Abstract
CD45, a receptor-type tyrosine phosphatase, is required for interleukin-6 (IL-6)-induced proliferation in human myeloma cells, which express the shortest isoform, CD45RO, but not the longest isoform, CD45RA. Here, we showed that IL-6 induced the translocation of CD45 to lipid rafts in an isoform-dependent manner. In myeloma cells, CD45RO was translocated to lipid rafts more rapidly than CD45RB, but exogenously expressed CD45RA was not translocated. When an IL-6Rα-transfected B-cell line was stimulated with IL-6, CD45RA was not translocated, although CD45RB was. We further confirmed that the translocated CD45 bound to IL-6Rα, Lyn, and flotillin-2, and this was followed by the dephosphorylation of the negative regulatory Tyr507 of Lyn. CD45 also bound to phosphoprotein associated with glycosphingolipid-enriched microdomains (PAGs), which were subsequently dephosphorylated, resulting in the release of C-terminal src kinase (Csk) from lipid rafts. Therefore, these results indicate that a rapid translocation of CD45RO to lipid rafts may be responsible for IL-6-induced proliferation, and that the change from CD45RA to CD45RO confers the ability to respond to IL-6 in human myeloma cells. (Blood. 2005;105:3295-3302)
Introduction
Multiple myeloma is a B-lineage cell malignancy characterized by the accumulation of monoclonal plasma cells in the bone marrow. We and other groups have found that interleukin-6 (IL-6) is a growth factor for human myeloma cells.1-3 IL-6 also plays a crucial role in the onset of plasma cell tumors in mice.4 Myeloma cells isolated from bone marrow aspirates of patients with multiple myeloma show heterogeneity in their phenotypes. Joshua et al5 reported that CD45+ immature myeloma cells had a higher labeling index than CD45- mature myeloma cells. Also, we reported that proliferating myeloma cells are found predominantly in immature (mature plasma cell-1 [MPC-1-] CD49e-) CD45+ cells rather than mature (MPC-1+ CD49e+) CD45- cells.6 Myeloma cell lines responding to IL-6 such as U266 and ILKM2 express CD45, whereas cell lines proliferating independent of IL-6 do not express CD45.7 Furthermore, only the U266 CD45+ subpopulation responded to IL-6 by proliferating; the CD45- subpopulation did not.8 IL-6-responsive proliferation of CD45+ U266 cells requires an active src-family kinase (SFK), Lyn.7 These results suggest that the interaction of CD45 with Lyn is essential for IL-6-induced proliferation in conjunction with the well-studied Janus kinase (JAK)-signal transducer and activator of transcription (STAT), and Ras-mitogen activated protein kinase (MAPK) pathways after the binding of IL-6 to IL-6 receptor alpha (IL-6Rα) and following the recruitment of gp130 in myeloma cells.
CD45, a receptor-type protein tyrosine phosphatase, is ubiquitously expressed in all nucleated hematopoietic cells.9 Genetic defects of CD45 in mice10 and humans11 cause severely combined immunodeficiency that demonstrates the essential role of CD45 in the immune system, especially for the activation and development of lymphocytes. CD45 regulates tyrosine phosphorylation in the signal transduction of the T-cell receptor (TCR) and B-cell receptor (BCR).9-13 It is generally accepted that CD45 is a major regulator of SFKs.13,14 One conventional model holds that when SFKs are phosphorylated at the negative regulatory COOH-terminal tyrosine residue by C-terminal src kinase (Csk), they adopt to the aforementioned closed and inactive conformation. The dominant role of CD45 is to dephosphorylate this negative regulatory tyrosine, setting the stage for antigen receptor signaling to activate SFKs by autophosphorylation.13,15-17
Considering the relationship between CD45 and its substrates, the cellular localization of CD45 and its accessibility to the substrates may define the activity on signaling. In the past few years, the localization of CD45 inside or outside lipid rafts has been an area of intensive research. Lipid raft microdomains represent cholesterol- and glycosphingolipid-enriched dynamic patches in the plasma membrane and organize the plasma membrane into functional units.18 These raft domains act as platforms for conducting a variety of cellular functions, including TCR, BCR, and cytokine signaling.19-21 Several protein families have been reported to modify lipid rafts structurally and functionally, including membrane integral proteins such as caveolins and flotillins, and lipid chain-modified proteins (eg, SFKs and Ras).22 Many reports have shown that CD45 is absent from membrane lipid rafts in T and B lymphocytes before and after antigen stimulation,19,23,24 although some studies have found CD45 to be weakly partitioned into rafts.25-27 These discrepant results might suggest heterogeneity of raft components within cells, and/or variations in raft composition in different cells.
In humans, CD45 is composed of several isoforms that vary in their extracellular region as a result of tissue or cell type-specific alternative splicing of exons 4, 5, and 6.9 Although the requirement of the intracellular PTPase domain of CD45 has been documented,13 the function of the extracellular domain that differs among these isoforms remains a major unresolved issue. Some reports have shown functional differences among isoforms.28-31 Interestingly, the isoform expressed in myeloma cells is CD45RO, whereas normal plasma cells express CD45RA.6 A change of isoform might occur during the progression of myeloma, but its significance remains unclear.
