Abstract
Clinical benefits from monoclonal antibody therapy for B-chronic lymphocytic leukemia (B-CLL) have increased interest in developing additional immunotherapies for the disease. CD40 ligand is an accessory signal for T-cell activation and can overcome T-cell anergy. The OX40-OX40 ligand pathway is involved in the subsequent expansion of memory antigen-specific T cells. We expressed both CD40L and OX40L on B-CLL cells by exploiting the phenomenon of molecular transfer from fibroblasts overexpressing these ligands. We analyzed the effects of the modified B-CLL cells on the number, phenotype, and cytotoxic function of autologous T cells in 7 B-CLL patients. Transfer of CD40L and OX40L was observed in all and was followed by the up-regulation of B7-1 and B7-2. The culture of CD40L/OX40L-expressing B-CLL cells with autologous T cells generated CD4+/CD8+ cytotoxic T-cell lines, which secreted interferon-γ (IFN-γ) and granzyme-B/perforin in response to autologous, but not to allogeneic, B-CLL cells or to autologous T-cell blasts. CD40L or OX40L alone was insufficient to expand tumor-reactive T cells. The combination of CD40L and OX40L on B-CLL cells may allow the generation of therapeutic immune responses to B-CLL, either by active immunization with modified tumor cells or by adoptive immunotherapy with tumor-reactive autologous T cells.
Introduction
B-chronic lymphocytic leukemia (B-CLL) cells express a range of tumor-associated and tumor-specific antigens, including immunoglobulins and lineage- and differentiation-specific markers. However, these cells do not appear to be immunogenic, either in vivo or when cultured ex vivo with autologous T cells. One means by which immunogenicity may be increased is by manipulation of the CD40/CD40 ligand (CD40L) pathway. CD40L interacts with CD40 on target cells to increase their antigen-presenting capacity by up-regulating the expression of costimulatory molecules (including CD80 and CD86), adhesion molecules, and major histocompatibility complex (MHC) molecules.1-5 CD40L also induces the maturation of dendritic cells (DCs), increasing their ability to take up and process antigens.6-9 These effects have encouraged exploration of the molecule for the immunotherapy of human B-cell malignancies.1-5,10-16
We and others have previously shown that the expression of CD40L on B-CLL cells can increase the cells' ability to present antigens and make them potentially recognizable by the immune system.2-4,16 Nevertheless, the expansion of autologous B-CLL–reactive T cells has to date proved problematic. Optimal recruitment and expansion of antigen-specific T cells after T-cell receptor (TCR) engagement require the sequential interaction of multiple costimulatory molecules. Among the first of these is the interaction of T-cell CD28 with CD80/86 on the antigen-presenting cell (APC), an event that will be favored by CD40 activation of B-CLL cells. However, several additional molecular pathways are subsequently triggered, depending on the functional nature of the stimulated T cell. Among these additional molecules is OX40 ligand (OX40L or CD134L), which is normally expressed on DCs17 and is a critical late accessory signal (after TCR and CD28 engagement) to augment the proliferation and survival of memory CD4+ T cells.18-23 Hence, the engagement of the OX40 receptor on T cells in vivo enhances antitumor activity by stimulating and expanding the natural repertoire of the host's tumor-specific CD4+ T cells.22,23 Because OX40/OX40L interactions occur late in the T-cell activation cascade, prior activation of the CD40 pathway is crucial if the immunostimulatory benefits of OX40 receptor activation are to be observed.24
We have determined whether combining the forced expression of the immunostimulatory molecules CD40L and OX40L by human B-CLL cells would enhance their immunogenicity and promote the expansion of autologous tumor-reactive T cells. Because direct gene transfer into primary leukemia cells, including B-CLL cells, has proved difficult and inconsistent, we developed an alternative means of inducing B-CLL cells to express human immunostimulatory molecules.16 Our approach exploits the phenomenon of molecular transference, in which fibroblasts overexpressing certain membrane proteins may donate them to recipient B-CLL cells. This mechanism requires cell-to-cell contact but does not involve discernible membrane fusion,16 and it is being exploited in a phase 1 clinical study in which hCD40L-expressing B-CLL cells are injected subcutaneously together with a fixed dose of human interleukin-2 (hIL-2)–secreting tumor cells.25 Our initial analysis showed that immunization was followed by the development of circulating leukemia-reactive autologous T cells that released interferon-γ (IFN-γ) and granzyme B after exposure to autologous leukemia cells. Unfortunately, this reactivity was short lived and became undetectable within a few weeks of completion of the vaccine schedule. To try to augment these specific, but transient, antitumor responses we and others observed ex vivo and in vivo with CD40L-modified B-CLL cells,1-5,16 we have modified the approach to transfer the additional, later-acting, costimulatory molecule OX40L.26 We now show that transfer of a combination of CD40L and OX40L molecules synergistically induces a cytotoxic T-cell immune response against autologous tumor cells and may be used to generate immunogenic tumor cells for active or adoptive immunotherapy of B-CLL.
Materials and methods
Leukemia cells and cell lines
After patients provided informed consent to a protocol approved by the institutional review board of the Baylor College of Medicine, B-CLL cells were isolated by Lymphoprep gradient separation (Nycomed, Oslo, Norway) from the peripheral blood of untreated patients with high leukocyte counts. The mean white blood cell count was 9.3 × 1010/L (range, 5.5-15.0 × 109/L). The purity of the tumor cell population (determined by immunostaining with CD5, CD19, CD20) was always greater than 90%. The average percentage of circulating T cells was 6.3% (range, 3%-10%).
MRC-5 cells (a human embryonic lung fibroblast cell line)27 and K562 cells were obtained from American Type Culture Collection (ATCC, Rockville, MD) and kept in culture using RPMI 1640 (BioWhittaker, Walkersville, MD) supplemented with 10% heat-inactivated fetal calf serum (FCS; HyClone, Perbio Science, Logan, UT) and 2 mM l-glutamine (BioWhittaker).
Adenoviral vector construction
Details of vector construction are provided elsewhere.4,5,16,28 In brief, both adenovectors (Ad-vectors) encoding human CD40L (Ad-hCD40L) and human OX40L (Ad-hOX40L) are based on a chimeric E1-E3 deletion mutant adenovirus Ad5/F35. hCD40L cDNA was obtained from ATCC (pBluescriptII SK phagemid pBS-hCD40L-6A9).29 hOX40L cDNA was obtained from InVivogen (product pORF5-hOX40L; San Diego, CA), and was cloned by polymerase chain reaction (PCR) into the pShuttleX plasmid (Clontech, Palo Alto, CA) after insertion of the NheI and NotI restriction enzyme sites. Procedures in the Clontech Adeno-X Expression System manual were then followed to produce the final Ad5/F35-hOX40L and Ad5/F35-hCD40L vectors. The titer of the viral preparation was 7.75 × 1010 infectious units (IU)/mL for Ad-hCD40L and 4.5 × 1010 IU/mL for Ad-hOX40L.
