HeLa and M-O7e cells, determined by amplified RNA analysis (n = 3). (H) TGF-β growth inhibition assay. 20 000 M-O7e cells, transduced with vector (□) or Smad7 (▪), were grown with 1 ng/mL TGF-β1 or without for 6 days. Total viable cells were enumerated at days 2, 4, and 6 of culture. Shown is the percent difference in growth of Smad7–M-O7e and vector–M-O7e at 1 ng/mL TGF-β1 compared with no TGF-β1 (dashed line), for triplicate samples in 2 independent experiments. Inset shows changes in cell number over time for all treatments.
To examine the biological role of Smad7 in human hematopoiesis, retroviral gene transfer was used to overexpress Smad7 in putative human hematopoietic stem cells, defined functionally by their ability to repopulate the bone marrow of NOD/SCID mice with human hematopoietic cells of multiple committed lineages.44,45 The human Smad7 gene was subcloned upstream of an enhanced green fluorescent protein (GFP) reporter to create a bicistronic retroviral vector for Smad7 overexpression (Figure 1D). Stable GaLV-pseudotyped retroviral producer lines expressing the Smad7 and control vector retroviruses were constructed as described (K.C., K. Jay, B. Murdoch, M.B., manuscript submitted). The GFP-selected producer lines were confirmed to produce infectious retrovirus by transduction of the human megakaryocytic leukemia cell lines MBA.135 and M-O7e.36 PG13-Smad7, MBA.1-Smad7, and M-O7e–Smad7 GFP+ cell lines were morphologically indistinguishable from their vector control counterparts (Figure 1E, and data not shown), suggesting that Smad7 did not alter cellular morphology in stably transduced cells. Western blot analysis of GFP+ PG13-Smad7 and MBA.1-Smad7 cell lysates demonstrated an approximate 2-fold increase in Smad7 protein compared with endogenous levels of Smad7 protein detected in PG13-vector and MBA.1-vector cells, respectively (Figure 1F). In addition, Smad7 was expressed at approximately 2- to 3-fold greater levels in HeLa-Smad7 and M-O7e–Smad7 compared with HeLa-vector and M-O7e vector cells, respectively, as detected using amplified RNA (Figure 1G, and data not shown). These results indicate that transduction of Smad7 causes an increase in both Smad7 mRNA and protein in transduced cells.
The M-O7e megakaryocytic cell line has previously been shown to be sensitive to TGF-β1 mediated growth inhibition.46 To determine if the retrovirally produced Smad7 was capable of inhibiting TGF-β signaling, M-O7e cells overexpressing Smad7 protein were cultured in a TGF-β growth inhibition assay with 1 ng/mL TGF-β1 or without. Regardless of TGF-β treatment, M-O7e–Smad7 cells demonstrated a higher rate of proliferation than M-O7e–vector cells, suggesting an effect of Smad7 on endogenous secreted TGF-β (Figure 1H, inset). Both M-O7e–Smad7 and M-O7e–vector cells exhibited growth inhibition in the presence of TGF-β (Figure 1H, inset). However, when comparing the percentage of growth inhibition of M-O7e cells by TGF-β, M-O7e–Smad7 cells were initially more refractory to TGF-β–mediated growth inhibition than M-O7e–vector cells. After 2 days of culture, M-O7e–Smad7 cells remained uninhibited by TGF-β, whereas M-O7e–vector showed a 25% reduction in proliferation (Figure 1H). This higher proliferative rate and abrogation of TGF-β–mediated growth inhibition in the day-2 TGF-β culture of M-O7e suggests that the Smad7 protein overexpressed in these cells can inhibit TGF-β signaling. Interestingly, increased cellular proliferation in the absence of exogenous TGF-β ligand was also observed in activated mature splenic T cells derived from mice engineered to misexpress Smad7 by means of a T-cell–specific promoter,26 supporting our observation that Smad7 overexpression is functional.
Ex vivo transduction of Smad7 into primitive human hematopoietic cells
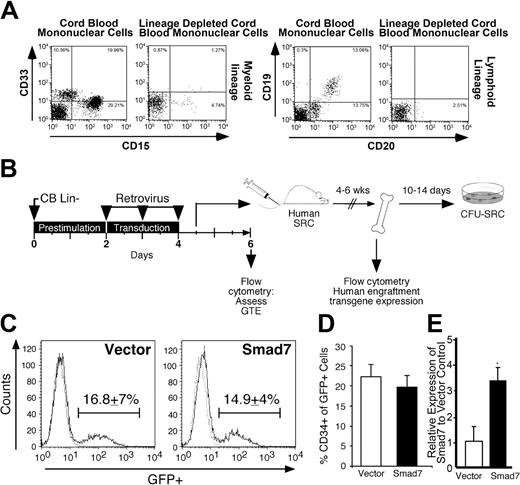
The CB Lin– cells used in this study are enriched for primitive hematopoietic cells with in vitro progenitor and in vivo repopulating function, present mostly within the CD34+CD38– subfraction,30 and have been shown to express Smad7 mRNA (Figure 1B). Efficiency of myeloid and B-cell lineage depletion is shown in a representative example of CB MNCs before and after lineage depletion using CD33 and CD15 for myeloid cells and CD19 and CD20 for B-lymphoid cells (Figure 2A). CB Lin– cells were transduced with either the Smad7 or vector retrovirus as outlined in Figure 2B. To determine the effect of Smad7 in human hematopoietic SRCs and their differentiated progeny, the total cells cultured from 4 × 105 Lin– cells were transplanted into each sublethally irradiated NOD/SCID mouse.
Transduction of CB Lin– cells with Smad7 retrovirus. (A) Depletion of myeloid (CD33 and CD15) and B-cell (CD19 and CD20) lineage markers from de novo isolated human CB to generate Lin– CB cells used for gene transfer. (B) Experimental outline for assessment of Smad7 function in hematopoietic repopulating cells, as described in “Materials and methods.” GTE indicates gene transfer efficiency; SRC, SCID repopulating cell assay; CFU-SRC, colony forming unit–SRC assay. (C) A representative example of CB Lin– cells 1.5 days after the final exposure to vector or Smad7 retrovirus, assessed by flow cytometric analysis of GFP expression. Dotted histogram indicates cells exposed to supernatant harvested from empty PG13 packaging cells, used to set the GFP+ marker. Value represents the average gene transfer efficiency into CB Lin– cells, measured as the percentage of GFP+ cells ± SEM, from 15 independent CB samples. (D) Expression of the primitive hematopoietic marker CD34 on transduced (GFP+) CB Lin– cells 1.5 days after the final virus exposure (± SEM). (E) Relative expression of Smad7 (± SEM) using Q-PCR in vector- (□) versus Smad7-transduced cells (▪).