In this paper, we showed that CD45RO and CD45RB could translocate to lipid rafts, while CD45RA could not, in response to stimulation with IL-6 in myeloma cells. We hypothesized that the IL-6-induced translocation to lipid rafts regulates the phosphatase activity of CD45 to induce activation of Lyn and thus the proliferation of myeloma cells, and discussed the meaning of a rapid translocation of CD45RO to lipid rafts in human myeloma cells.
Materials and methods
Cell culture
Human myeloma cell lines, U266, ILKM2, and NOP2, and the Epstein-Barr virus (EBV)-transformed human B-cell line, KUS, were cultured as described.7 U266 CD45+ and U266 CD45- cells were separated with a cell sorter (Epics Elite; Immunotech Coulter, Hialeah, FL) and further cultured independently.
Flow cytometry
Cells were collected, stained with phycoerythrin (PE)-conjugated antibodies, and analyzed with a cell sorter. Antibodies used were as follows: human immunoglobulin G (IgG; 679.1Mc7), CD45RO (UCLH1), CD45RA (2H4) CD126 (M91) (Immunotech Coulter) and CD45RB (MT4[6B6]); BD Biosciences Pharmingen, San Diego, CA).
Primary myeloma cell analysis
Bone marrow mononuclear cells from bone marrow aspirates were obtained from myeloma patients and healthy donors with informed consent according to the Helsinki protocol and Faculty Board of Yamaguchi University School of Medicine, stained with fluorescein isothiocyanate (FITC) anti-CD38 and PC5 anti-CD45 as described previously,6 and applied to the cell sorter. CD38 strongly positive (CD38++) CD45-, or CD38++CD45+ cells were collected, cultured for 48 hours with or without IL-6 (2 ng/mL), and pulse labeled with [3H]-thymidine deoxyribose (TdR, 185 GBq/mmol; Amersham, Buckinghamshire, United Kingdom) for the last 16 hours of culture.
DNA cloning and retrovirus-mediated gene transfer
By reverse-transcriptase-polymerase chain reaction (RT-PCR), CD45 cDNA was amplified from U266 CD45+ and KUS cells. We amplified CD45 cDNA in 2 parts and then cloned each fragment into the vector pCR2.1 TOPO.
The primer pairs used were 5′-TATATTctcgagGGGCACAAGGTGGCAGGATGTATTTGTGGCTTAAACTC-3′ and 5′-TGACAACATAACCATAAACATCC-3′ for the former fragment, and 5′-AAACTGAGAAGGAGAGTGAATGC-3′ and 5′-ggtaccTCCAATGAACCTTGATTTAAG GC-3′ for the latter. The human STAT1 5′-untranslated region (UTR) (underlined) instead of the original sequence was used to enhance the cell surface expression of protein. After DNA sequencing confirmation, the former fragments were digested with XhoI (restriction site: ctcgag) and ApaLI, and the latter fragments were digested with ApaLI and KpnI (ggtacc). The 2 fragments were then subcloned into pEGFP-N1 (Clontech, Palo Alto, CA) between the XhoI and KpnI sites to yield pCD45RO-, CD45RB-, or CD45RA-enhanced green fluorescent protein (EGFP). Then, these CD45-EGFP fusion genes were excised with XhoI (blunt-ended) and NotI (blunt-ended), and cloned into the retroviral vector pQCXIP (Clontech) between the EcoRI (blunt-ended) site. pREP (a gift from Dr Harumi Suzuki, Department of Microbiology, Yamaguchi University School of Medicine) was used as a mock vector, which expresses EGFP and is resistant to puromycin. Cloning of IL-6Rα was conducted as reported previously,32 except that the expression vector was pQCXIN (Clontech). Retrovirus-mediated gene transfer was performed as described in our previous report.32
Isolation of lipid rafts by sucrose density gradient ultracentrifugation
The separation of lipid rafts was performed as described previously with some modification.33 Cells (1 × 108) were washed with ice-cold phosphate-buffered saline (PBS) containing 2 mM Na3VO4 and 2 mM EDTA (ethylenediaminetetraacetic acid), and lysed with 1 mL lysis buffer7 containing 0.5% Triton X-100 or 1% Brij 58 on ice for 30 minutes. After homogenization, the lysates were centrifuged at 800g at 4°C for 10 minutes, and the postnuclear supernatant was mixed with the same volume of 80% sucrose in lysis buffer and overlaid with 2.7 mL of 35% sucrose in buffer followed by 1.6 mL of 5% sucrose in buffer. After ultracentrifugation for 18 hours at 55 000 rpm (212 000g) in a Beckman TY 80Ti rotor (Beckman Coulter, Hialeah, FL), 12 450-μL fractions were collected starting from the top of the gradient.