Monoclonal antibodies and flow cytometry
The following were purchased from Becton Dickinson (BD) Biosciences (San Jose, CA): mouse immunoglobulin G1 (IgG1)–specific monoclonal antibodies (mAbs); fluorescein isothiocyanate (FITC)–labeled mAb specific for human CD5, CD45RA, TCR α/β, and OX40 (CD134); phycoerythrin (PE)–labeled mAbs specific for human CD19, CD20, CD80 (B7-1), CD86 (B7-2), CD40, CD56, CD154 (CD40L), CD45RO, CD62L, CCR7, and TCR γ/δ; peridin chlorophyll protein (PerCP)–labeled mAbs specific for human CD3, CD4, CD19, and CD20; and APC-labeled mAbs specific for human CD8. An anti–human OX40L antibody (CD134L) was obtained from MBL (clone TAG-34; Naka-Ku Nagoya, Japan). Indirect staining using rat antimouse (RAM) PE-conjugated Ab was performed using standard techniques. We used an anti–human perforin PE-conjugate mAb purchased from BD Biosciences to stain for intracytoplasmic perforin. Cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences). All gates were set using appropriate isotype control antibodies.
Transduction of MRC-5 with Ad-hCD40L and Ad-hOX40L vectors and coculture with B-CLL cells
Complete details of the coculture conditions are described elsewhere.16 Briefly, MRC-5 cells were incubated for 2 hours at 37°C in 5% CO2 in the presence of Ad-hCD40L, Ad-hOX40L vectors alone, or the combination of both at a multiplicity of infection of 250 IU/cell for each vector. The day after transduction, B-CLL cells were seeded onto the transduced MRC-5 cells at an MRC-5/B-CLL ratio between 1:20 and 1:25 and were maintained in culture for a further 24-hour period at 37°C in 5% CO2. The B-CLL cells were then harvested, analyzed, and frozen for further use.
Autologous T-cell expansion
Autologous T cells were obtained from peripheral blood mononuclear cells by using positive selection with CD4 and CD8 magnetic beads and magnetic cell sorting (MACS) technology (Miltenyi Biotech, Bergisch Gladbach, Germany). Purity was always greater than 90%. Contamination with monocytes, B-CLL, and NK cells was always 2% or less for each. Mitogen-stimulated T-cell lines were generated using phytohemagglutinin (PHA) at 5 μg/mL and then were kept in culture, adding recombinant human IL-2 (rhIL-2; Proleukin; Chiron, Emeryville, CA) at 100 U/mL every 2 to 3 days.
T cells (2 × 106/mL) were first stimulated in 24-well plates at a 1:1 ratio with unmanipulated, hCD40L-, hOX40L-, or hCD40L/hOX40L-expressing B-CLL cells without IL-2. Restimulation was then performed weekly with each specific target at a 1:3 target-effector (T/E) ratio, adding rhIL-2 at 40 U/mL. Target B-CLL cells were always irradiated at 40 Gy before stimulation. Half the medium was replaced every 2 to 3 days, and fresh IL-2 was added. Cell concentration was always maintained at approximately 2 × 106/mL. Cells were grown in 45% RPMI 1640 and 45% Click medium (Irvine Scientific, Santa Ana, CA) supplemented with 10% heat-inactivated FCS and 2 mM L-glutamine. Cells were counted weekly by trypan blue exclusion, and CD3, CD4, CD8, and CD56 immunophenotyping was performed weekly. Memory marker expression (CD45RA/R0, CCR7, and CD62L) was also analyzed weekly, whereas OX40 expression on CD4/CD8 cells was analyzed 24 and 48 hours after each stimulation.
A cytokine bead array (CBA; BD Biosciences) was performed at week 3 on supernatant samples to measure the secretion of IFN-γ, tumor necrosis factor-α (TNF-α), IL-2, IL-4, IL-5, and IL-10.
For OX40/OX40L blocking experiments, hCD40L/hOX40L-expressing B-CLL cells were incubated for 45 minutes before each stimulation with a CD134/Fc human recombinant protein with hOX40L-blocking activity at 1 μg/mL (CD134/Fc human recombinant; Alexis Biochemicals, Qbiogene, Carlsbad, CA) or with a control protein (control/Fc fusion protein human recombinant; Alexis Biochemicals).
ELISPOT assay
Cytotoxic T-cell lines (CTLs) expanded under each condition we describe were cocultured with 40 Gy–irradiated unmanipulated autologous B-CLL cells, autologous PHA T cells, mismatched allogeneic B-CLL cells, and K562 cells in an enzyme-linked immunospot (ELISPOT) assay for IFN-γ (BD Biosciences) and granzyme B (Diaclone; Cell Sciences, Canton, MA), according to the manufacturer's instructions. The same CTLs, stimulated with 0.1 μg/mL each phorbol myristate acetate (PMA)/ionomycin (Sigma, St Louis, MO), were used as internal positive control. T cells (2 × 104) were cultured in duplicate with 2 × 105 of each specific target in each well. Blocking experiments were performed using CD4 (clone 13B8.2) and CD8 (clone B9.11) monoclonal antibodies (Immunotech; Beckman Coulter, Fullerton, CA) at 2 μg/well, incubating the effector cells for 30 minutes at room temperature. After 24 hours, spots were developed according to the manufacturer's instructions and were enumerated using the Series 1 ImmunoSpot Image Analyzer and software (Cellular Technologies, Cleveland, OH). The assay was performed at time 0, week 3, and week 4.
CFSE-based cytotoxicity assay
A carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes Europe BV, Leiden, The Netherlands)–based cytotoxicity assay was used to measure the cytotoxicity of CD40L/OX40L-stimulated CTLs, and flow cytometry was adopted as read-out.30 Briefly, 2 × 107/mL B-CLL cells were incubated for 10 minutes at room temperature with 1.5 μM CFSE in phosphate-buffered saline (PBS) supplemented with 1% FCS. An equal amount of warmed FCS was added for 10 minutes, and the cells were washed twice. These target cells were cocultured with CTLs at a 1:10 T/E ratio for 18 hours at 37°C in 5% CO2 in 48-well plates, without the addition of rhIL-2. CTLs alone, autologous CFSE-labeled B-CLL cells alone, or mismatched allogeneic CFSE-labeled B-CLL cells alone or incubated with CTLs were used as specificity controls. Cytotoxic activity was also tested against autologous CFSE-labeled PHA blasts. Before and 18 hours after incubation, the CFSE-labeled cells were counted by fluorescence-activated cell sorter (FACS) gating on the viable population using a fixed number (5000) of unconjugated beads (BD Biosciences) as a standard to allow a precise and consistent quantification of cell death between experiments.30 The percentage of CTL-specific killing was calculated as follows: [100 - (a/b × 100)] - [100 - c/b × 100], where a is the viable B-CLL absolute cell count 18 hours after culture with CTL, b the viable absolute count at time 0, and c is the viable absolute B-CLL cell count after 18 hours of culture alone, without CTL.
To confirm that the antitumor T-cell activity was MHC class 1 or 2 restricted, we incubated target cells with anti–human HLA-A, -B, or -C (clone W6/32) or anti–HLA-DP, -DQ, or -DR (clone CR3/43) antibodies (DAKO, Glostrup, Denmark) or with isotype control antibody, all at 20 μg/mL for 30 minutes at room temperature before we added effector cells.
