Abstract
Endothelial progenitor cells (EPCs) can be isolated from adult peripheral and umbilical cord blood and expanded exponentially ex vivo. In contrast, human umbilical vein endothelial cells (HUVECs) or human aortic endothelial cells (HAECs) derived from vessel walls are widely considered to be differentiated, mature endothelial cells (ECs). However, similar to adult- and cord blood–derived EPCs, HUVECs and HAECs derived from vessel walls can be passaged for at least 40 population doublings in vitro. Based on this paradox, we tested whether EPCs reside in HUVECs or HAECs utilizing a novel single cell deposition assay that discriminates EPCs based on their proliferative and clonogenic potential. We demonstrate that a complete hierarchy of EPCs can be identified in HUVECs and HAECs derived from vessel walls and discriminated by their clonogenic and proliferative potential. This study provides evidence that a diversity of EPCs exists in human vessels and provides a conceptual framework for determining both the origin and function of EPCs in maintaining vessel integrity.
Introduction
Endothelial progenitor cells (EPCs) can be isolated from adult peripheral and umbilical cord blood (UCB).1-9 EPCs are thought to originate from bone marrow and circulate in peripheral blood.1,2 Experimental evidence to support the use of EPCs for angiogenic therapies or as biomarkers to assess cardiovascular disease risk and progression is compelling.1,2,10-13 Currently, there is no uniform definition of an EPC, which makes interpretation of these studies difficult, and EPCs are primarily defined by expression of cell surface antigens.1,2 However, a hallmark of stem and progenitor cells is their ability to proliferate and give rise to functional progeny,14 and progenitor cells are identified by their clonogenic potential.14 Based on these principles, we recently developed a single cell clonogenic assay to define a novel hierarchy of EPCs based on their proliferative and clonogenic potential.15
In contrast to EPCs, human umbilical vein endothelial cells (HUVECs) and human aortic endothelial cells (HAECs), which are derived from vessel walls, are considered to be differentiated, mature endothelial cells (ECs) and are used as “controls” for EPC studies.4,5,16,17 However, similar to adult- and UCB-derived EPCs, HUVECs and HAECs can be passaged for at least 40 population doublings.4,16,17 Given that HUVECs and HAECs display similar growth kinetics to EPCs, we tested whether EPCs reside in vessel walls using single cell assays to test the proliferative and clonogenic potential of single HUVECs and HAECs.
Study design
Umbilical cord blood samples
Human UCB samples from term newborns were collected as previously described.15 The Institutional Review Board at the Indiana University School of Medicine approved all protocols, and informed consent was obtained from all adult donors and parents of newborns.
Buffy coat cell preparation of umbilical cord blood samples
Mononuclear cells (MNCs) were isolated as previously described15 and washed with endothelial growth medium-2 (EGM-2) (Cambrex, Walkersville, MD) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 2% penicillin/streptomycin (Invitrogen, Grand Island, NY), and 0.25 μg/mL amphotericin B (Invitrogen) (complete EGM-2).
Culture of umbilical cord blood endothelial cells
MNCs were seeded onto tissue culture plates precoated with type I rat tail collagen (BD Biosciences, Bedford, MA) in complete EGM-2 at 37°C, 5% CO2 in a humidified incubator and cultured for EC colony growth as previously described.15 EC colonies appeared between 5 and 10 days of culture. The ECs were expanded in complete EGM-2 as previously described.15
Culture of HUVECs and HAECs
Cryopreserved HUVECs and HAECs were obtained from Cambrex (Walkersville, MD) at passage 3. Cells were seeded in 75 cm2 tissue culture flasks precoated with type I rat tail collagen in complete EGM-2 for passage.
Immunophenotyping of endothelial cells
Early-passage (3 to 4) ECs (5 × 105) were incubated with primary or isotype control antibody and analyzed by fluorescence-activated cell sorting (FACS) (Becton Dickinson, San Diego, CA) as previously described.15 We used directly conjugated primary murine monoclonal antibodies (all BD Pharmingen, San Diego, CA, unless indicated) against human CD31–fluorescein isothiocyanate (FITC), human CD14-FITC, human CD45-FITC, human CD146-phycoerythrin (PE), human CD141-FITC (Cymbus Biotechnology, Chandlers Ford, United Kingdom), human CD105-AlexaFluor 647 (Molecular Probes, Eugene, OR), and human CD144-AlexaFluor 647 (Molecular Probes, Eugene, OR). We used directly conjugated mouse immunoglobulin G1κ (IgG1)κ (BD Pharmingen) for isotype controls. We used purified anti–human fibroblast growth factor receptor 1 (FGFR1) antibody (R&D Systems, Minneapolis, MN) with a goat anti–mouse IgG allophycocyanin (APC) secondary antibody (Molecular Probes) and purified mouse IgG2aκ (BD Pharmingen) for an isotype control.