Transduction of CB Lin– cells with Smad7 retrovirus. (A) Depletion of myeloid (CD33 and CD15) and B-cell (CD19 and CD20) lineage markers from de novo isolated human CB to generate Lin– CB cells used for gene transfer. (B) Experimental outline for assessment of Smad7 function in hematopoietic repopulating cells, as described in “Materials and methods.” GTE indicates gene transfer efficiency; SRC, SCID repopulating cell assay; CFU-SRC, colony forming unit–SRC assay. (C) A representative example of CB Lin– cells 1.5 days after the final exposure to vector or Smad7 retrovirus, assessed by flow cytometric analysis of GFP expression. Dotted histogram indicates cells exposed to supernatant harvested from empty PG13 packaging cells, used to set the GFP+ marker. Value represents the average gene transfer efficiency into CB Lin– cells, measured as the percentage of GFP+ cells ± SEM, from 15 independent CB samples. (D) Expression of the primitive hematopoietic marker CD34 on transduced (GFP+) CB Lin– cells 1.5 days after the final virus exposure (± SEM). (E) Relative expression of Smad7 (± SEM) using Q-PCR in vector- (□) versus Smad7-transduced cells (▪).
To assess the gene transfer efficiency into the bulk Lin– population, a fraction of Lin– cells were cultured for an additional 36 hours after transduction to allow for retroviral integration and GFP expression. Two days after the final transduction, the gene transfer efficiency into the bulk Smad7 and vector Lin– populations was not significantly different, as assessed by flow cytometry. Approximately 17% of vector-transduced Lin– cells and 15% of Smad7 transduced Lin– cells were GFP+ as determined by comparison to GFP– Lin– cells exposed to supernatant not containing retroviral particles (Figure 2C). To determine if Smad7 altered the primitive state of Lin– cells, transduced (GFP+) cells were examined for expression of the primitive hematopoietic marker CD34. Approximately 20% of the GFP+ cells 36 hours after gene transfer remained primitive based on retention of CD34 expression, regardless of the vector treatment (Figure 2D), suggesting that Smad7 did not enhance or inhibit the differentiation of the transduced Lin– cells in vitro. In addition, cells transduced with Smad7 versus vector alone demonstrated a more than 3-fold increase in expression of Smad7 (Figure 2E), thereby supporting effective Smad7 overexpression in transgenic human hematopoietic cells to be used in subsequent functional assays.
Effect of Smad7 on CB hematopoietic repopulating cell capacity
Previous studies using retroviral gene marking have determined that the cells within the Lin– population that engraft the BM of NOD/SCID mice with differentiated myeloid and lymphoid progeny, termed SRCs, are distinct from the less-primitive hematopoietic progenitors detected in the in vitro colony-forming unit (CFU) assay.31,47 To evaluate whether Smad7 plays a role in regulating the human multilineage repopulating cells detected in this assay, sublethally irradiated NOD/SCID mice were given transplants of equivalent numbers of transduced Smad7 and vector Lin– cells. Mice were killed 5 to 8 weeks after transplantation for analysis of human hematopoietic reconstitution by flow cytometric analysis of the bone marrow (BM) using the human-specific CD45 panleukocyte marker (Figure 3Ai), and those human cells expressing the transgene were detected by GFP fluorescence as shown (Figure 3Aii). From 5 independent CB samples transplanted into 13 mice, the level of human engraftment did not differ significantly between SRCs transduced with Smad7 compared with vector alone (Figure 3B). In addition, the fraction of gene-marked (GFP+) human (CD45+) cells engrafting the NOD/SCID mouse was similar under vector and Smad7 treatments (Figure 3C), suggesting that expression of Smad7 did not alter the level of hematopoietic chimerism. Since mice were given transplants of similar numbers of vector- or Smad7-transduced cells, this data suggests that overexpression of Smad7 did not result in increased expansion or proliferation of SRCs.
Smad7 alters the balance of lymphoid and myeloid cells following CB Lin– SRC reconstitution. (A) A representative example of human engraftment in NOD/SCID mice repopulated with vector- (left column) or Smad7-transduced SRCs (right column) from the same CB sample. (i) Level of human engraftment in the NOD/SCID bone marrow, detected by the human specific pan-leukocyte marker CD45. (ii) Human CD45+ cells expressing the vector or Smad7 transgene were gated according to co-expression of GFP. (Inset) Dotplot for a mouse engrafted with nontransduced Lin– cells, used to set quadrants for GFP+ human cells. (iii,iv) B-lymphoid and myeloid composition of the human hematopoietic graft in vector- and Smad7-transduced SRC (gated CD45+ in Figure 3Ai). Transduced B-lymphoid component expressed as percentage of CD19+ cells from total GFP+ cells (corresponding to GFP+ gate in Figure 3Aii). Transduced myeloid component expressed as percentage of CD33+ cells from total GFP+ cells. Nontransduced GFP– human cells are shown in the left quadrants for comparison. Quadrant settings were based on CD45+ isotype controls from a mouse given transplants of nontransduced (GFP–) CB Lin– cells (inset). (B) Summary of the level of engraftment of human hematopoietic cells (% CD45+ cells) in the bone marrow of transduced NOD/SCID mice, calculated from gates shown in panel Ai, from 5 independent CB samples (13 mice total). Horizontal lines indicate mean level of engraftment; dots represent individual mice. (C) Summary of level of gene marking in the human hematopoietic graft, determined as percentage of GFP+ cells from total CD45+ cells, calculated from gates shown in panel Aii. Horizontal lines indicate mean level of gene marking; dots indicate the level of marking in individual mice. (D) Summary of engraftment patterns from vector (□) and Smad7 CB-paired mice (▪), from 5 independent CB samples transplanted into NOD/SCID mice (13 mice: 6 vector, 7 Smad7). Shown is the B-lymphoid (CD19+), myeloid (CD33+), and primitive (CD34+) populations expressed as percentage of total human (CD45+) GFP+ cells (i), or human (CD45+) GFP– cells (ii) (± SEM). (E) Summary of engraftment patterns from vector (□) and Smad7 CB-paired mice (▪), from 4 independent CB samples transplanted into NOD/SCID/β2–/– mice (18 mice: 8 vector, 10 Smad7). (F) Paired analysis of CD19 and CD33 engraftment patterns for vector and Smad7 SRCs from each of 5 CB samples transplanted into NOD/SCID mice. Bar indicates the mean percentage of CD19+ or CD33+ cells of the total GFP+ cells. *P ≤ .01. Error bars indicate SEM.