Statistical analysis
Paired Student t test was used to determine statistical significance. Data are presented as mean plus SD or mean plus range.
Results
hCD40L and hOX40L expression on transduced MRC-5 fibroblasts and cocultured B-CLL cells
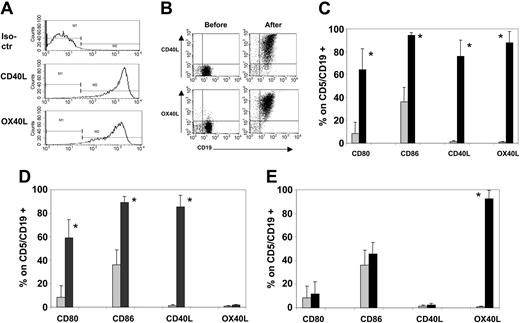
Nontransduced MRC-5 fibroblasts were negative for hCD40L and hOX40L expression. Within 24 hours of transduction with the Ad-hCD40L or Ad-hOX40L vectors, individually or together, more than 95% of MRC-5 expressed the appropriate transgenes by FACS analysis. There were no differences in expression of each transgene or of cell viability when the vectors were used individually or in combination (Figure 1A).
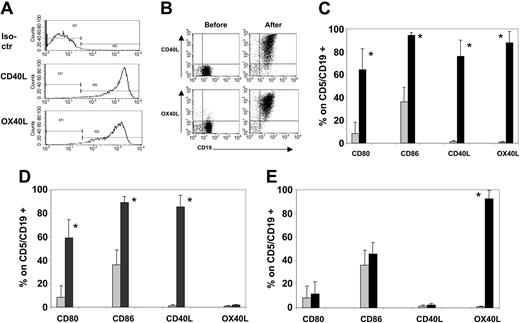
B-CLL cells efficiently express CD40L, OX40L, CD80, and CD86 after coculture with CD40L/OX40L-expressing MRC-5. (A) Expression of hCD40L and hOX40L on transduced MRC-5 24 hours after transduction with both vectors simultaneously. No difference was observed when transducing the MRC-5 with each single vector separately (data not shown). (B) Representative sample (of 7 tested) of hCD40L and hOX40L expression on B-CLL cells before and after the coculture on CD40L/OX40L-expressing MRC-5. (C-E) Expression of CD80, CD86, CD40L, and OX40L before (time 0, ▦) and 24 hours after (▪) the coculture with CD40L/OX40L-expressing (C) MRC-5, (D) CD40L-expressing MRC-5, and (E) OX40L-expressing MRC-5, respectively. Each bar represents the mean + SD percentage of positive viable cells gated on CD5+/CD19+ B-CLL cells (7 donors tested). *P < .005.
B-CLL cells efficiently express CD40L, OX40L, CD80, and CD86 after coculture with CD40L/OX40L-expressing MRC-5. (A) Expression of hCD40L and hOX40L on transduced MRC-5 24 hours after transduction with both vectors simultaneously. No difference was observed when transducing the MRC-5 with each single vector separately (data not shown). (B) Representative sample (of 7 tested) of hCD40L and hOX40L expression on B-CLL cells before and after the coculture on CD40L/OX40L-expressing MRC-5. (C-E) Expression of CD80, CD86, CD40L, and OX40L before (time 0, ▦) and 24 hours after (▪) the coculture with CD40L/OX40L-expressing (C) MRC-5, (D) CD40L-expressing MRC-5, and (E) OX40L-expressing MRC-5, respectively. Each bar represents the mean + SD percentage of positive viable cells gated on CD5+/CD19+ B-CLL cells (7 donors tested). *P < .005.
These transduced MRC-5 fibroblasts were cocultured with B-CLL cells from 7 different patients. Flow cytometry analysis (Figure 1B-C) showed that there was a consistent transfer of hCD40L and hOX40L from hCD40L/hOX40L-expressing MRC-5 to patient B-CLL cells. Mean fluorescence intensity was also significantly up-regulated for CD40L (from an average value of 8.2 to 878; range, 270-2078; P < .005) and for OX40L (from an average value of 9 to 631; range, 123-1000; P < .005). As we reported previously,16 there was also CD80 and CD86 up-regulation on the B-CLL cells subsequent to engagement of their own CD40 receptor by transferred CD40L (Figure 1C-D). By contrast, after culture on hOX40L-expressing MRC-5, no up-regulation of CD80 or of CD86 occurred (Figure 1E). In all 7 samples tested, conditions required for clinical vaccine preparation (freezing, storage, thawing, and irradiation of manipulated cells) did not diminish expression of any of these molecules. Moreover, B-CLL cells stably expressed all these transferred and costimulatory molecules for as long as they survived (1 week in vitro),16 and expression was not modulated by exposure to unmodified B-CLL cells or to T cells (data not shown).
These data show that CD40L and OX40L are equally well transferred from MRC-5 cells to B-CLL cells, that the presence of these molecules is stable, and that the observed up-regulation of CD80 and CD86 on the B-CLL cells is a consequence exclusively of CD40 activation and does not involve OX40 stimulation.
T-cell proliferation in response to modified B-CLL cells
Autologous T cells were cocultured with B-CLL cells expressing hCD40L or hOX40L alone or in combination or with unmodified B-CLL cells. Stimulation with unmodified, hCD40L-, or hOX40L-expressing B-CLL cells produced little or no expansion of autologous T cells. A mean 41-fold T-cell expansion was, however, observed at week 4 in the presence of B-CLL cells expressing both hCD40L and hOX40L (Figure 2A; P < .05 comparing CD40L/OX40L with each molecule alone or unmodified B-CLL cells). To confirm the contribution of the OX40/OX40L interaction to this increased proliferative response, we repeated these experiments in the presence of a CD134/Fc human recombinant protein with hOX40L-blocking activity. Proliferation was significantly reduced, falling to levels comparable to those for CD40L stimulation alone (Figure 2B). A control human recombinant fusion protein had no effect (data not shown).
hCD40L/hOX40L-expressing B-CLL cells efficiently expand autologous T cells. (A) Growth rate of viable autologous T cells stimulated with each different B-CLL target (unmanipulated [•], hCD40L- [▴], hOX40L- [▪], or hCD40L/hOX40L-expressing B-CLL cells [ ]). At each time point, the mean + SD cell number for all 7 donors tested is represented. Cells were counted by trypan blue exclusion. At week 4, no restimulation was performed and no exogenous IL-2 was added. (B) Effect of a CD134/Fc human recombinant protein with hOX40L-blocking activity added to hCD40L/hOX40L-expressing B-CLL cells (• 3 donors tested; mean ± SD) compared with CD40L/OX40L combination (▪) and CD40L alone (
]). At each time point, the mean + SD cell number for all 7 donors tested is represented. Cells were counted by trypan blue exclusion. At week 4, no restimulation was performed and no exogenous IL-2 was added. (B) Effect of a CD134/Fc human recombinant protein with hOX40L-blocking activity added to hCD40L/hOX40L-expressing B-CLL cells (• 3 donors tested; mean ± SD) compared with CD40L/OX40L combination (▪) and CD40L alone ( ). The 3 donors tested in this experiment presented a normal profile of CD4-CD8-CD56- cells.