Western blotting, immunoprecipitation, and dot-blotting
After being starved of IL-6 for 12 hours, cells unstimulated or stimulated with IL-6 (10 ng/mL) for 5 minutes were lysed with the buffer used for sucrose fractionations. The lysates or sucrose fractions were subjected to Western blotting as described.7 For immunoprecipitation, cell lysates were precleared by incubation with protein G agarose beads (Santa Cruz Biotech, Santa Cruz, CA) for 2 hours at 4°C. Sucrose fractions were diluted with lysis buffer containing 1% octyl-glucoside (final sucrose concentration, < 8%) before the preclearing. These precleared lysates were immunoprecipitated by adding 10 μg of specific antibody for 2 hours at 4°C, followed by incubation overnight with protein G agarose beads. Immunoprecipitates were washed with lysis buffer, suspended in sodium dodecyl sulfate (SDS) sample buffer, and subjected to Western blotting. The antibodies used were CD45 (35-Z6), D45RA (4KB5), gp130 (C-20), IL-6Rα (H-300), Lyn (H-6), CD71 (H-300), Csk (C-20), phosphoprotein associated with glycosphingolipid-enriched microdomain (PAG, H-100; Santa Cruz Biotech), antiphosphotyrosine (4G10; Upstate Biotechnology, Lake Placid, NY), CD45RO (OPD4; DAKO, Copenhagen, Denmark), CD45RB (MT4[6G6]), flotillin-2 (29) (BD Biosciences Pharmingen), phospho-Lyn (P-Lyn Tyr507), and phospho-Src family (P-Src Tyr416), which corresponds to P-Lyn at Tyr396 (Cell Signaling Technology, Beverly, MA). To identify the raft-enriched fractions in sucrose gradient fractions, aliquots of each fraction were spotted onto a nitrocellulose membrane, which was subsequently hybridized with horseradish peroxidase-conjugated cholera toxin B (CTB; List Biology Laboratory, Campbell, CA) to label endogenous GM1 ganglioside, and the signal was detected similarly to Western blotting.
Immunofluorescence staining
IL-6-treated or untreated cells were washed with PBS and allowed to attach to poly-l-lysine-coated glass-bottom dishes (Matsunami Glass, Tokyo, Japan) by incubation on ice for 45 minutes (5 × 105 cells/slip). CTB-FITC or CTB-tetramethylrhodamine isothiocyanate (TRITC) conjugate (List Biology Laboratory) was used to label endogenous GM1 ganglioside at 10 μg/mL in PBS with 0.1% bovine serum albumin (BSA). To induce membrane raft patching, CTB-labeled cells were cross-linked by incubation with anti-CTB antibody (1/250 dilution in PBS/0.1% BSA; Calbiochem, San Diego, CA) for 30 minutes on ice and then 20 minutes at 37°C. For immunofluorescence staining, cells attached to glass-bottom dishes were fixed with 3.7% paraformaldehyde in PBS for 20 minutes on ice or 5 minutes at room temperature, and then blocked with 2% BSA/PBS for 10 minutes. Cells were then incubated with primary antibody (1-10 μg/mL in 2% BSA/PBS) for 30 minutes, washed in PBS, and incubated with rhodamine-conjugated anti-mouse or anti-rabbit IgG (Santa Cruz Biotech) for 45 minutes. The primary antibodies used were as follows: CD45 (Immu-19.2), CD126 (M91), CD45RA (2H4; Immunotech Coulter), CD45RB (MT4[6B6]), CD71 (M-A712; BD Biosciences Pharmingen), and CD45RO (OPD4; DAKO). Fluorescence images were acquired with a laser confocal microscope (LSM510, magnification × 630; numerical aperture of the objective lens, 1.4; camera mode, multi-tracking; acquisition software, LSM510 v2.8, Carl Zeiss, Jena, Germany). To destroy lipid rafts, cells were pretreated with 10 mM methyl-β-cyclodextrin (MCD; Sigma-Aldrich, St Louis, MO) for 30 minutes at 37°C before being treated with IL-6.
Results
CD45+ myeloma cells responded to IL-6 by proliferating
First, we examined the expression profile of CD45 isoforms in bone marrow aspirates of patients with myeloma and healthy donors. Plasma cells are clearly distinguishable from other bone marrow cells in that they express a high level of CD38, but normal plasma cells and myeloma cells have quite different expression patterns for CD45 and CD45 isoforms5,6,34,35 : normal plasma cells express CD45RA and CD45RB but not CD45RO, whereas myeloma consists of CD45-negative (CD45-) and CD45-positive (CD45+) cells expressing CD45RO and CD45RB but not CD45RA (Figure 1A). With regard to the IL-6-induced proliferation, CD45+ myeloma cells responded, but CD45- myeloma cells and CD45+ normal plasma cells did not (Figure 1B); although there exist no significant differences in IL-6Rα (CD126) expression among these cells.2 Therefore, we hypothesized that the expression pattern of CD45 isoforms could determine the proliferative response to IL-6 in these cells.
CD45RO+ myeloma cells responded to IL-6 by proliferating. (A) Expression profiles of CD45 isoforms in myeloma and normal plasma cells from bone marrow aspirate of a representative myeloma patient and healthy donor were analyzed by flow cytometry. CD38++ CD45+ cells (regions a and b) were analyzed with CD45RA, CD45RB, or CD45RO antibody. (B) Incorporation of [3H]-TdR into normal plasma cells (region a in panel A), and CD45+ and CD45- immature myeloma cells (regions b and c) isolated from a healthy donor and myeloma patient, respectively, by the cell sorter in the presence (▪) or absence (□) of IL-6 (n = 5). FS indicates forward scatter; SS, side scatter.