Smad7 alters the balance of lymphoid and myeloid cells following CB Lin– SRC reconstitution. (A) A representative example of human engraftment in NOD/SCID mice repopulated with vector- (left column) or Smad7-transduced SRCs (right column) from the same CB sample. (i) Level of human engraftment in the NOD/SCID bone marrow, detected by the human specific pan-leukocyte marker CD45. (ii) Human CD45+ cells expressing the vector or Smad7 transgene were gated according to co-expression of GFP. (Inset) Dotplot for a mouse engrafted with nontransduced Lin– cells, used to set quadrants for GFP+ human cells. (iii,iv) B-lymphoid and myeloid composition of the human hematopoietic graft in vector- and Smad7-transduced SRC (gated CD45+ in Figure 3Ai). Transduced B-lymphoid component expressed as percentage of CD19+ cells from total GFP+ cells (corresponding to GFP+ gate in Figure 3Aii). Transduced myeloid component expressed as percentage of CD33+ cells from total GFP+ cells. Nontransduced GFP– human cells are shown in the left quadrants for comparison. Quadrant settings were based on CD45+ isotype controls from a mouse given transplants of nontransduced (GFP–) CB Lin– cells (inset). (B) Summary of the level of engraftment of human hematopoietic cells (% CD45+ cells) in the bone marrow of transduced NOD/SCID mice, calculated from gates shown in panel Ai, from 5 independent CB samples (13 mice total). Horizontal lines indicate mean level of engraftment; dots represent individual mice. (C) Summary of level of gene marking in the human hematopoietic graft, determined as percentage of GFP+ cells from total CD45+ cells, calculated from gates shown in panel Aii. Horizontal lines indicate mean level of gene marking; dots indicate the level of marking in individual mice. (D) Summary of engraftment patterns from vector (□) and Smad7 CB-paired mice (▪), from 5 independent CB samples transplanted into NOD/SCID mice (13 mice: 6 vector, 7 Smad7). Shown is the B-lymphoid (CD19+), myeloid (CD33+), and primitive (CD34+) populations expressed as percentage of total human (CD45+) GFP+ cells (i), or human (CD45+) GFP– cells (ii) (± SEM). (E) Summary of engraftment patterns from vector (□) and Smad7 CB-paired mice (▪), from 4 independent CB samples transplanted into NOD/SCID/β2–/– mice (18 mice: 8 vector, 10 Smad7). (F) Paired analysis of CD19 and CD33 engraftment patterns for vector and Smad7 SRCs from each of 5 CB samples transplanted into NOD/SCID mice. Bar indicates the mean percentage of CD19+ or CD33+ cells of the total GFP+ cells. *P ≤ .01. Error bars indicate SEM.
Smad7 alters the developmental capacity of human hematopoietic repopulating cells
When Lin– populations containing SRCs engraft the BM of NOD/SCID mice, these primitive cells divide and differentiate to give rise to committed cells of multiple hematopoietic lineages in a distinctive pattern of hematopoietic engraftment consisting of a predominantly B-lymphoid graft, with lesser contributions from committed myeloid cells and primitive cells.41 To determine if Smad7 affects the hematopoietic developmental capacity of transduced SRCs, mice successfully engrafted with gene-marked SRCs, which then gave rise to GFP+CD45+ cells in the mouse BM, were further analyzed for the presence of multilineage cell surface markers. B-lymphoid (CD19+), myeloid (CD33+), and primitive (CD34+) lineages were examined in combination with CD45 and GFP expression to assess the contribution of GFP+ cells to each of these hematopoietic lineages. In a representative experiment (Figure 3A), vector-transduced SRCs gave rise to committed cells with a typical pattern of engraftment,41 where the majority of hematopoietic cells within the graft expressed the B-lymphoid marker CD19 (Figure 3Aiii) and a smaller population of cells expressed the pan-myeloid marker CD33 (Figure 3Aiv). However, reconstitution of Smad7 SRCs demonstrated equal lymphoid to myeloid graft composition. Overall, in 13 mice from 5 independent CB samples, a shift from the lymphoid (CD19+ cells) to myeloid (CD33+ cells) arm of the hematopoietic system was observed, representing 20% of the bulk GFP+ population derived from Smad7 SRCs (vector 73.2: 26.3% vs Smad7, 53.0: 44.9%, lymphoid: myeloid progeny, P ≤ .01; Figure 3Aiii,iv). The shift toward myeloid cells was apparent only in the GFP+ fraction of cells derived from the Smad7-transduced SRCs (Figure 3Di). Acting as an internal control, the GFP– fraction of Smad7 cells, presumably not expressing the Smad7 transgene, displayed an engraftment pattern indistinguishable from the GFP– vector-transduced cells (Figure 3Dii). Since this lineage shift occurs only in GFP+ Smad7 cells, we suggest that Smad7 overexpression affects the development from multipotent SRCs to differentiated progeny.
To address any potential variability between independent primary human CB samples, the percentages of lymphoid and myeloid cells were analyzed from paired mice given transplants of SRCs from 5 separate CB samples (Figure 3F). In all 5 CB samples, the engraftment pattern demonstrates a shift toward increased myeloid composition in Smad7 GFP+ cells. Despite the changes in lymphoid and myeloid contributions to the human hematopoietic graft, the proportion of primitive CD34+ cells was not altered in Smad7 mice, regardless of GFP expression (Figure 3D), suggesting that Smad7 alters the lineage commitment decision during hematopoiesis, but does not alter the total levels of differentiation or cellular expansion.
Smad7 only affects in vivo reconstitution arising from the most primitive long-term hematopoietic repopulating cells engrafting the NOD/SCID mouse
Smad7-transduced Lin– cells were transplanted into β2-microglobulin null NOD/SCID mice (NOD/SCIDβ2m–/–)39,40 that possess decreased NK cell activity compared with the parental NOD/SCID strain,39 making this host more permissive to engraftment by more mature human hematopoietic cells with short-term myeloid and lymphoid-myeloid repopulating capacity. Glimm et al48 have previously demonstrated that at 6 weeks after transplantation with Lin– cells, the predominant myeloid-lymphoid engraftment pattern in NOD/SCID β2–/– mice arises from a distinct short-term repopulating (STR) hematopoietic cell subset, while the parental NOD/SCID mice are engrafted only with the more primitive long-term repopulating (LTR) hematopoietic cell subset, both present within the same Lin– population. In 18 mice from 4 CB samples, transplanted Smad7-transduced Lin– cells gave rise to a lymphoid/myeloid pattern of engraftment indistinguishable from vector control cells, in both the GFP+ (Figure 3Ei) and GFP– subfractions (Figure 3Eii), suggesting that Smad7 does not alter the myeloid-lymphoid repopulating function of STR hematopoietic cells, in contrast to the LTR-derived reconstitution observed in NOD/SCID recipients. We suggest that Smad7 acts on the LTR cells, which contain bipotent myeloid and lymphoid precursors.