). The 3 donors tested in this experiment presented a normal profile of CD4-CD8-CD56- cells.
hCD40L/hOX40L-expressing B-CLL cells efficiently expand autologous T cells. (A) Growth rate of viable autologous T cells stimulated with each different B-CLL target (unmanipulated [•], hCD40L- [▴], hOX40L- [▪], or hCD40L/hOX40L-expressing B-CLL cells [ ]). At each time point, the mean + SD cell number for all 7 donors tested is represented. Cells were counted by trypan blue exclusion. At week 4, no restimulation was performed and no exogenous IL-2 was added. (B) Effect of a CD134/Fc human recombinant protein with hOX40L-blocking activity added to hCD40L/hOX40L-expressing B-CLL cells (• 3 donors tested; mean ± SD) compared with CD40L/OX40L combination (▪) and CD40L alone (
]). At each time point, the mean + SD cell number for all 7 donors tested is represented. Cells were counted by trypan blue exclusion. At week 4, no restimulation was performed and no exogenous IL-2 was added. (B) Effect of a CD134/Fc human recombinant protein with hOX40L-blocking activity added to hCD40L/hOX40L-expressing B-CLL cells (• 3 donors tested; mean ± SD) compared with CD40L/OX40L combination (▪) and CD40L alone ( ). The 3 donors tested in this experiment presented a normal profile of CD4-CD8-CD56- cells.
). The 3 donors tested in this experiment presented a normal profile of CD4-CD8-CD56- cells.
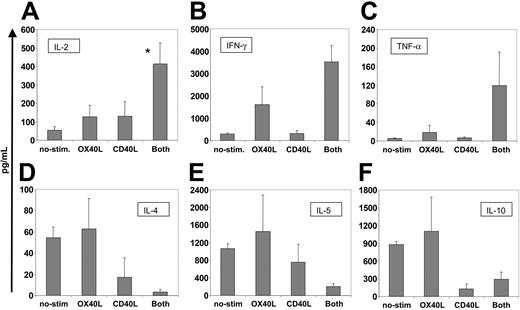
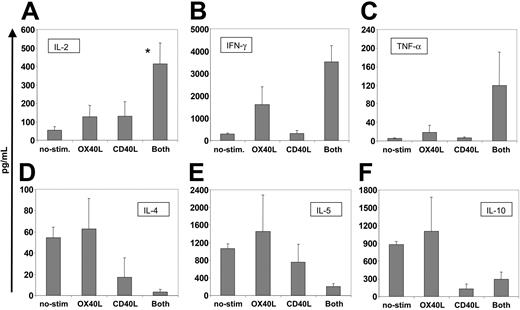
Physiologic OX40-OX40L interaction triggers IL-2 production by T cells.18-20 To determine whether activation by our transferred OX40L molecule could mimic the effects of conventionally expressed OX40L, we measured the amount of IL-2 in the cell culture supernatants. Figure 3A shows that T cells exposed to hCD40L/hOX40L-expressing B-CLL cells secreted significantly more IL-2 than T cells exposed to OX40L or CD40L alone or to nonmanipulated B-CLL cells (P < .05). Secretion of other cytokines was also influenced by the CD40L/OX40L combination. Secretion of T-helper 1 (TH1) cytokines IFN-γ and TNF-α was increased, whereas production of the TH2 cytokines IL-4, IL-5, and IL-10 was decreased in comparison to T cells stimulated by single agents (Figure 3B-F). Hence, the transferred CD40L and OX40L molecules appeared to be fully functional and could act with true synergism to promote the proliferation and development of a TH1 effector phenotype.
CD40L/OX40L-stimulated CTLs present increased amounts of proinflammatory TH1 cytokines and lower amounts of TH2 cytokines and of IL-10. The figure shows the amount of cytokines secreted at week 3 (mean + SD in picograms per milliliter) by CTLs stimulated by the different B-CLL target cells (nonmanipulated, OX40L-, CD40L-, and CD40L/OX40L-expressing cells). Cell concentration was maintained at approximately 2 × 106/mL for all the conditions and samples tested. (A-F) IL-2, IFN-γ, TNF-α, IL-4, IL-5, and IL-10, respectively. *Significant difference (P < .05). For TNF-α and IL-4, P = .06 and P = .07, respectively.
CD40L/OX40L-stimulated CTLs present increased amounts of proinflammatory TH1 cytokines and lower amounts of TH2 cytokines and of IL-10. The figure shows the amount of cytokines secreted at week 3 (mean + SD in picograms per milliliter) by CTLs stimulated by the different B-CLL target cells (nonmanipulated, OX40L-, CD40L-, and CD40L/OX40L-expressing cells). Cell concentration was maintained at approximately 2 × 106/mL for all the conditions and samples tested. (A-F) IL-2, IFN-γ, TNF-α, IL-4, IL-5, and IL-10, respectively. *Significant difference (P < .05). For TNF-α and IL-4, P = .06 and P = .07, respectively.
Phenotypic characterization of T-cell lines generated after culture with modified B-CLL cells
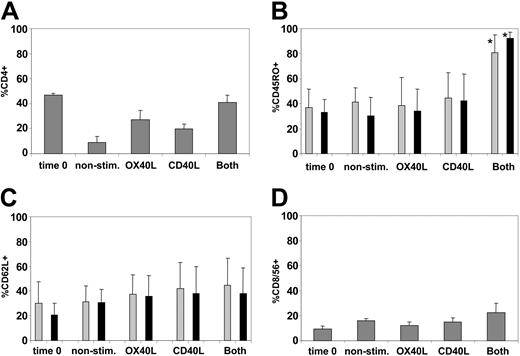
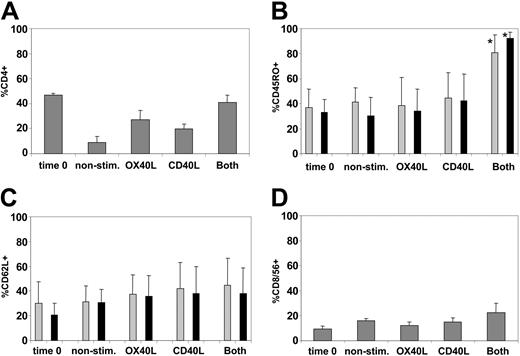
By week 3, all cultures consisted predominantly of TCR αβ–expressing T cells (greater than 95% in all conditions). No NK-cell expansion was observed (less than 5% in all conditions). The CD3+ T cells were a mixture of CD4+ and CD8+ cells, but the proportion of CD4+ cells was highest after stimulation with CD40L and OX40L in combination (Figure 4A). The proportion of CD45RO+ T cells was greatest in the CD40L/OX40L-stimulated cultures (Figure 4B). CD62L expression31 was little changed by any culture condition (Figure 4C). CCR731 was poorly expressed at the beginning of the coculture on CD4 and CD8 (less than 2%) and did not significantly change under any of the culture conditions. CD8+CD56+ NK-like T cells expanded to essentially the same degree in all conditions (Figure 4D).