CD45RO+ myeloma cells responded to IL-6 by proliferating. (A) Expression profiles of CD45 isoforms in myeloma and normal plasma cells from bone marrow aspirate of a representative myeloma patient and healthy donor were analyzed by flow cytometry. CD38++ CD45+ cells (regions a and b) were analyzed with CD45RA, CD45RB, or CD45RO antibody. (B) Incorporation of [3H]-TdR into normal plasma cells (region a in panel A), and CD45+ and CD45- immature myeloma cells (regions b and c) isolated from a healthy donor and myeloma patient, respectively, by the cell sorter in the presence (▪) or absence (□) of IL-6 (n = 5). FS indicates forward scatter; SS, side scatter.
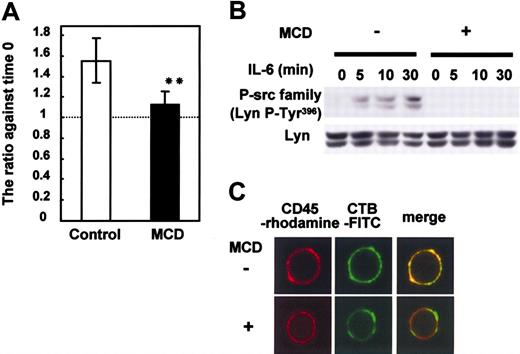
Lipid rafts were required for IL-6-induced proliferation and CD45-Lyn pathway
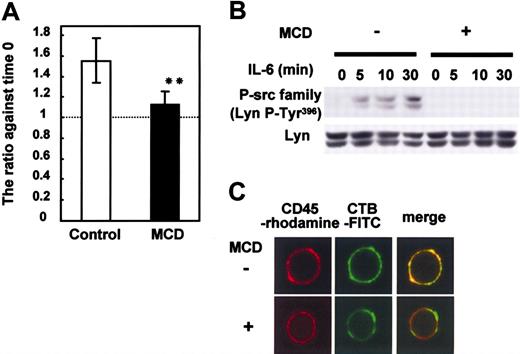
To characterize the role of lipid rafts in the IL-6-mediated proliferation and in the signaling of CD45 in myeloma cells, we treated U266 CD45+ cells that proliferate in response to IL-67,8 with MCD, a lipid raft inhibitor that depletes cholesterol and destroys lipid rafts. As expected, MCD treatment significantly inhibited the IL-6-induced proliferation of U266 (Figure 2A). Then, we checked the activation of SFK as a downstream molecule of the CD45 signaling pathway. The phosphorylation of Lyn Tyr396 at the kinase domain, which correlates with increased kinase activity,9,15 was greatly diminished in MCD-treated cells (Figure 2B). As MCD inhibited the activation of SFK, we checked the possibility that CD45 localized in lipid rafts in the presence of IL-6 by immunostaining. CD45 colocalized with lipid rafts as represented by merged yellow spots throughout the cell membrane (Figure 2C). MCD treatment destroyed the colocalization. Thus, we concluded that the IL-6-mediated proliferation signaling relevant to CD45 in myeloma cells depended on the presence of intact lipid rafts.
Lipid rafts are required for IL-6-induced proliferation and the CD45-Lyn pathway. U266 CD45+ cells starved of IL-6 for 12 hours were preincubated with or without 10 mM MCD for 30 minutes and then stimulated with IL-6 (10 ng/mL). (A) Cells cultured with or without IL-6 were collected after 24 hours, and live cells were enumerated with a cell sorter. Data are shown as a ratio compared with before stimulation. □ indicates control (no MCD); ▪, MCD. The data are expressed as the mean ± SD from 3 different experiments, and ** indicates P < .01 compared with no MCD treatment. (B) Cells stimulated with IL-6 were recovered at 5, 10, and 30 minutes. Anti-P-Src family (Tyr416) was used to detect active Src by immunoblotting. Immunoblotting of Lyn confirmed equal protein loading. (C) Cells pretreated with or without 10 mM MCD were stimulated with IL-6. After being fixed, cells were stained with FITC-conjugated CTB and anti-CD45 followed by rhodamine-conjugated anti-mouse IgG. Representative images obtained by LCM are shown.
Lipid rafts are required for IL-6-induced proliferation and the CD45-Lyn pathway. U266 CD45+ cells starved of IL-6 for 12 hours were preincubated with or without 10 mM MCD for 30 minutes and then stimulated with IL-6 (10 ng/mL). (A) Cells cultured with or without IL-6 were collected after 24 hours, and live cells were enumerated with a cell sorter. Data are shown as a ratio compared with before stimulation. □ indicates control (no MCD); ▪, MCD. The data are expressed as the mean ± SD from 3 different experiments, and ** indicates P < .01 compared with no MCD treatment. (B) Cells stimulated with IL-6 were recovered at 5, 10, and 30 minutes. Anti-P-Src family (Tyr416) was used to detect active Src by immunoblotting. Immunoblotting of Lyn confirmed equal protein loading. (C) Cells pretreated with or without 10 mM MCD were stimulated with IL-6. After being fixed, cells were stained with FITC-conjugated CTB and anti-CD45 followed by rhodamine-conjugated anti-mouse IgG. Representative images obtained by LCM are shown.