Smad7 does not alter hematopoietic maturation subsequent to lineage commitment
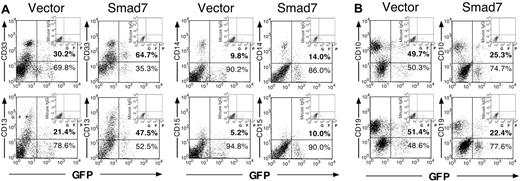
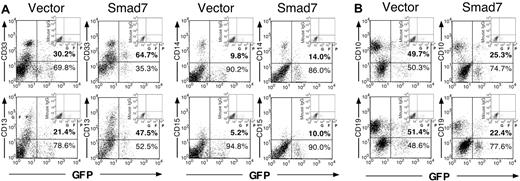
To determine if the increase in myeloid cells and decrease in lymphoid cells is due to impaired development of myeloid cells, and hence accumulation of primitive blastlike myeloid cells, progression down the myeloid lineage was examined in greater detail by flow cytometric analysis of NOD/SCID mice engrafted from a representative CB sample (Figure 4A). Bipotent granulocyte/monocyte precursors (CD33+CD15–), as well as mature monocytic and granulocytic progeny determined by the expression of CD13, CD14, and CD15 in addition to CD33 expression, were observed within both vector and Smad7 GFP+ human grafts, suggesting that Smad7 does not impair normal myeloid differentiation and maturation patterns. Furthermore, the fold increases in the various myeloid lineages in Smad7-versus vector-transduced cells were similar (1.5-2.2 fold; Table 1), suggesting that Smad7 overexpression does not favor, or inhibit, the development of any particular subset of committed myeloid cells. Since all subsets of committed myeloid cells were altered similarly by Smad7 expression, this suggests that Smad7 overexpression in SRCs prior to myeloid cell fate commitment does not impair the ability of committed myeloid cells to differentiate normally.
Multilineage lymphoid and myeloid analysis of Smad7- and vector-transduced SRCs. FACS analysis of lymphoid and myeloid commitment markers on CD45+ human hematopoietic cells transduced with vector or Smad7 retrovirus, engrafted in the BM of a representative pair of NOD/SCID mice. (A) Analysis of myeloid markers CD33, CD13, CD14, and CD15 on CD45+ gated cells. (B) Analysis of lymphoid markers CD10 and CD19 on CD45+ gated cells.
Multilineage lymphoid and myeloid analysis of Smad7- and vector-transduced SRCs. FACS analysis of lymphoid and myeloid commitment markers on CD45+ human hematopoietic cells transduced with vector or Smad7 retrovirus, engrafted in the BM of a representative pair of NOD/SCID mice. (A) Analysis of myeloid markers CD33, CD13, CD14, and CD15 on CD45+ gated cells. (B) Analysis of lymphoid markers CD10 and CD19 on CD45+ gated cells.
Alternatively, to determine if the increase in myeloid progeny could be caused by a relative decrease in lymphoid cells due to an early block in lymphoid commitment, thus preventing cellular maturation to the pro–B cell stage associated with expression of CD19, we also examined GFP+ human cells for the expression of CD10, a marker expressed on uncommitted lymphoid precursors. The fold decrease in lymphoid cells in Smad7 versus vector SRCs was comparable based on expression of CD10 and CD19 (1.96-fold compared with 2.27-fold, respectively; Figure 4B, Table 1), and was reproducible in all independent CB samples examined. These results suggest that Smad7 does not block progression of uncommitted lymphoid cells toward the B-cell lineage prior to CD19 acquisition, but instead reduces the frequency of lymphoid cell fate commitment decisions prior to CD10 acquisition during SRC differentiation into lineage-restricted progeny.
Mice engrafted with Smad7 SRCs demonstrate an increased frequency of functional myeloid progenitors
Our data suggest that mice engrafted with Smad7 SRCs give rise to a greater proportion of committed myeloid cells compared with vector SRCs based on expression of myeloid cell surface markers. To determine if Smad7 also affects the frequency of functional myeloid progenitors, primitive CD34+ cells fractionated on GFP expression were isolated from the BM of engrafted mice by FACS (Figure 5A). Aliquots of 5000 selected CD34+GFP+ or CD34+GFP– cells were placed into clonogenic progenitor assays to detect myeloid and erythroid CFUs arising from SRCs that had engrafted the NOD/SCID mouse, here termed CFU-SRCs. Representative granulocytic vector and Smad7 GFP+ CFU-SRCs are shown in Figure 5B, as visualized by light and fluorescent microscopy. Both colonies demonstrate a similar morphology, suggesting that Smad7 did not alter the process of myeloid maturation. Figure 5C summarizes the CFU-SRC capacity of vector compared with Smad7 GFP+ cells. In 4 out of 5 experiments, mice given transplants of Smad7 SRCs gave rise to more CFU-SRCs per 5000 CD34+GFP+ cells than did vector SRCs from the same CB sample (average, 4.6-fold increase). On average, Smad7 SRCs gave rise to 41 GFP+ CFU-SRCs, compared with only 18 CFU-SRCs from the corresponding vector control. Similar to phenotypic data, the increased frequency of CFU-SRCs was apparent only in GFP+ Smad7 cells, as demonstrated by similar frequencies of GFP– Smad7 and vector CFU-SRCs, suggesting the effect is specific to overexpression of the Smad7 transgene (Figure 5C, inset). Both vector- and Smad7-transduced cell populations gave rise to the full distribution of erythroid and myeloid CFU-SRC types at approximately equal proportions, both in the GFP+ population (Figure 5D) as well as the GFP– population (Figure 5D, inset), suggesting that while Smad7 may influence the frequency of myeloid progenitors, Smad7 does not alter subsequent maturation of committed myeloid colony types.
Smad7-transduced SRCs give rise to more clonogenic progenitors than do vector-transduced SRCs. (A) Sorting gates used to select human CD34+GFP+ or CD34+GFP– cells from the BM of NOD/SCID mice. Cells of the appropriate phenotype were sorted, and 5000 cells of each population were placed into CFU assay for detection of CFUs arising from SRCs (CFU-SRC). (B) Representative granulocytic CFU-SRCs (corrected to line 1, dashed line) transduced with vector (left) or Smad7 (right) retrovirus, visualized by light (inset) and fluorescent microscopy, scored between days 10 and 14. (C) Frequency of Smad7 CFU-SRCs relative to corresponding frequency of vector CFU-SRCs, from equal cell inputs. Shown below the graph is mean CFU-SRCs ± SEM of 5 independent pairings, per 5000 input cells, from the 4 independent CB samples showing an increased Smad7 CFU-SRC frequency compared with vector. Inset shows percentage of CFU-SRCs from GFP+ and GFP– Smad7 treatments compared with corresponding vector control. (D) Distribution of multiple hematopoietic lineages (erythroid burst-forming units [CFU-E], granulocyte CFUs [CFU-G], macrophage CFUs [CFU-M], granulocytemacrophage CFUs [CFU-GM], and granulocyte-erythrocyte-macrophage-megakaryocyte CFUs [CFU-GEMM]) within the total GFP+ CFU-SRCs, or GFP– CFU-SRCs (inset). □ indicates vector GFP+; ▪, Smad7 GFP+. Bars represent the percentage of total CFU-SRCs ± SEM, from 5 independent CB pairings.
