Comment on Flierman et al, page 2991
Nonmyeloablative conditioning regimens are the logical and safer approach for both autologous and allogeneic hematopoietic stem cell transplantation for autoimmune diseases.
In the animal model reported by Flierman and colleagues, stable long-term high-percentage donor chimerism (> 95%) without clinical graft-versus-host disease (GVHD) was achieved with a nonmyeloablative stem cell transplantation (NST) regimen of reduced intensity total body irradiation (TBI) and costimulatory blockade with anti-CD40L. The result was durable remission of collagen-induced arthritis (CIA). Consequent with other reports, this animal preclinical study adds support for use of allogeneic NST for autoimmune disorders. CIA is an environmentally induced (initiated by immunizing the immune system to collagen) autoimmune disease. This paper and other recent publications have shown that allogeneic NST-induced mixed chimerism results in remission of both environmentally induced and genetically predetermined spontaneous onset autoimmune diseases.1-3 In addition, clinical trials using allogeneic matched sibling hematopoietic stem cell (HSC) donors in NST protocols have already begun. Recently, a matched sibling NST for the indication of rheumatoid arthritis (RA) resulted in 50% donor mixed chimerism and a 2-year rheumatoid factor–negative remission of RA without GVHD4 (see figure).
The rationale for autologous HSC transplantation for autoimmune diseases is that the disease is not a genetic stem cell defect but rather an environmentally induced disorder. For autoimmune diseases, the goal of an autologous transplant conditioning regimen is, therefore, immune ablation, while myeloablation would be an unwanted side effect. Following NST, autologous HSCs are infused to shorten the duration of conditioning regimen–related cytopenias. Compared with myeloablative HSC transplantation regimens, NST regimens have a lower treatment-related mortality, which in terms of risk/benefit from treatment is a significant advantage for autoimmune diseases that have a lower disease-related mortality than malignancies. Examples of conditioning agents that suppress the immune system without myeloablation include cyclophosphamide, fludarabine, antithymocyte globulin, rituximab, and alemtuzumab. Even traditional myeloablative agents such as busulfan, when administered at reduced doses, may be used without myeloablation. Despite lack of myeloablation, aggressive combinations of NST agents are highly immune suppressive and may result in profound immune suppression and lethal opportunistic infections similar to aggressive myeloablative regimens. Therefore, NST regimens must be tailored for the degree of immune suppression desired. The NST conditioning agents should also be designed for each autoimmune disease in order to avoid further injury to already disease-impaired organs, which the treatment is designed to salvage.5 FIG1
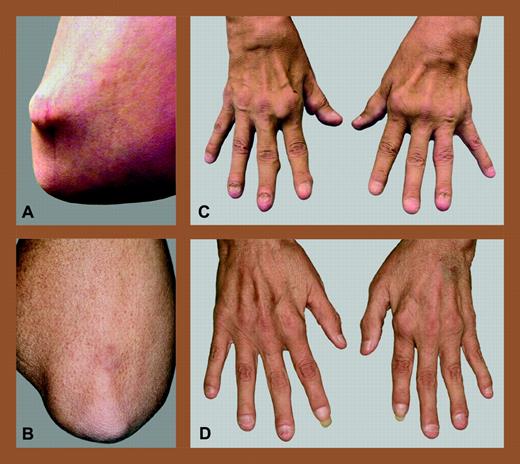
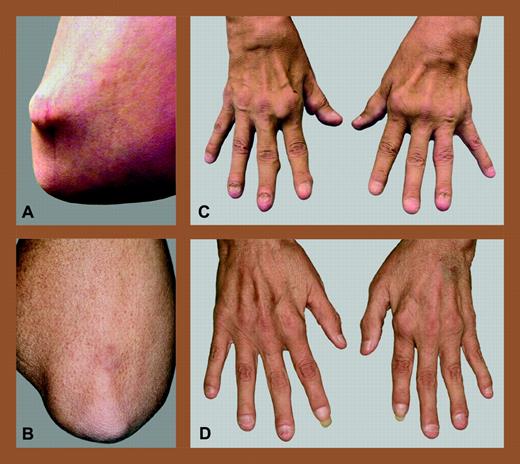
Allogeneic NST for refractory rheumatoid arthritis. Elbow and hands of a patient with refractory RA before (A,C) and after (B,D) allogeneic NST from an HLA-matched sibling. Reprinted from Burt et al4with permission from Arthritis & Rheumatism, copyright (2004).
Allogeneic NST for refractory rheumatoid arthritis. Elbow and hands of a patient with refractory RA before (A,C) and after (B,D) allogeneic NST from an HLA-matched sibling. Reprinted from Burt et al4with permission from Arthritis & Rheumatism, copyright (2004).
Autologous transplantation generally induces disease remission and improved quality of life, but disease relapse may occur. Based on preclinical animal models, allogeneic HSCs appear more likely to cure autoimmune diseases.6,7 Similar to autologous NST, an allogeneic NST regimen will ablate the recipient's immune system but, in addition, the allogeneic HSCs will also alter the genetic predisposition to autoimmune disease recurrence. It is, however, of no benefit to trade one crippling autoimmune disease for GVHD, a common immune-mediated complication of allogeneic transplantation. Allogeneic NST regimens for autoimmune diseases must, therefore, be designed to prevent GVHD. Prevention of GVHD may be accomplished by donor lymphocyte and antigen-presenting cell (APC) depletion and/or costimulatory blockade using methods such as ex vivo CD34+ selection, in vivo depletion of donor lymphocytes with alemtuzumab, or, as demonstrated by Flierman et al, costimulatory blockade with anti-CD40L (ligand) to induce anergy. When combining an NST regimen with donor lymphocyte depletion or costimulatory blockade it is likely that the result will be mixed chimerism (ie, coexistence of both donor and recipient hematopoiesis). This raises several questions. (1) What NST regimen maximizes donor engraftment without clinical GVHD? (2) Is mixed chimerism sufficient to prevent autoimmune disease recurrence? (3) What percentage of donor chimerism is required to prevent autoimmune disease relapse? (4) Is mixed chimerism durable or will either recipient or donor hematopoiesis eventually be rejected? These questions remain unanswered and require further research. ▪