For von Willebrand factor (VWF) and flk-1, cells were permeabilized as previously described15 and incubated with 2 μg/mL anti–human VWF (Dako, Carpinteria, CA) or biotinylated anti–human flk-1 (Sigma, St Louis, MO). The secondary antibodies for VWF and flk-1 were goat antirabbit-FITC and strepavidin-APC (BD Pharmingen), respectively. The primary isotype control antibodies for VWF and flk-1 were rabbit Ig (Dako) and biotinylated mouse IgG1κ (BD Pharmingen), respectively.
Generation of gibbon ape leukemia virus (GALV)–pseudotyped MFG-EGFP and retroviral transduction of ECs
The MFG-EGFP retrovirus vector expressing the enhanced green fluorescent protein (EGFP) under the control of the Moloney murine leukemia virus long terminal repeat was employed as previously described.18 Supernatant from MFG-EGFP clone 5 with a titer of 0.5 × 106 to 1 × 106 infectious units per milliliter was used. ECs were transduced with MGF-EGFP supernatant as previously described and analyzed for EGFP expression by FACS.15
Single cell assays
ECs transduced with the MFG-EGFP retrovirus were sorted by FACS for EGFP expression. The FACS Vantage Sorter (Becton Dickinson) was used to place one single EGFP-positive EC per well of a 96-well tissue culture plate precoated with type I collagen containing 200 μL complete EGM-2. Cells were cultured as previously described.15 At day 14, wells were examined for EC growth. We scored as positive those wells in which 2 or more ECs were identified using a fluorescent microscope. Cells were counted as previously described.15 Wells containing more than 50 cells were subcultured to a 24-well tissue culture plate. On day 7, wells were examined for secondary colony growth or cell confluence.
Telomerase activity assay
Telomerase activity was measured by the telomeric repeat amplification protocol (TRAP) as previously described19 using the TRAP-eze telomerase detection kit (Chemicon, Temecula, CA). Cell lysates from 103 cells were used for each assay. The polymerase chain reaction (PCR) product and a 6 base pair incremental ladder were electrophoresed on a 12.5% nondenaturing polyacrylamide gel and visualized by SYBR gold staining (Molecular Probes).
Fluorescence imaging
Adherent ECs transduced with EGFP were imaged in complete EGM-2 at room temperature. Fluorescence images were collected using a Zeiss Axiovert 2 inverted microscope with a Fix CP-ACHROMAT/0.12 NA objective (Zeiss, Thornwood, NY). Images were acquired using a SPOT RT color camera (Diagnostic Instruments, Sterling Heights, MI) with the manufacturer's software. Composite images were assembled in Adobe Photoshop version 8.0.
Results and discussion
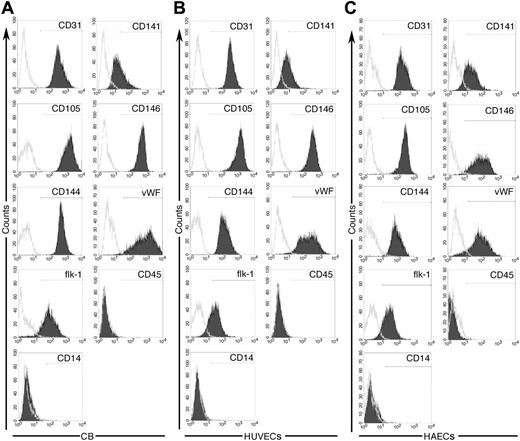
To compare the clonogenic capacity of single ECs derived from EPC colonies and vessel walls, we established monolayers of UCB-derived EPCs, HUVECs, and HAECs as recently described.15 UCB-derived EPCs, HUVECs, and HAECs could be passaged for at least 40 population doublings (data not shown). Cord blood EPCs, HUVECs, and HAECs expressed the EC surface antigens CD31, CD141, CD105, CD146, CD144, VWF, and flk-1 but not the hematopoietic cell surface antigens CD45 and CD14 (Figure 1A-C). Thus, UCB-derived EPCs, HUVECs, and HAECs expressed a similar profile of EC-specific antigens.
Immunophenotypic analysis of cord blood EPC-derived endothelial cells, HUVECs, and HAECs. Immunophenotyping of cell monolayers derived from cord blood EPCs (A), umbilical veins (B), or human aortas (C) by fluorescence cytometry. Cord blood EPC-derived ECs, HUVECs, and HAECs express CD31, CD141, CD105, CD146, CD144, VWF, and Flk-1 but do not express CD45 and CD14. Shown are representative data from 5 independent experiments using 5 different cord blood EC monolayers, 5 different HUVEC samples, and 5 different HAEC samples with similar results. Isotype controls are overlaid in white on each histogram for each surface antigen tested.