Smad7-transduced SRCs give rise to more clonogenic progenitors than do vector-transduced SRCs. (A) Sorting gates used to select human CD34+GFP+ or CD34+GFP– cells from the BM of NOD/SCID mice. Cells of the appropriate phenotype were sorted, and 5000 cells of each population were placed into CFU assay for detection of CFUs arising from SRCs (CFU-SRC). (B) Representative granulocytic CFU-SRCs (corrected to line 1, dashed line) transduced with vector (left) or Smad7 (right) retrovirus, visualized by light (inset) and fluorescent microscopy, scored between days 10 and 14. (C) Frequency of Smad7 CFU-SRCs relative to corresponding frequency of vector CFU-SRCs, from equal cell inputs. Shown below the graph is mean CFU-SRCs ± SEM of 5 independent pairings, per 5000 input cells, from the 4 independent CB samples showing an increased Smad7 CFU-SRC frequency compared with vector. Inset shows percentage of CFU-SRCs from GFP+ and GFP– Smad7 treatments compared with corresponding vector control. (D) Distribution of multiple hematopoietic lineages (erythroid burst-forming units [CFU-E], granulocyte CFUs [CFU-G], macrophage CFUs [CFU-M], granulocytemacrophage CFUs [CFU-GM], and granulocyte-erythrocyte-macrophage-megakaryocyte CFUs [CFU-GEMM]) within the total GFP+ CFU-SRCs, or GFP– CFU-SRCs (inset). □ indicates vector GFP+; ▪, Smad7 GFP+. Bars represent the percentage of total CFU-SRCs ± SEM, from 5 independent CB pairings.
Potential gene targets of Smad7 in primitive human hematopoietic cells
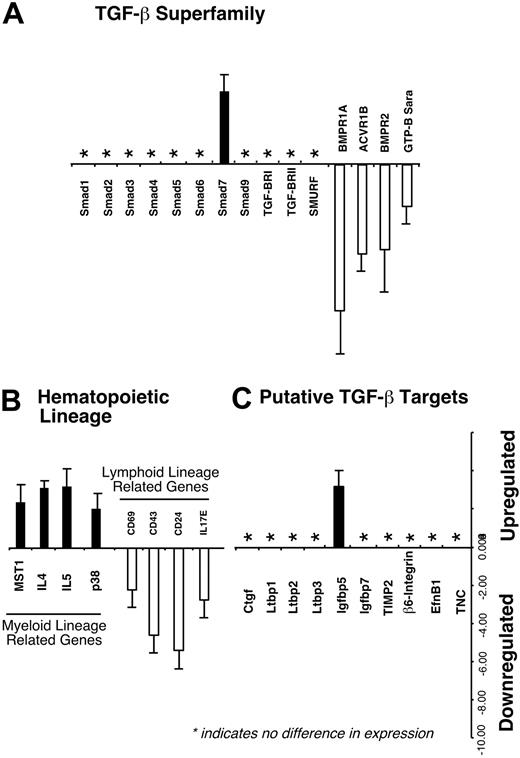
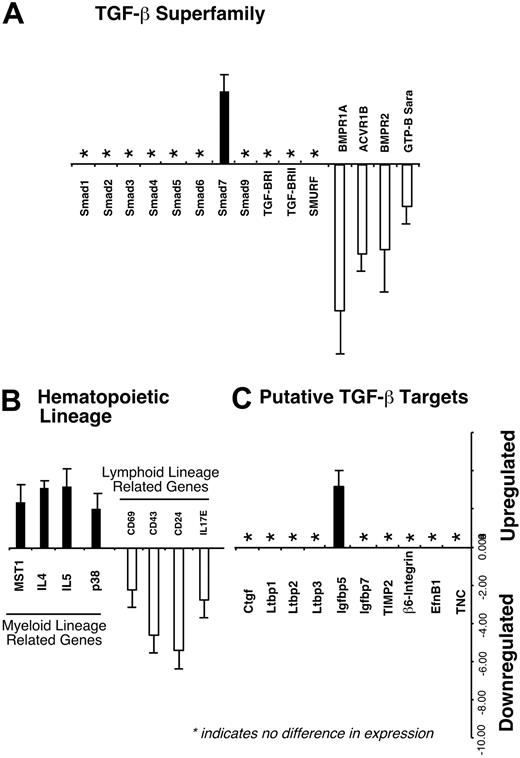
Downstream gene regulation by Smad7 has not been characterized in primitive human hematopoietic cells. Given the effects of Smad7 on repopulating cells, we isolated primitive Smad7-transduced hematopoietic cells (CD34+SMAD7–GFP+) and compared their gene expression profile with similar cells transduced with vector alone (CD34+vector–GFP+). Genes associated with the TGF-β superfamily, including all known human Smads, were analyzed for differential regulation upon Smad7 overexpression (Figure 6A). With the exception of Smad7 (retrovirally transduced into the cells), Smads 1 through 9 were not regulated, suggesting the effects of Smad7 overexpression do not alter transcriptional regulation of other related SMAD genes. However, TGF-β/BMP receptors (BMPR1A, ACVR1B, amd BMPR2) and Smad anchor for receptor activation (SARA) were down-regulated in response to Smad7 overexpression. Furthermore, consistent with our in vivo effects of Smad7 on cell fate of repopulating cells, specific genes involved in supporting myeloid lineages were up-regulated, whereas other genes related to lymphoid differentiation were down-regulated (Figure 6B). Recently, putative downstream targets of the TGF-β/BMP pathway have been identified.49 Of these candidates, overexpression of Smad7 in primitive human hematopoietic cells is capable of up-regulating insulin growth factor binding protein-5 (Igfbp5), whereas other recently reported targets were not regulated by Smad7 (Figure 6C), illustrating the specificity of Smad7 and its cell-specific effects. Taken together, these analyses provide new insights into potential Smad pathway-related gene targets and mechanism of Smad7 to alter cell fate of primitive human hematopoietic cells.