CD40L-OX40L combination maintains CD4 T cells in culture and increases the proportion of CD45RO-expressing CD4 and CD8 T cells. (A) Mean ± SD percentage of CD4+ cells for each condition tested. (B-C) Mean + SD percentages of CD4 (▦) and CD8 (▪) positive for CD45RO and CD62L, respectively. *Significant difference (P < .05). (D) Percentages of CD8 T cells positive for CD56. For all panels, expression at time 0 is compared with expression for each condition at week 3.
CD40L-OX40L combination maintains CD4 T cells in culture and increases the proportion of CD45RO-expressing CD4 and CD8 T cells. (A) Mean ± SD percentage of CD4+ cells for each condition tested. (B-C) Mean + SD percentages of CD4 (▦) and CD8 (▪) positive for CD45RO and CD62L, respectively. *Significant difference (P < .05). (D) Percentages of CD8 T cells positive for CD56. For all panels, expression at time 0 is compared with expression for each condition at week 3.
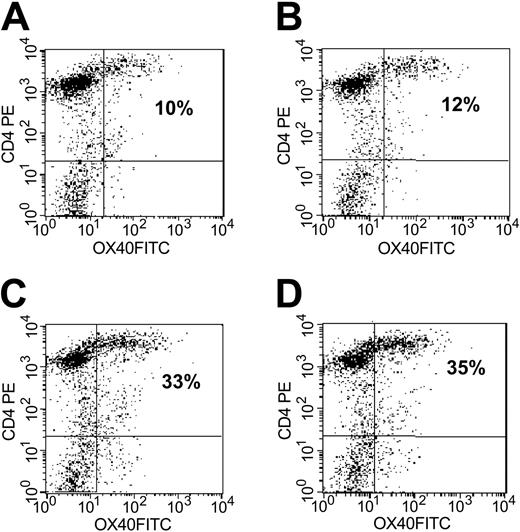
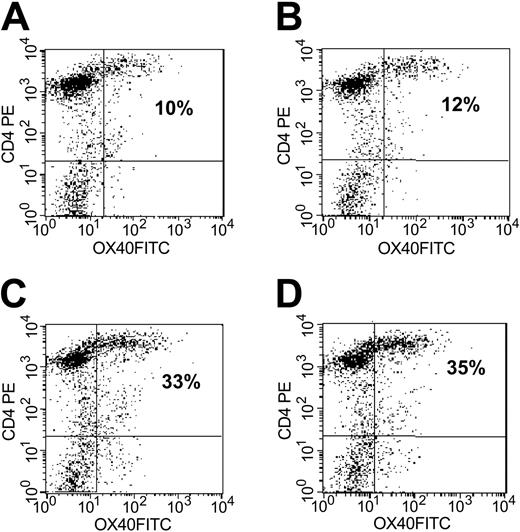
At the initiation of culture, the patient T cells were OX40-, but expression of this receptor progressively increased in the presence of CD40L costimulation and became maximal on CD4+ T cells at week 2 (Figure 5). At this time, a mean 25% (range, 17%-33%; n = 7) CD4+ cells were OX40+. The presence of OX40L on stimulator B-CLL cells did not further influence the expression of OX40, and CD4+ T-cell lines grown in the presence of both transferred molecules were a mean 29% OX40+ (range, 19%-38%; n = 7). OX40L alone did not increase OX40 expression above control levels. No OX40 expression was detected on CD8 T cells (data not shown).
Significant up-regulation of OX40 expression on CD4 is observed after stimulation with CD40L- or CD40L/OX40L-expressing B-CLL cells. Results are representative of 7 experiments. OX40 expression was efficiently up-regulated on CD4 cells after CD40L stimulation, with the highest levels reached at 24 hours after the third stimulation. (A-D) CD4 T cells stimulated with unmanipulated leukemia cells, OX40L-expressing, CD40L-expressing, and CD40L/OX40L-expressing B-CLL cells, respectively. Numbers indicate percentages of OX40-positive CD4 T cells.
Significant up-regulation of OX40 expression on CD4 is observed after stimulation with CD40L- or CD40L/OX40L-expressing B-CLL cells. Results are representative of 7 experiments. OX40 expression was efficiently up-regulated on CD4 cells after CD40L stimulation, with the highest levels reached at 24 hours after the third stimulation. (A-D) CD4 T cells stimulated with unmanipulated leukemia cells, OX40L-expressing, CD40L-expressing, and CD40L/OX40L-expressing B-CLL cells, respectively. Numbers indicate percentages of OX40-positive CD4 T cells.
Hence, in the presence of CD40L, OX40 expression was up-regulated, and the combination of CD40L and OX40L could successfully expand CD4 and CD8 effector CD45RO+ T cells in 5 of 7 patients. In the remaining 2 donors, we were unable to expand cells with a classic CD4 or CD8 T-cell phenotype, obtaining instead cultures that were predominantly CD8+CD56+ NK-like T cells (70% and 80% of all T cells).
T-cell lines have antileukemic function
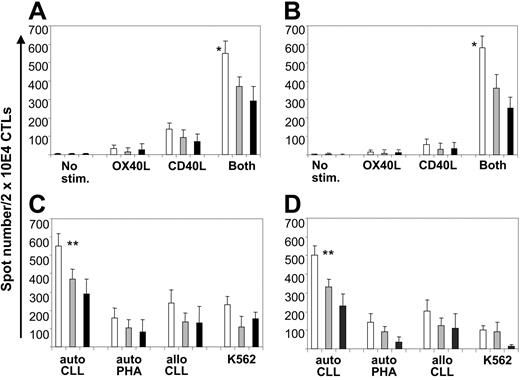
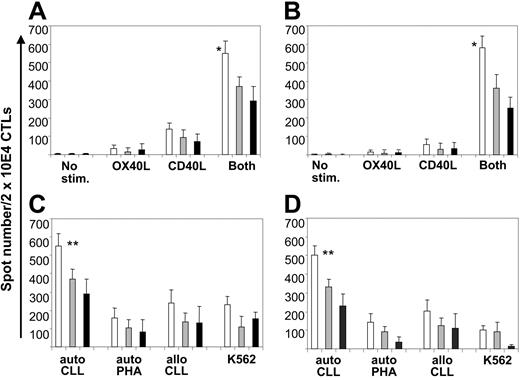
To evaluate the specificity of the T-cell lines generated using manipulated B-CLL cells as stimulators, we used the ELISPOT assay to measure the release of IFN-γ and granzyme B by T cells in response to unmanipulated B-CLL cells. Initial autologous cocultures of patient T cells and unmanipulated B-CLL cells produced no measurable IFN-γ or granzyme B release (frequency of responder cells 0.001% or less). By week 4, however, precursor frequency significantly rose, and the highest levels were found in the cultures stimulated with CD40L/OX40L-expressing B-CLL cells (Figure 6A-B) (mean frequency of IFN-γ and granzyme B spot–forming cells of 1 [2.7%] of 36 and 1 [2.9%] of 34, respectively; P < .05 compared with stimulation with CD40L or OX40L alone). Blocking experiments demonstrated that CD4+ and CD8+ T cells contributed to IFN-γ and granzyme B release (Figure 6A-B).