Immunophenotypic analysis of cord blood EPC-derived endothelial cells, HUVECs, and HAECs. Immunophenotyping of cell monolayers derived from cord blood EPCs (A), umbilical veins (B), or human aortas (C) by fluorescence cytometry. Cord blood EPC-derived ECs, HUVECs, and HAECs express CD31, CD141, CD105, CD146, CD144, VWF, and Flk-1 but do not express CD45 and CD14. Shown are representative data from 5 independent experiments using 5 different cord blood EC monolayers, 5 different HUVEC samples, and 5 different HAEC samples with similar results. Isotype controls are overlaid in white on each histogram for each surface antigen tested.
Progenitor cells are defined by their clonogenic and proliferative potential.14 The most rigorous test for the clonogenic potential of progenitors is to determine whether a single cell will form a colony in the absence of other cells.14 We recently developed a single cell clonogenic assay to quantitate the clonogenic potential of individual ECs.15 Using this assay, we defined a hierarchy of endopoiesis where single EPCs are discriminated by their clonogenic potential, analogous to the hematopoietic system.15 To test whether HUVECs and HAECs contained EPCs, we compared the clonogenic potential of single HUVECs, HAECs, and cord blood ECs.
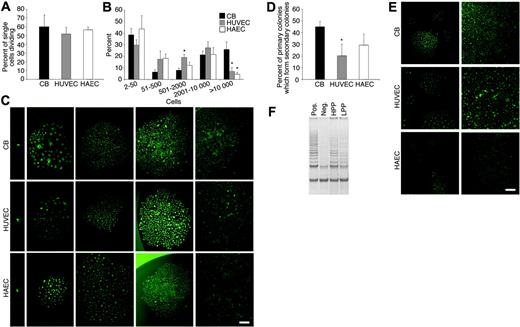
As shown previously, the percentage of single cord blood ECs undergoing at least one cell division was 55% (Figure 2A). Similarly, 52% of single HUVECs and 53% of single HAECs divided after 14 days (Figure 2A). Forty-seven percent of the single cord blood ECs that divided formed well-circumscribed colonies containing more than 2000 cells (Figure 2B). Strikingly, 28% of single HUVECs and 27% of single HAECs that divided formed colonies containing more than 2000 cells. In addition, single cord blood ECs, HUVECs, and HAECs formed smaller colonies, which contained between 50 and 500 cells or less than 50 cells (Figure 2B). We previously described these colonies as low proliferative potential–endothelial colony-forming cells (LPP-ECFCs) and endothelial cell clusters (endo-clusters), respectively.15 Photomicrographs of the clusters and colonies derived from single cord blood ECs, HUVECs, and HAECs are shown in Figure 2C.
Quantitation of the clonogenic and proliferative potential of single cord blood endothelial cells derived from EPC colonies, HUVECs, and HAECs. (A) The percentage of single cord blood (CB) EPC-derived ECs, HUVECs, or HAECs undergoing at least one cell division after 14 days of culture. Results represent the average of 5 independent experiments using single ECs derived from different donors. (B) Number of cell progeny derived from a single CB EPC-derived EC (□), HUVEC (▦), or HAEC (□) in an individual well after 14 days of culture. *P < .01 by Student paired t test for comparison of a single CB-derived EC versus either a single HUVEC or HAEC. (C) Representative photomicrographs (× 50 magnification) of the different EC clusters (less than 50 cells) or colonies (more than 50 cells) derived from a single cord blood EPC-derived EC, HUVEC, or HAEC. Results are representative of 4 other independent experiments utilizing cells from different donors. Scale bar in photomicrographs represents 100 μm. (D) Percent of the cell progeny derived from a single cord blood EPC-derived EC, HUVEC, or HAEC that formed secondary colonies or rapidly grew to cell confluence after 7 days of culture in a 24-well tissue culture plate. Results represent the average ± SEM of 4 independent experiments using cells derived from 4 different donors. *P < .01 by Student paired t test for comparison of a single CB-derived EC versus either a single HUVEC or HAEC. (E) Representative photomicrographs (× 50 magnification) of the secondary EC colonies or confluent cell monolayers derived from the cell progeny of a single plated cord blood EPC-derived EC, HUVEC, or HAEC in a 24-well plate after 7 days in culture. Scale bar in photomicrographs represents 100 μm. (F) Telomerase activity in an HPP-ECFC (HPP) and LPP-ECFC (LPP) colony derived from HUVECs. “Pos” indicates telomerase activity in HeLa cells, which were used as a positive control, and “neg” indicates a negative control. Results are representative of 4 other independent experiments. Similar differences in telomerase activity were observed between HPP-ECFC and LPP-ECFC colonies isolated from cord blood ECs and HAECs (data not shown).