Differential regulation of potential Smad7 target genes in primitive human hematopoietic cells. Smad7- and vector-transduced GFP+ cells expressing CD34 were isolated and used for RNA extraction and subsequent microarray analysis as detailed in “Materials and methods.” (A) A summarized subset of known Smads 1-9 and other genes involved in TGF-β/BMP pathway are shown, along with (B) genes previously associated with supporting either the myeloid or lymphoid lineage and (C) putative TGF-β target genes. Abbreviations: Smad ubiquitination regulatory factor (SMURF), activin receptor type 1 (ACVR1B), macrophage-stimulating protein 1 (MST1), interleukin (IL), connective tissue growth factor (Ctgf), latent TGF-β binding protein (Ltbp), tissue inhibitor of metalloproteinases 2 (TIMP2), ephrin-B1 (Efn B1), tenascin C (TNC).
Differential regulation of potential Smad7 target genes in primitive human hematopoietic cells. Smad7- and vector-transduced GFP+ cells expressing CD34 were isolated and used for RNA extraction and subsequent microarray analysis as detailed in “Materials and methods.” (A) A summarized subset of known Smads 1-9 and other genes involved in TGF-β/BMP pathway are shown, along with (B) genes previously associated with supporting either the myeloid or lymphoid lineage and (C) putative TGF-β target genes. Abbreviations: Smad ubiquitination regulatory factor (SMURF), activin receptor type 1 (ACVR1B), macrophage-stimulating protein 1 (MST1), interleukin (IL), connective tissue growth factor (Ctgf), latent TGF-β binding protein (Ltbp), tissue inhibitor of metalloproteinases 2 (TIMP2), ephrin-B1 (Efn B1), tenascin C (TNC).
Discussion
It is well documented that numerous ligands of the TGF-β superfamily are involved in various stages of hematopoietic regulation, both in humans and small animal models. However, the effects of downstream regulators of this signaling pathway by Smad effectors and inhibitors have yet to be evaluated. Here, we examined the role of a TGF-β superfamily inhibitor, Smad7, in primitive human hematopoiesis through retroviral modification of multipotent human hematopoietic cells with in vivo lymphoid and myeloid repopulating function (SRCs). Surprisingly, overexpression of Smad7 in CB Lin– cells caused a lineage shift of SRC reconstitution toward myelopoiesis (Figure 3D,F).41 This observation suggests that Smad7 either alters the cell fate decisions of multipotent cells with myeloid and lymphoid differentiation capacity, or influences a subset of committed myeloid or lymphoid progeny to produce altered frequencies of mature cells. To determine at which stage of hematopoiesis Smad7 was acting, Smad7-transduced SRCs were transplanted into NOD/SCID/β2m–/– mice39,40 that allow preferential engraftment of more mature STR hematopoietic progenitors, while the parental NOD/SCID strain only permits the engraftment of the more primitive LTR hematopoietic cells.48 NOD/SCID/β2m–/– mice given transplants of Smad7-transduced Lin– cells gave rise to myeloid/lymphoid progeny in a repopulation pattern indistinguishable from vector control cells 6 to 8 weeks after transplantation (Figure 3E), suggesting that Smad7 has no effect on this more mature STR hematopoietic progenitor population. This suggests that Smad7 influences cell fate commitment decisions only in the most primitive human hematopoietic repopulating cells within the Lin– subfraction of CB MNCs.
Our study indicates that Smad7 did not block myeloid progression of SRCs to cause an accumulation of early CD33+ myeloid blasts. Consistent with the phenotypic data supporting an increased production of normal myeloid cells from Smad7 SRCs, we also observed an increase in the frequency of functional myeloid progenitors arising from the CD34+ cells within the BM of SRC-repopulated mice overexpressing Smad7 (Figure 5C). Since the early stages of lymphoid commitment and B-lymphoid maturation were not compromised in Smad7 SRCs, and the decrease in committed lymphoid cells was due to a decrease in the frequency of lymphoid cell fate commitment decisions, our data suggest that Smad7 does not alter the behavior of cells once they have committed to a particular hematopoietic lineage. We suggest that Smad7 acts only to alter hematopoietic cell fate commitment decisions in the most primitive cells with in vivo lymphoid and myeloid differentiation potential. Since the NOD/SCID assays for human repopulating cells does not readily detect differentiation into the T-cell lineage, use of alternative recipients capable of supporting human T lymphopoiesis will be required to determine whether Smad7 effects lymphoid T-cell development.
This role for Smad7 in human SRCs is similar in principle to observations of the role of Smad7 in Xenopus embryogenesis, where the ectopic expression of Smad7 in Xenopus embryos and animal pole explants was found to inhibit BMP-4– and activin-driven mesoderm formation, instead promoting/inducing neural cell fate decisions.27,50 There is no previous indication from the literature that Smad7 can alter cell fate commitment decisions in such a fashion in primary mammalian tissue, or outside of early embryonic development, thus identifying a novel role for Smad7 in multipotent human hematopoietic stem cell populations. Our hypothesis that Smad7 plays an important role in stem cell regulation is supported by the recent report that Smad7 expression is enhanced in murine multipotent HSC and pluripotent ESC populations, identified through oligonucleotide microarray analysis of genes preferentially expressed in various stem cell populations.28,29
Only 2 other genes, both encoding leukemogenic transcription factors,51-53 have been shown to alter the hematopoietic balance from committed lymphoid to myeloid progeny in mice repopulated with retrovirally transduced human SRCs. Retroviral-mediated overexpression of HoxA10 in SRCs gave rise to an engraftment pattern with increased myeloid and decreased lymphoid graft contribution, coupled with an absence of in vitro erythroid CFUs and an increase in the frequency of SRC-derived blast-CFUs incapable of proper myeloid differentiation.54 In our study, the myeloid differentiation capacity of cells arising from Smad7-transduced SRCs was unimpaired, as demonstrated by the expression of mature myeloid markers at equal proportions compared with vector-transduced cells, and by the presence of multiple lineages of mature myeloid CFU-SRCs (including erythroid CFUs) isolated from the BM of engrafted mice. Thus, Smad7 does not likely operate upstream of a committed myeloid factor such as HoxA10. Overexpression of the dominant negative Ikaros 6 (Ik6) isoform in SRCs resulted in a decrease in lymphoid cells and concomitant increase in myeloid cells55 in a pattern superficially similar to that seen by Smad7 expression in SRCs. Since Ikaros is believed to be an essential transcription factor for regulating the proliferation and differentiation of cells within the T and B lymphoid lineages,53 Ik6 overexpression was proposed to cause an inhibition of B-cell development, thus leading to the development of a relative increase in the proportion of committed myeloid cells.55 As the clonogenic capacity of myeloid CFU-SRCs was increased with Smad7 overexpression, it seems unlikely that Smad7 operates upstream of genes responsible for regulating committed lymphoid lineages, but rather acts in a novel manner to modulate the expression of genes involved in the earliest hematopoietic lineage commitment decisions. The mechanism by which Smad7 achieves this effect on cell fate decisions remains to be elucidated and these efforts will be facilitated by preliminary microarray data that identify regulated genes in response to Smad7 overexpression.