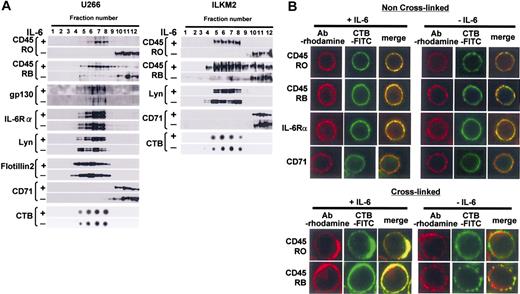
CD45RO and CD45RB translocated to lipid rafts in response to IL-6
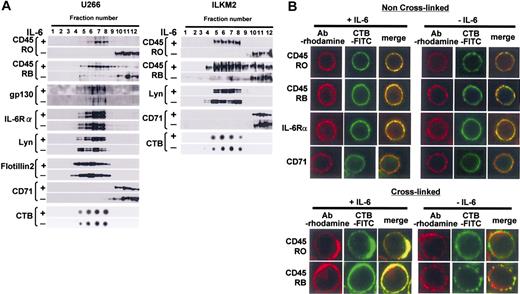
Expression patterns of CD45 isoforms in U266 CD45+ cells and ILKM2 cells are similar to those in primary myeloma cells (data not shown). To investigate the distribution of IL-6 signaling molecules in the cell membrane, cell lysates were separated using discontinuous sucrose gradient ultracentrifugation followed by immunoblotting with specific antibodies. Among light buoyant density fractions, those positive for GM1 gangliosides as identified by CTB binding (around fractions 5-8) were considered to contain lipid rafts (Figure 3A). On the other hand, CD71, which has been used as a nonraft marker,27 was found to be virtually undetectable in these raft-containing fractions, but present in soluble fractions (fractions 10-12). Taken together, it was confirmed that our protocol to separate lipid raft fractions by sucrose density gradient ultracentrifugation was successful. Our results showed that gp130, IL-6Rα, and Lyn were detected in the raft fractions before and after IL-6 treatment, indicating that these proteins constitutively localize to the lipid rafts in U266 CD45+ cells and ILKM2 cells (Figure 3A). Flotillin-2 was also constitutively expressed in lipid rafts as reported previously.36
IL-6 induced translocation of CD45 to lipid rafts. (A) Lysates of U266 CD45+ or ILKM2 cells were separated by sucrose density gradient centrifugation, and continuous fractions (fractions 1 to 12) were examined by immunoblotting. Raft fractions were identified by dot-blotting with horseradish peroxidase-conjugated CTB. (B) For the non-cross-linked condition, cells were fixed and then stained with antibody (Ab) against CD45RO, CD45RB, IL-6Rα, or CD71, and subsequently with rhodamine-conjugated secondary antibody. Lipid rafts were stained with FITC-labeled CTB. Representative images were obtained by LCM. For the cross-linking condition, lipid rafts were pretreated with CTB-FITC followed by anti-CTB monoclonal antibody. Then, treated cells were fixed and stained similarly to panel A except for CTB staining.
IL-6 induced translocation of CD45 to lipid rafts. (A) Lysates of U266 CD45+ or ILKM2 cells were separated by sucrose density gradient centrifugation, and continuous fractions (fractions 1 to 12) were examined by immunoblotting. Raft fractions were identified by dot-blotting with horseradish peroxidase-conjugated CTB. (B) For the non-cross-linked condition, cells were fixed and then stained with antibody (Ab) against CD45RO, CD45RB, IL-6Rα, or CD71, and subsequently with rhodamine-conjugated secondary antibody. Lipid rafts were stained with FITC-labeled CTB. Representative images were obtained by LCM. For the cross-linking condition, lipid rafts were pretreated with CTB-FITC followed by anti-CTB monoclonal antibody. Then, treated cells were fixed and stained similarly to panel A except for CTB staining.
Surprisingly, the distribution of both CD45RO and CD45RB changed markedly in response to IL-6. Without IL-6, CD45RO did not exist in the raft fraction, but a small percentage of CD45RB did. Importantly, IL-6 treatment resulted in a significant translocation of not only all the CD45RO but also most of the CD45RB in lipid rafts, which was mirrored by a drastic decrease in the soluble compartment. The translocation of CD45RO and CD45RB to lipid rafts was confirmed using lysate prepared with a different concentration (0.2%) of Triton X-100, or different detergent (1% Brij 58), and thus this phenomenon was not confined to the particular experimental conditions (data not shown).
Next, we investigated if this phenomenon actually occurred on the cell membrane using immunostaining. As revealed in Figure 3B, without IL-6 stimulation, CD45RO did not colocalize with lipid rafts, although a relatively small portion of CD45RB did as shown by the small yellow region in the cell membrane of the merged image. However, IL-6 induced the colocalization of CD45RO or CD45RB with lipid rafts. Both CD45 and the rafts looked to gather into small and large patches. In order to obtain more information on the colocalization, we performed the cross-linking of lipid rafts by treatment with CTB followed by anti-CTB monoclonal antibody after IL-6 stimulation. In cross-linked cells, we observed a stronger red and green fluorescence, which overlapped considerably in IL-6-treated cells (large coalescent signal) compared with un-cross-linked cells (Figure 3B). On the other hand, IL6Rα, but not CD71, always colocalized with rafts in the presence or absence of IL-6 in un-cross-linked cells. These results correspond exactly to the sucrose density gradient fractionation data. Taken together, our results showed that both CD45RO and CD45RB were translocated to lipid rafts where the other IL-6 signal molecules IL-6Rα, gp130, and Lyn already existed.