Quantitation of the clonogenic and proliferative potential of single cord blood endothelial cells derived from EPC colonies, HUVECs, and HAECs. (A) The percentage of single cord blood (CB) EPC-derived ECs, HUVECs, or HAECs undergoing at least one cell division after 14 days of culture. Results represent the average of 5 independent experiments using single ECs derived from different donors. (B) Number of cell progeny derived from a single CB EPC-derived EC (□), HUVEC (▦), or HAEC (□) in an individual well after 14 days of culture. *P < .01 by Student paired t test for comparison of a single CB-derived EC versus either a single HUVEC or HAEC. (C) Representative photomicrographs (× 50 magnification) of the different EC clusters (less than 50 cells) or colonies (more than 50 cells) derived from a single cord blood EPC-derived EC, HUVEC, or HAEC. Results are representative of 4 other independent experiments utilizing cells from different donors. Scale bar in photomicrographs represents 100 μm. (D) Percent of the cell progeny derived from a single cord blood EPC-derived EC, HUVEC, or HAEC that formed secondary colonies or rapidly grew to cell confluence after 7 days of culture in a 24-well tissue culture plate. Results represent the average ± SEM of 4 independent experiments using cells derived from 4 different donors. *P < .01 by Student paired t test for comparison of a single CB-derived EC versus either a single HUVEC or HAEC. (E) Representative photomicrographs (× 50 magnification) of the secondary EC colonies or confluent cell monolayers derived from the cell progeny of a single plated cord blood EPC-derived EC, HUVEC, or HAEC in a 24-well plate after 7 days in culture. Scale bar in photomicrographs represents 100 μm. (F) Telomerase activity in an HPP-ECFC (HPP) and LPP-ECFC (LPP) colony derived from HUVECs. “Pos” indicates telomerase activity in HeLa cells, which were used as a positive control, and “neg” indicates a negative control. Results are representative of 4 other independent experiments. Similar differences in telomerase activity were observed between HPP-ECFC and LPP-ECFC colonies isolated from cord blood ECs and HAECs (data not shown).
In hematopoiesis, the most proliferative progenitor that can be cultured in the absence of a stromal cell monolayer is termed the high proliferative potential–colony-forming cell (HPP-CFC).14,20 We recently showed that a similar cell exists in endopoiesis, which we termed the high proliferative potential–endothelial colony-forming cell (HPP-ECFC).15 Single HPP-ECFCs yield macroscopic colonies that form secondary and tertiary colonies upon replating.15 To test whether HUVECs or HAECs contained HPP-ECFCs, the clonal progeny derived from a single cord blood EC, HUVEC, or HAEC were replated and cultured into 24-well plates. Forty-five percent of the clonal progeny of single plated cord blood EPC-derived cells formed secondary colonies or rapidly grew to confluence in 24-well plates (Figure 2D). Twenty percent of the clonal progeny of single plated HUVECs and 29% of single plated HAECs formed secondary colonies or became confluent (Figure 2D). A photomicrograph of the secondary EC colonies or confluent cell monolayers derived from the progeny of a single cord blood EC, HUVEC, or HAEC is shown in Figure 2E. Consistent with their high proliferative potential, clonal progeny derived from HPP-ECFCs isolated from cord blood ECs, HUVECs, or HAECs contained higher telomerase activity compared with the progeny derived from LPP-ECFCs (Figure 2F). However, fibroblast growth factor (FGF) receptor expression was not different in the HPP-ECFC colonies compared with the LPP-ECFC colonies (data not shown).
HUVECs and HAECs derived from vessel walls are considered to be differentiated, mature ECs.4,16,17 However, we show that a complete hierarchy of EPCs can be identified in HUVEC and HAEC monolayers and discriminated by their clonogenic potential. These data explain why HUVECs and HAECs can be passaged for at least 40 population doublings given that HPP-ECFCs and LPP-ECFCs exist in HUVEC and HAEC populations. We are now testing whether EPCs also exist in other human adult vessels, which would provide a new conceptual framework for determining both the origin and function of EPCs in maintaining vessel integrity.
Prepublished online as Blood First Edition Paper, December 7, 2004; DOI 10.1182/blood-2004-08-3057.
Supported by the Division of Neonatal and Perinatal Medicine at the Indiana University School of Medicine.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.