Prepublished online as Blood First Edition Paper, October 21, 2004; DOI 10.1182/blood-2004-03-0881.
Supported by a research grant from the Canadian Institutes of Health Research (CIHR) and Krembil Foundation, Canada Research Chair in Stem Cell Biology and Regenerative Medicine (M.B.), and a postgraduate scholarship award from CIHR to K.C.
K.C. and F.S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Jeffrey Wrana for providing us with the Smad7 construct, Dr Robert Hawley for providing us with the MIEV retroviral vector, and Drs Kysta Levac and Francis Karanu for their helpful discussions and insights toward completion of this work.

![Figure 1. Smad7 expression, retroviral constructs, and packaging lines. (A) Expression of Smad7 in human fetal blood (FB) and cord blood (CB) hematopoietic cell populations, including the primitive CD34+CD38– cell fraction devoid of lineage commitment markers (Lin–), mature CD33+ myeloid cells, CD19+ B lymphocytes, and CD3+ T lymphocytes, as determined by RT-PCR analysis. An RT-PCR reaction was performed on human fetal head cDNA as a positive control, and on a sample containing no cDNA (H2O) as a negative control. RT-PCR for β-glucuronidase (β-gluc), a housekeeping gene expressed at a single copy per cell, was used to assess the quality of the cDNA templates. Quantitative PCR was used to measure the expression of Smad7 in (B) highly purified Lin–CD34+CD38– and Lin–CD34+CD38+ subsets from human cord blood (summarized as relative expression in Lin–CD34+CD38– [▪] compared with Lin–CD34+CD38+ cells [□], right) and (C) in mature human CB subsets containing the myeloid (▪) and B-cell lineages (□). *Smad7 not detectable by Q-PCR. (D) Control vector and Smad7 retroviral constructs. The gene for Smad7 was subcloned into the control vector backbone using EcoRI and BamHI cloning sites, upstream of an internal ribosomal entry site (IRES). An enhanced green fluorescent protein (EGFP) gene downstream of the IRES acts as a reporter for selection of stable cell lines and tracking of transduced cells. (E) (i) PG13 packaging cell lines transduced with vector or Smad7 retrovirus were isolated by fluorescence-activated cell sorting (FACS) based on GFP expression. (ii) Validation of the PG13 retroviral packaging cell lines by retroviral transduction of the human hematopoietic cell line MBA.1 with vector- or Smad7-containing retrovirus. Transduced cells were selected based on GFP expression and verified by fluorescence microscopy. (F) Western blot analysis of cells transduced with vector or Smad7 retrovirus, and selected based on GFP expression. Equal amounts of protein were loaded per lane in all experiments. PG13 and MBA.1 cell lines transduced with Smad7 retrovirus express a higher level of Smad7 protein than cells transduced with vector retrovirus. Values indicated are protein band intensity relative to vector control. (G) Fold increase in Smad7 mRNA expression in Smad7 compared with vector-transduced HeLa and M-O7e cells, determined by amplified RNA analysis (n = 3). (H) TGF-β growth inhibition assay. 20 000 M-O7e cells, transduced with vector (□) or Smad7 (▪), were grown with 1 ng/mL TGF-β1 or without for 6 days. Total viable cells were enumerated at days 2, 4, and 6 of culture. Shown is the percent difference in growth of Smad7–M-O7e and vector–M-O7e at 1 ng/mL TGF-β1 compared with no TGF-β1 (dashed line), for triplicate samples in 2 independent experiments. Inset shows changes in cell number over time for all treatments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/5/10.1182_blood-2004-03-0881/6/m_zh80050574950001.jpeg?Expires=1766559402&Signature=ISf5HV1hWAtLypnzsUDnVIvz6LkUnPYkwPJ-9cB3UHD-EHP9agfGfVlA86At1~ORDcjs6BQjyxiqVx7lXkXuRTOJcr-qxcNs2FuAKteOdk2RhNiX8-Nr1T-6sxGkHaHqt-c36sDyFNaeTwcPyRLQ-cJSgtiQrmtaWEs5o9W~W4kaCEei9aaULpCKHLma3kH9ZfVTNvsb-ZskmyxHxIRo1yshypKfW0~zfF5e1nOCv4B~jl2X16MtTD1IFqxP9j5jY61XglY3o5GaqmR~KfW0ny8fwYbGoV6Emws~5bTHHcDe2hyEuNSXkltl1FQaQZn3jyyl-iKgny~jylr9-5f04g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. Smad7-transduced SRCs give rise to more clonogenic progenitors than do vector-transduced SRCs. (A) Sorting gates used to select human CD34+GFP+ or CD34+GFP– cells from the BM of NOD/SCID mice. Cells of the appropriate phenotype were sorted, and 5000 cells of each population were placed into CFU assay for detection of CFUs arising from SRCs (CFU-SRC). (B) Representative granulocytic CFU-SRCs (corrected to line 1, dashed line) transduced with vector (left) or Smad7 (right) retrovirus, visualized by light (inset) and fluorescent microscopy, scored between days 10 and 14. (C) Frequency of Smad7 CFU-SRCs relative to corresponding frequency of vector CFU-SRCs, from equal cell inputs. Shown below the graph is mean CFU-SRCs ± SEM of 5 independent pairings, per 5000 input cells, from the 4 independent CB samples showing an increased Smad7 CFU-SRC frequency compared with vector. Inset shows percentage of CFU-SRCs from GFP+ and GFP– Smad7 treatments compared with corresponding vector control. (D) Distribution of multiple hematopoietic lineages (erythroid burst-forming units [CFU-E], granulocyte CFUs [CFU-G], macrophage CFUs [CFU-M], granulocytemacrophage CFUs [CFU-GM], and granulocyte-erythrocyte-macrophage-megakaryocyte CFUs [CFU-GEMM]) within the total GFP+ CFU-SRCs, or GFP– CFU-SRCs (inset). □ indicates vector GFP+; ▪, Smad7 GFP+. Bars represent the percentage of total CFU-SRCs ± SEM, from 5 independent CB pairings.