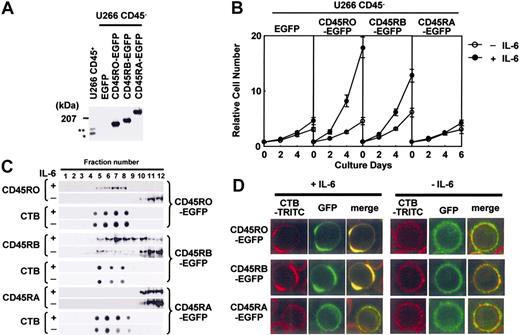
IL-6 induced translocation of exogenously expressed CD45RO- and CD45RB-EGFP, but not CD45RA-EGFP, to lipid rafts
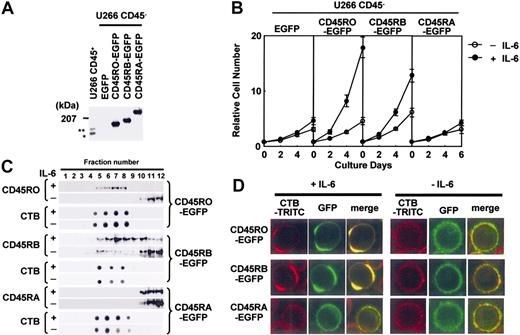
The data shown in the part of translocation of CD45RO and CD45RB translocation to lipid rafts were obtained by observing the movement of endogenous CD45. It is difficult to separate the cell membrane from the membrane compartments inside cells using biochemical methods (fractionation based on detergent-resistant properties followed by Western blotting), and immunostaining is sometimes misleading because of the nonspecific binding of antibodies. Therefore, we made EGFP-tagged CD45RO, CD45RB, or CD45RA, which enabled us to analyze the localization of CD45 without antibody. These constructs were stably expressed in U266 CD45- cells, and analyzed by Western blotting and flow cytometry to confirm the protein expression and isoform-specific expression (Figure 4A; data not shown). U266 cells with CD45RO-EGFP and CD45RB-EGFP (to a lesser extent) but not CD45RA-EGFP or EGFP alone showed enhanced proliferation in response to IL-6, and thus we confirmed the positive role of endogenous CD45RO in the IL-6-induced proliferation (Figure 4B). Sucrose gradient fractions prepared from these CD45-EGFP cells also revealed that a significant percentage of CD45RO- and CD45RB-EGFP moved into lipid raft fractions with IL-6 stimulation, similar to the endogenous CD45 (Figure 4C). Then, U266 CD45RO-, CD45RB-, or CD45RA-EGFP cells were stained with TRITC-labeled CTB followed by cross-linking with anti-CTB antibody, fixed, and observed by LCM. Before IL-6 treatment, lipid rafts and CD45-EGFP distributed throughout the plasma membrane, but were located in different regions as indicated by the absence of yellow. After IL-6 stimulation, a substantial fraction of CD45RO- or CD45RB-EGFP but not CD45RA-EGFP was associated with lipid rafts (Figure 4D). These results indicated that both CD45RO- and CD45RB-EGFP were translocated to lipid rafts in the cell membrane, which was consistent with the movement of endogenous CD45 in response to IL-6. Our results also suggested that the movement and function of CD45RA after IL-6 stimulation differed from those of CD45RO or CD45RB in the exogenously expressed system.
Exogenously expressed CD45RO- and CD45RB-EGFP translocated to lipid rafts in response to IL-6. (A) Lysates from U266 cells expressing EGFP-tagged CD45RO, CD45RB, or CD45RA at the carboxy terminus were subjected to immunoblotting using anti-CD45 Ab. * and ** indicate endogenous CD45RO and CD45RB, respectively. (B) Cells were inoculated at 104 cells/mL without (○) or with IL-6 (•) and counted at 0, 2, 4, and 6 days using the cell sorter. Values shown in these figures are the mean ± 1 SD. (C) Cells were subjected to sucrose density gradient ultracentrifugation followed by immunoblotting with the antibodies indicated. (D) Lipid rafts were cross-linked as in Figure 3B to clearly show the colocalization of CD45-EGFP and lipid rafts. The location of lipid rafts was visualized using TRITC-labeled CTB, and CD45 could be observed by EGFP fluorescence.
Exogenously expressed CD45RO- and CD45RB-EGFP translocated to lipid rafts in response to IL-6. (A) Lysates from U266 cells expressing EGFP-tagged CD45RO, CD45RB, or CD45RA at the carboxy terminus were subjected to immunoblotting using anti-CD45 Ab. * and ** indicate endogenous CD45RO and CD45RB, respectively. (B) Cells were inoculated at 104 cells/mL without (○) or with IL-6 (•) and counted at 0, 2, 4, and 6 days using the cell sorter. Values shown in these figures are the mean ± 1 SD. (C) Cells were subjected to sucrose density gradient ultracentrifugation followed by immunoblotting with the antibodies indicated. (D) Lipid rafts were cross-linked as in Figure 3B to clearly show the colocalization of CD45-EGFP and lipid rafts. The location of lipid rafts was visualized using TRITC-labeled CTB, and CD45 could be observed by EGFP fluorescence.