CD40L/OX40L-expressing B-CLL cells elicit increased IFN-γ and granzyme B release by autologous peripheral blood CD4 and CD8 T lymphocytes. (A-B) Number of IFN-γ and granzyme B spots/2 × 104 CTLs, respectively, against the autologous B-CLL target for each different condition tested at week 4 (mean + SD). □ indicates total amount of spots; ▦, number of spots after CD8 blocking; ▪, number of spots after CD4 blocking. *Significant difference (P < .05) comparing the CD40L/OX40L combination to each molecule alone or no stimulation. (C) Number of IFN-γ spots/2 × 104 CD40L/OX40L-stimulated CTLs against the autologous B-CLL target and the different control targets (mean + SD). (D) Same experiment as in panel C after CD56 depletion. **Significant difference (P < .05) comparing the response against autologous CLL cells with responses against allogeneic CLL cells, autologous PHA blasts, and K562.
CD40L/OX40L-expressing B-CLL cells elicit increased IFN-γ and granzyme B release by autologous peripheral blood CD4 and CD8 T lymphocytes. (A-B) Number of IFN-γ and granzyme B spots/2 × 104 CTLs, respectively, against the autologous B-CLL target for each different condition tested at week 4 (mean + SD). □ indicates total amount of spots; ▦, number of spots after CD8 blocking; ▪, number of spots after CD4 blocking. *Significant difference (P < .05) comparing the CD40L/OX40L combination to each molecule alone or no stimulation. (C) Number of IFN-γ spots/2 × 104 CD40L/OX40L-stimulated CTLs against the autologous B-CLL target and the different control targets (mean + SD). (D) Same experiment as in panel C after CD56 depletion. **Significant difference (P < .05) comparing the response against autologous CLL cells with responses against allogeneic CLL cells, autologous PHA blasts, and K562.
To determine whether these responses to autologous B-CLL cells were mediated by conventional MHC-restricted, antigen-specific T cells, we cocultured the CD40L/OX40L-stimulated T cells with allogeneic B-CLL or with autologous PHA (ie, T-cell) blasts or K562 cells (an NK cell target) (Figure 6C). Significant IFN-γ release was found only in the cultures stimulated by autologous B-CLL cells, an effect made even more apparent by removal of CD56+ cells (Figure 6D).
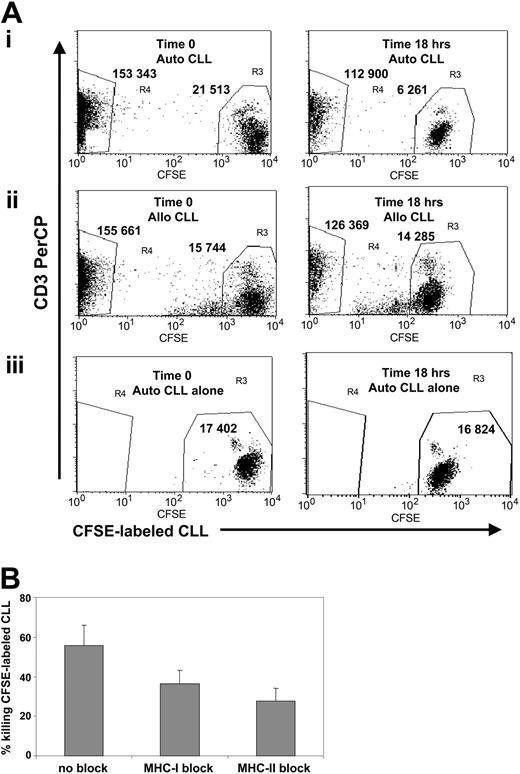
In addition to measures of cytokines and granzyme B release, sufficient T cells grew in the cultures stimulated by CD40L/OX40L-expressing B-CLL cells to test direct cytotoxicity against autologous target cells using a CFSE-based assay. The average rate of killing of autologous B-CLL cells was 48% (range, 31%-68%; n = 5; Figure 7A). This killing likely uses perforin given that intracytoplasmic flow cytometry analysis showed high levels of perforin in CD8+ (mean positive cells, 45%; range, 31%-56%; n = 5) and CD4+ T cells (mean positive cells, 33%; range, 22%-42%; n = 5). Adding MHC-blocking antibodies showed that the killing of autologous B-CLL cells was class 1 and class 2 restricted (Figure 7B). In the 2 donors who had an expansion predominantly of CD8+CD56+ NK-like T cells, there was no discernible specific killing of autologous B-CLL cells.
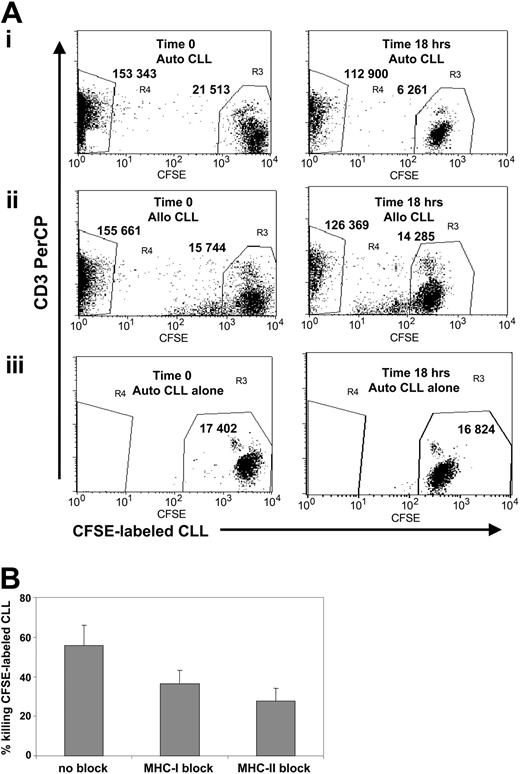
CD40L/OX40L-stimulated T cells have cytotoxic activity against autologous leukemia cells in a CFSE-based flow cytometry assay. (A) The x-axis represents CFSE-labeled B-CLL cells, gated on viable cells, and the y-axis represents the CD3 PerCP-positive population. Numbers represent the events counted in each gate (R3 for B-CLL cells and R4 for CTLs). (left panel) Time 0. (right panel) 18-hour analysis. (i) CFSE-labeled B-CLL cells cocultured with autologous CD40L/OX40L-stimulated T cells. The B-CLL cells are reduced by 71%. (ii) CTLs cocultured with mismatched allogeneic B-CLL cells. B-CLL cells are reduced by 9.3%. (iii) Autologous CFSE-labeled B-CLL cells alone. B-CLL cells are reduced by 3%. (B) Reduction in B-CLL cell numbers (mean + SD; 4 donors tested) with and without MHC class 1 and 2 blocking. Difference was statistically significant (P < .05 for MHC class 1 and class 2 blocking).