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/5/10.1182_blood-2004-03-0881/6/m_zh80050574950005.jpeg?Expires=1766559402&Signature=CzdlywfD1A04c84JwDljaL~zWV9d81y0-6BCYKMW7oBaanhZKZIFsqYqURY2-K3MqncFwEfhPwVGG26TfjzGDoARECb421qTFjJ9GDSb3z1lB7Iz6U~NF8djS1khpHhNCzxy5nHgWue~utd~N1BZdyMrIS-k4y6~Vl9h80~cy5XPDV9M~~JBsoZB4NLcOPR1VMJhP2al5BwG1j1qSojCuku2AAYWJ9BRPKYJTXZMg0KpAZPvhgGdsnfvDYS7ofIZJWqHwLhU-av9UUw82ABQdaR0KYiZuRZSiTWtvf60p42EUR4uLK5mJFM-~RaBLhGOEmdsKpio2BQR7mj1mIeiRA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Smad7 expression, retroviral constructs, and packaging lines. (A) Expression of Smad7 in human fetal blood (FB) and cord blood (CB) hematopoietic cell populations, including the primitive CD34+CD38– cell fraction devoid of lineage commitment markers (Lin–), mature CD33+ myeloid cells, CD19+ B lymphocytes, and CD3+ T lymphocytes, as determined by RT-PCR analysis. An RT-PCR reaction was performed on human fetal head cDNA as a positive control, and on a sample containing no cDNA (H2O) as a negative control. RT-PCR for β-glucuronidase (β-gluc), a housekeeping gene expressed at a single copy per cell, was used to assess the quality of the cDNA templates. Quantitative PCR was used to measure the expression of Smad7 in (B) highly purified Lin–CD34+CD38– and Lin–CD34+CD38+ subsets from human cord blood (summarized as relative expression in Lin–CD34+CD38– [▪] compared with Lin–CD34+CD38+ cells [□], right) and (C) in mature human CB subsets containing the myeloid (▪) and B-cell lineages (□). *Smad7 not detectable by Q-PCR. (D) Control vector and Smad7 retroviral constructs. The gene for Smad7 was subcloned into the control vector backbone using EcoRI and BamHI cloning sites, upstream of an internal ribosomal entry site (IRES). An enhanced green fluorescent protein (EGFP) gene downstream of the IRES acts as a reporter for selection of stable cell lines and tracking of transduced cells. (E) (i) PG13 packaging cell lines transduced with vector or Smad7 retrovirus were isolated by fluorescence-activated cell sorting (FACS) based on GFP expression. (ii) Validation of the PG13 retroviral packaging cell lines by retroviral transduction of the human hematopoietic cell line MBA.1 with vector- or Smad7-containing retrovirus. Transduced cells were selected based on GFP expression and verified by fluorescence microscopy. (F) Western blot analysis of cells transduced with vector or Smad7 retrovirus, and selected based on GFP expression. Equal amounts of protein were loaded per lane in all experiments. PG13 and MBA.1 cell lines transduced with Smad7 retrovirus express a higher level of Smad7 protein than cells transduced with vector retrovirus. Values indicated are protein band intensity relative to vector control. (G) Fold increase in Smad7 mRNA expression in Smad7 compared with vector-transduced HeLa and M-O7e cells, determined by amplified RNA analysis (n = 3). (H) TGF-β growth inhibition assay. 20 000 M-O7e cells, transduced with vector (□) or Smad7 (▪), were grown with 1 ng/mL TGF-β1 or without for 6 days. Total viable cells were enumerated at days 2, 4, and 6 of culture. Shown is the percent difference in growth of Smad7–M-O7e and vector–M-O7e at 1 ng/mL TGF-β1 compared with no TGF-β1 (dashed line), for triplicate samples in 2 independent experiments. Inset shows changes in cell number over time for all treatments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/5/10.1182_blood-2004-03-0881/6/m_zh80050574950001.jpeg?Expires=1766583938&Signature=QknOQotrG3Wd4dIcoGhyBzvwm1VWSFFUS9eEXlI0CUql5lEfuS6fiXGy7uQ18gNh~RFrnI7T0WDitccDDCDlIxJ1j1e2esBahPR6OFZc5QiqpYwhlcnpoTXpqmxP6UCvv0f7Dd7RUpOJrIDvrsQh9Z5K6Q5oN41uY-8vJphKedWYj--BNB7kLs3F~0c1U9HPWYQWApLQ5WvVIvd7hWzz-B5RRjh93uTB0mMXxZQ98k84ELpmLL0J2Mjl5uqarajkY6lE6OCdLXN64oFon0ej1UL9clrO8NPjL62UTlbzP3~exg2j2Byg7eq6B8qQkg-9NzHqxS5WpmZD4AvHqYEFBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 5. Smad7-transduced SRCs give rise to more clonogenic progenitors than do vector-transduced SRCs. (A) Sorting gates used to select human CD34+GFP+ or CD34+GFP– cells from the BM of NOD/SCID mice. Cells of the appropriate phenotype were sorted, and 5000 cells of each population were placed into CFU assay for detection of CFUs arising from SRCs (CFU-SRC). (B) Representative granulocytic CFU-SRCs (corrected to line 1, dashed line) transduced with vector (left) or Smad7 (right) retrovirus, visualized by light (inset) and fluorescent microscopy, scored between days 10 and 14. (C) Frequency of Smad7 CFU-SRCs relative to corresponding frequency of vector CFU-SRCs, from equal cell inputs. Shown below the graph is mean CFU-SRCs ± SEM of 5 independent pairings, per 5000 input cells, from the 4 independent CB samples showing an increased Smad7 CFU-SRC frequency compared with vector. Inset shows percentage of CFU-SRCs from GFP+ and GFP– Smad7 treatments compared with corresponding vector control. (D) Distribution of multiple hematopoietic lineages (erythroid burst-forming units [CFU-E], granulocyte CFUs [CFU-G], macrophage CFUs [CFU-M], granulocytemacrophage CFUs [CFU-GM], and granulocyte-erythrocyte-macrophage-megakaryocyte CFUs [CFU-GEMM]) within the total GFP+ CFU-SRCs, or GFP– CFU-SRCs (inset). □ indicates vector GFP+; ▪, Smad7 GFP+. Bars represent the percentage of total CFU-SRCs ± SEM, from 5 independent CB pairings.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/5/10.1182_blood-2004-03-0881/6/m_zh80050574950005.jpeg?Expires=1766583938&Signature=ARoqk-VDYHg-koUh035OJ2Fj17kc236pcVMZrle5aWTxE1MG1w~6~tksQHVEhVS5tv1jTpSIg-mfXN1O-iUOro0c8eDXWrUPxxClAtbneZktb15ZL19BSYSX3blRNc4hgINAf9ROO6gXAlqwl8WyfjsyFsV3-p~aImO~f8hVQRJ0ZDUkMT-hM7hfuoaeugcLAbURuH9k1TVT1VatkoyqmKyU1X42XKQEdeh3tD878G88W1gGRxYKN1UGqi9gU~qfPjkLxug95V24r0E1IdaoWD4VJo01Kt0We9uesVRb7Gpw-9CViRB9dCS8A7UE~27hQmTHrz9ymCT8KCHcQn7vRg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)