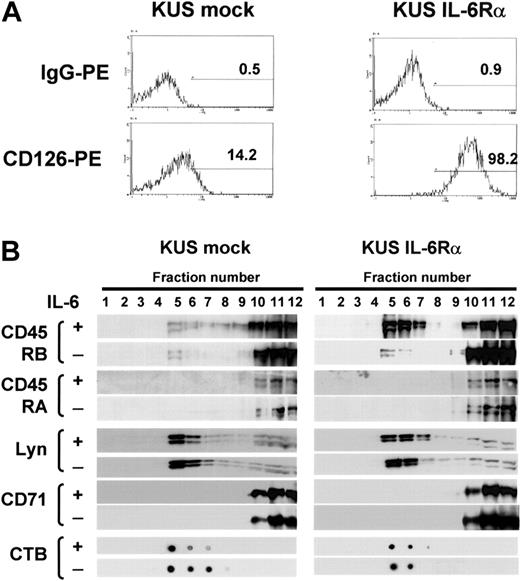
CD45RA, not CD45RB, was unable to translocate to lipid rafts in IL-6Rα-expressing B cells
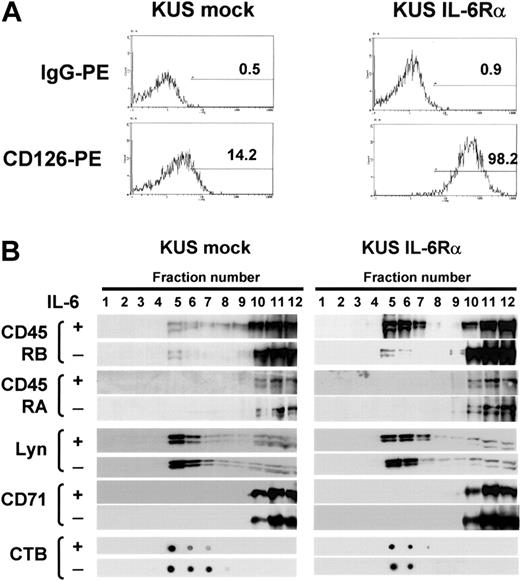
The findings shown in the part of IL-6-induced translocation of exogenously expressed CD45 isoforms raised the possibility that the difference in the translocation of CD45 isoforms might be specific to IL-6 signaling in myeloma cells, and so, we further checked if these isoforms translocate to lipid rafts on IL-6 stimulation in EBV-transformed B cells, KUS. As parent KUS cells expressed IL-6Rα at very low levels (Figure 5A), and the addition of IL-6 did not enhance proliferation, we established IL-6Rα-transfected KUS (KUS IL-6Rα) cells, which respond to IL-6 with greater proliferation than vector-transfected (mock) cells (data not shown). By sucrose density fractionation, neither CD45RA nor CD45RB moved to lipid rafts with the addition of IL-6 in KUS mock cells. However, CD45RB but not CD45RA moved to lipid rafts after IL-6 treatment in KUS IL-6Rα cells, while Lyn always existed on rafts and CD71 always existed outside rafts even in the presence of IL-6 (Figure 5B). These results suggested that the translocation of CD45 in response to IL-6 was different between CD45RA and CD45RB even in a B-cell line, and thus, the inability of CD45RA to translocate to lipid rafts might be a feature of the longest isoform of CD45.
IL-6 induced translocation of CD45RB but not CD45RA to lipid rafts in IL-6Rα-transformed B cells. (A) KUS cells transduced with IL-6Rα (KUS IL-6Rα) or with vector alone (KUS mock) were stained with anti-CD126 PE and analyzed by flow cytometry. The percentage of positive cells relative to the control is given in each histogram. (B) KUS mock and KUS IL-6Rα cells with or without IL-6 treatment were subjected to sucrose density fractionation, followed by immunoblotting with the antibodies indicated.
IL-6 induced translocation of CD45RB but not CD45RA to lipid rafts in IL-6Rα-transformed B cells. (A) KUS cells transduced with IL-6Rα (KUS IL-6Rα) or with vector alone (KUS mock) were stained with anti-CD126 PE and analyzed by flow cytometry. The percentage of positive cells relative to the control is given in each histogram. (B) KUS mock and KUS IL-6Rα cells with or without IL-6 treatment were subjected to sucrose density fractionation, followed by immunoblotting with the antibodies indicated.