CD40L/OX40L-stimulated T cells have cytotoxic activity against autologous leukemia cells in a CFSE-based flow cytometry assay. (A) The x-axis represents CFSE-labeled B-CLL cells, gated on viable cells, and the y-axis represents the CD3 PerCP-positive population. Numbers represent the events counted in each gate (R3 for B-CLL cells and R4 for CTLs). (left panel) Time 0. (right panel) 18-hour analysis. (i) CFSE-labeled B-CLL cells cocultured with autologous CD40L/OX40L-stimulated T cells. The B-CLL cells are reduced by 71%. (ii) CTLs cocultured with mismatched allogeneic B-CLL cells. B-CLL cells are reduced by 9.3%. (iii) Autologous CFSE-labeled B-CLL cells alone. B-CLL cells are reduced by 3%. (B) Reduction in B-CLL cell numbers (mean + SD; 4 donors tested) with and without MHC class 1 and 2 blocking. Difference was statistically significant (P < .05 for MHC class 1 and class 2 blocking).
Hence, simultaneous delivery of CD40L and OX40L increases IFN-γ and granzyme B release in response to autologous leukemia cells on CD4 and CD8 effector T cells from B-CLL patients and produces autologous cytotoxic CD4/CD8 T cells.
Discussion
Our data show that stimulation of autologous T cells with B-CLL cells expressing CD40L and OX40L in combination greatly enhances the number and frequency of tumor-reactive T cells, compared with exposure to B-CLL cells expressing just one or another of these immunostimulatory molecules. The responding cells were CD4+ and CD8+ effector T cells, and their IFN-γ and granzyme B release and cytotoxic effects were all restricted to autologous tumor cells.
Expression of CD40L by B-CLL cells, together with the subsequent up-regulation of B7 molecules, can augment the antigen-presenting capacity of leukemia cells, breaking the anergic state of peripheral blood circulating T cells and thereby inducing the generation of leukemia-reactive T lymphocytes.2-4,16 This observation has been used as a rationale for developing a B-CLL “vaccine” expressing either murine or human CD40L.3,16 However, because stimulation with this single moiety does not substitute for all other costimulatory signals involved in antigen presentation, only a limited degree of expansion of reactive precursor cells is produced, which is likely insufficient for optimal in vivo activity. It is certainly inadequate for the ex vivo generation of sufficient tumor-reactive T cells for use in adoptive T-cell immunotherapy, an approach showing considerable promise for hematologic malignancies and solid tumors.32,33
Although several other approaches have been used for ex vivo activation and selective expansion of low-frequency, leukemia-specific precursor cells, they have resulted in equally limited benefit. High doses of IL-212 or the addition of IL-7 and IL-1534,35 have all tended to expand T cells nonspecifically. OX40L, by contrast, appears to have the appropriate characteristics to selectively expand antigen-specific memory-effector CD4+ T cells after their encounter with specific antigens and with CD40L. After the initial T-cell activation by MHC-restricted receptor recognition of antigen, CD40L is expressed by T cells and promotes antigen presentation by up-regulating B7 molecule expression on the APC (in this case the B-CLL cell). B7 molecules activate the CD28 pathway in the T cell to induce IL-2 secretion and initial T-cell proliferation. After 24 to 48 hours, OX40 is up-regulated on antigen-stimulated CD4+ T cells, and OX40L appears on the surfaces of dendritic cells17 to trigger the second phase of costimulation. The interaction of OX40 and OX40L is responsible for continued IL-2 secretion, improved survival of memory CD4+ T cells, and sustained T-cell proliferation.18-20
By coexpressing CD40L and OX40L on B-CLL cells, we have ensured that a B-CLL antigen-specific T-cell encounters a CD40-activated target B-CLL cell already expressing the CD80 and CD86 costimulatory molecules for early T-cell activation. Subsequently, OX40L expressed on the B-CLL cell provides a second phase of signals required by antigen-stimulated T cells to trigger sustained growth and activation of the tumor-reactive clones. The consequence is that, within 4 weeks of culture, the CD40L and OX40L combination produces a greater than 40-fold expansion of total T cells and a greater than 2500-fold expansion of leukemia-reactive T cells (from less than 1 of 100 000 to 1 of 36).
Tumor-reactive T-cell expansion by itself may be insufficient for an effective in vivo antitumor response. Many different systems in multiple species have confirmed that optimum effector response generally requires a combination of CD8+ effector and CD4+ T cells, which may themselves have direct effector activity or may provide cognate help to the CD8+ T cells. Hence, the ideal immune response to a tumor such as B-CLL will likely involve the recruitment of CD4 and CD8 cells. The CD40L/OX40L combination studied here successfully induces each type of effector cell. It contributes to the growth and survival of CD4+ T cells,18-23 which secrete cytokines in a TH1 pattern and have direct cytotoxicity against B-CLL target cells, likely mediated by perforins.36 In addition, coculture of T cells with CD40L/OX40L-expressing B-CLL cells results in the expansion of IFN-γ/granzyme B–secreting CD8 T cells with cytotoxic properties. These specific CD8 T cells likely benefit from a “helper” effect from OX40-expressing antigen-specific CD4+ T cells that are costimulated by exposure to the ligand on B-CLL cells.37,38 Despite the coinduction of limited IL-10 release, the overall effect of the CD40L/OX40L-expressing B-CLL cells is to favor the production of proinflammatory TH1 cytokines, such as IL-2, IFN-γ, and TNF-α. We do not know whether either or both of these populations will have antitumor activity in vivo. However, a number of precedents from other human studies suggest that cognate interactions of CD4 and CD8 lymphocytes in vivo are critical to the success of immunotherapeutic strategies in cancer patients.33 Two of the 7 patients did not respond in this way. Instead, their cocultures with CD40L/OX40L B-CLL cells grew out CD8 NK-like T cells that had no specific antileukemic activity. In both patients, the starting populations of cells contained more than 50% CD8+CD56+ T cells, whereas the remaining 5 patients had less than 10% of these cells. It will be of interest to discover whether these patients represent a distinct subset in terms of primary disease, disease stage, or previous treatment. No obvious characteristics yet link the 2 patients in the nonresponding subset.
How may the expression of stimulatory CD40L and OX40L molecules best be obtained on B-CLL cells? Because these B lymphocytes are resistant to transduction with available vectors, we exploited the phenomenon of molecular transference (MT) of TNF-receptor superfamily molecules from fibroblasts (in this case, the MRC-5 cell line) to the B-CLL cells. Although we do not yet know the physiologic purpose of MT, we do know it is a robust and highly reproducible phenomenon that transfers CD40L16 and OX40L26 molecules with high efficiency and in correct orientation from donor cells to B-CLL cells, where they appear to exert full activity after binding to the CD40 or OX40 receptors on target cells. Although an approach using MT may appear less straightforward than directly introducing transgenes into B-CLL cells, in fact it has considerable advantages for clinical development. MT allows the generation of just one standardized gene-modified cell line (the transduced MRC-5 donor cells), and it allows each patient sample to be cocultivated with a well-characterized transduced line. Hence, MT can readily be adapted for clinical immunotherapy of B-CLL. A phase 1 immunotherapy protocol is ongoing at our institution using MT to produce hCD40L-expressing autologous vaccines.16,25
Although we do not yet know whether the approach we have outlined will be successful in vivo, we have already confirmed that the approach is feasible for clinical study and that a correlation exists between immune responses obtained in vitro and those that can be developed in B-CLL patients in vivo. We have developed a phase 1 protocol for patients affected by B-CLL, in which a fixed dose of hIL-2–secreting autologous B-CLL cells is administered as a subcutaneous injection, together with an escalating dose of hCD40L-expressing autologous B-CLL cells prepared using the MT approach described here.25 Within these vaccines, a mean of 92% B-CLL cells was CD40L positive (compared with less than 1% before treatment), and costimulatory molecules were also up-regulated on the tumor cells (CD80 from a mean of 6%-74% and CD86 from a mean of 16%-73% positive cells). The mean secretion of IL-2 (measured by enzyme-linked immunosorbent assay [ELISA] at 72 hours after transduction) was 1780 pg/mL per 106 cells (range, 175-3400 pg/mL per 106 cells). To date, 8 patients have received between 3 and 8 subcutaneous injections. As predicted from previous in vitro studies,4,5,16 the results showed that a combination of hIL-2–secreting and hCD40L-expressing B-CLL cells produced adequate costimulation for a sufficient length of time to induce measurable CD4- and CD8-dependent anti–B-CLL immune responses. Total T-cell numbers were significantly increased in 3 patients, and an anti–B-CLL immune response was detected in 5 of 6 patients analyzed to date. Using ELISPOT assays with autologous B-CLL cells as stimulators, we found an increase in IFN-γ spot-forming T cells (less than 1 of 100 000 before to more than 1 of 1500 after vaccination) and in granzyme B–expressing T cells (less than 1 of 100 000 before to more than 1 of 2500 after vaccination). These in vivo immune responses were transient and became undetectable at 2 to 3 months after the last vaccine administration, leading us to explore methods by which the responses could be enhanced and prolonged. The data presented here suggest that the combination of CD40L and OX40L will fulfill that need, producing a greater expansion of leukemia-reactive T cells with a considerable increase of cytotoxic effector functions.
In conclusion, our data suggest that the combination of an early-(CD40L) and a later-acting costimulatory molecule (OX40L) meets the requirements for a high-level, sustained immune response against B-CLL cells in a cancer-vaccine approach. Additionally, the transfer of hCD40L and hOX40L onto B-CLL cells permits the generation of immunostimulatory target cells to expand tumor-reactive autologous T cells ex vivo that can be subsequently used for adoptive cellular immunotherapy.
Prepublished online as Blood First Edition Paper, November 9, 2004 DOI 10.1182/blood-2004-07-2556.
Supported by National Institutes of Health grant CA78792. G.D. is supported by The Methodist Hospital Foundation award and is the recipient of a Chao Scholar grant. S.G. is the recipient of a Doris Duke Clinical Scientist Development Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Tatiana Goltsova for technical support in FACS analysis. E.B. thanks the Comitato Maria Letizia Verga per lo studio e la cura della leucemia del bambino (Monza, Italy) and the Fondazione Cassa di Risparmio di Genova ed Imperia—Associazione Cristina Bassi contro le leucemie acute dell'adulto (Genova, Italy) for their valuable support.

![Figure 2. hCD40L/hOX40L-expressing B-CLL cells efficiently expand autologous T cells. (A) Growth rate of viable autologous T cells stimulated with each different B-CLL target (unmanipulated [•], hCD40L- [▴], hOX40L- [▪], or hCD40L/hOX40L-expressing B-CLL cells []). At each time point, the mean + SD cell number for all 7 donors tested is represented. Cells were counted by trypan blue exclusion. At week 4, no restimulation was performed and no exogenous IL-2 was added. (B) Effect of a CD134/Fc human recombinant protein with hOX40L-blocking activity added to hCD40L/hOX40L-expressing B-CLL cells (• 3 donors tested; mean ± SD) compared with CD40L/OX40L combination (▪) and CD40L alone (). The 3 donors tested in this experiment presented a normal profile of CD4-CD8-CD56- cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/6/10.1182_blood-2004-07-2556/6/m_zh80060575670002.jpeg?Expires=1767897455&Signature=ucqNfL~X2BOMoYHif9F-~jv6~11xMA~dzQXVRfu3dIq2T-QOypGk9WZtmF7vhw1qad9~AI4MJl0pkO-kDhC1eNzqyyGtHHfDrZV4vg0H~qY2gQOIO3yxw6c-FVUZn6TfrKKTTN9YD7ABTgfdJOnSiCMKdDJecPOhSagTNvMQaBlb5EqboKHyYbkgdZZaa~bKdbTLGYacjOeQN4vXavEHm9HS1qYaltuKFF2ylqi8obzwyVO799z~3-0cksAe0Hm5VxumX3BhyOmHD4aDNp0OJL6xPUWbEcVkHqduKlN1QcScvGQf5dM2GcVp04EBDKXVB2ZfEUPbl8heJ5jK8POhfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. hCD40L/hOX40L-expressing B-CLL cells efficiently expand autologous T cells. (A) Growth rate of viable autologous T cells stimulated with each different B-CLL target (unmanipulated [•], hCD40L- [▴], hOX40L- [▪], or hCD40L/hOX40L-expressing B-CLL cells []). At each time point, the mean + SD cell number for all 7 donors tested is represented. Cells were counted by trypan blue exclusion. At week 4, no restimulation was performed and no exogenous IL-2 was added. (B) Effect of a CD134/Fc human recombinant protein with hOX40L-blocking activity added to hCD40L/hOX40L-expressing B-CLL cells (• 3 donors tested; mean ± SD) compared with CD40L/OX40L combination (▪) and CD40L alone (). The 3 donors tested in this experiment presented a normal profile of CD4-CD8-CD56- cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/6/10.1182_blood-2004-07-2556/6/m_zh80060575670002.jpeg?Expires=1767897456&Signature=fp6DmgH~olBOfxQJhV7ZCRypjethRrPN~CQU9KMZ1WyM1VRhwEiL7DU9mJgKlSwYqNAjh7ra9vCtDjEV1anYsziA8SN3vRmvmWhNvdVEtPHDIr9f6VvdvljK5mrMJozX3S7irXnHWsKLutPVIow9GbJqs4YFraP-thz0j4Um1Afd4n12RGeyIPx7Ndop8QWEPG2WEqBqn~vAfKFjPi5ogqlEF8K83SaZv2PFj8M4QeE6AYCSSS0cgREIO5u0lvVpdNbQ7CpSGA1AGrPpxEJQnmNzHzkQEuOobEhv1sj~xaGNUWQVYapNwDaekRRyIsuLHyyBWGvsKiykup3TCNpqlQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
 ]). At each time point, the mean + SD cell number for all 7 donors tested is represented. Cells were counted by trypan blue exclusion. At week 4, no restimulation was performed and no exogenous IL-2 was added. (B) Effect of a CD134/Fc human recombinant protein with hOX40L-blocking activity added to hCD40L/hOX40L-expressing B-CLL cells (• 3 donors tested; mean ± SD) compared with CD40L/OX40L combination (▪) and CD40L alone (
]). At each time point, the mean + SD cell number for all 7 donors tested is represented. Cells were counted by trypan blue exclusion. At week 4, no restimulation was performed and no exogenous IL-2 was added. (B) Effect of a CD134/Fc human recombinant protein with hOX40L-blocking activity added to hCD40L/hOX40L-expressing B-CLL cells (• 3 donors tested; mean ± SD) compared with CD40L/OX40L combination (▪) and CD40L alone (