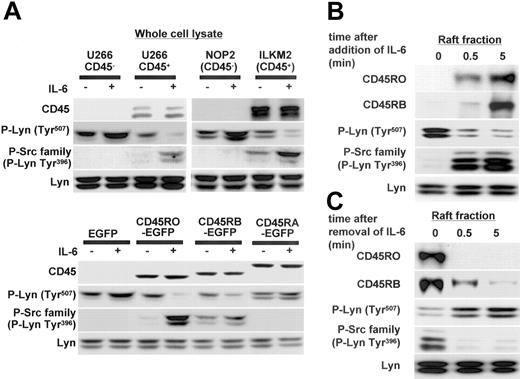
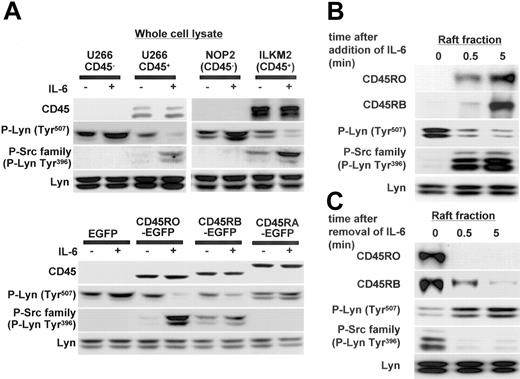
Lyn Tyr507 dephosphorylation and Tyr396 phosphorylation occurred simultaneously with quick translocation of CD45RO to lipid rafts
Lyn is considered to be the major molecule regulated by CD45 in myeloma cells, and our previous report showed that IL-6-induced proliferation of CD45+ U266 cells was significantly suppressed by Lyn-specific antisense oligodeoxynucleotides.7 Here, we further assessed the phosphorylation status of Lyn after IL-6 stimulation (negative regulatory COOH-terminal Tyr507 and positive regulatory Tyr396 in the kinase domain as described in preceding paragraphs). In CD45+ cells (U266 CD45+ and ILKM2), IL-6 induced dephosphorylation of Tyr507, and then Tyr396 was phosphorylated. In CD45- cells (U266 CD45- and NOP2), however, neither Tyr507 dephosphorylation nor Tyr396 phosphorylation of Lyn was observed (Figure 6A). In addition, CD45RO- or CD45RB-EGFP U266 cells had a similar phosphorylation status to CD45+ cells, while CD45RA-EGFP U266 cells were similar to EGFP U266 cells or CD45- cells. Tyr507 dephosphorylation and Tyr396 phosphorylation of Lyn were significantly greater in CD45RO-EGFP than the CD45RB-EGFP cells, indicating a stronger effect of CD45RO on the activation of Lyn. Thus, these results showed that CD45 functioned as an active phosphatase to dephosphorylate the inhibitory site of Lyn, resulting in a triggering of the activation of Lyn in response to IL-6.
Lyn Tyr507 dephosphorylation and Tyr396 phosphorylation occurred simultaneously with the rapid translocation of CD45RO to lipid rafts. (A) U266 CD45-, U266 CD45+, NOP2, ILKM2, U266 EGFP, and U266 CD45RO-, CD45RB-, and CD45RA-EGFP cells stimulated with or without IL-6 (10 ng/mL) for 5 minutes were collected and whole-cell lysates were subjected to immunoblotting with antibodies against CD45, P-Lyn Tyr507, and the P-Src family (Tyr416). Control blots were performed with antibodies against Lyn. (B) U266 CD45+ cells starved of IL-6 for 12 hours were stimulated with 10 ng/mL IL-6 for 0.5 and 5 minutes, and then samples of the raft fraction from each point were immunoblotted with antibodies against CD45RO, CD45RB, P-Lyn Tyr507, the P-Src family, and Lyn. (C) U266 CD45+ cells cultured with IL-6 were starved of IL-6 by replacing the medium using centrifugation, and the cells refed with culture medium were recovered at 0.5 and 5 minutes. Samples of the raft fraction from each point were immunoblotted using the same antibodies as in panel B.
Lyn Tyr507 dephosphorylation and Tyr396 phosphorylation occurred simultaneously with the rapid translocation of CD45RO to lipid rafts. (A) U266 CD45-, U266 CD45+, NOP2, ILKM2, U266 EGFP, and U266 CD45RO-, CD45RB-, and CD45RA-EGFP cells stimulated with or without IL-6 (10 ng/mL) for 5 minutes were collected and whole-cell lysates were subjected to immunoblotting with antibodies against CD45, P-Lyn Tyr507, and the P-Src family (Tyr416). Control blots were performed with antibodies against Lyn. (B) U266 CD45+ cells starved of IL-6 for 12 hours were stimulated with 10 ng/mL IL-6 for 0.5 and 5 minutes, and then samples of the raft fraction from each point were immunoblotted with antibodies against CD45RO, CD45RB, P-Lyn Tyr507, the P-Src family, and Lyn. (C) U266 CD45+ cells cultured with IL-6 were starved of IL-6 by replacing the medium using centrifugation, and the cells refed with culture medium were recovered at 0.5 and 5 minutes. Samples of the raft fraction from each point were immunoblotted using the same antibodies as in panel B.
Although CD45+ myeloma cells express both CD45RO and CD45RB, a functional difference seemed to exist between them. Thus, we further compared the kinetics of translocation to rafts between CD45RO and CD45RB in U266 CD45+ cells. The results revealed that the translocation of CD45RO was more rapid and reached a maximum at 5 minutes after IL-6 stimulation, while maximal CD45RB accumulation in the rafts was found to occur after 15 minutes of stimulation (Figure 6B; data not shown). Lyn Tyr507 phosphorylation was quickly decreased and Tyr396 phosphorylation was increased even 0.5 minute after IL-6 treatment, consistent with the translocation of CD45RO to lipid rafts. In addition, we analyzed the effect of removing IL-6 on the translocation of CD45RO or CD45RB in U266 CD45+ cells. Figure 6C showed that CD45RO was rapidly excluded from rafts after the removal of IL-6 even at 0.5 minute, while CD45RB showed a slower exclusion. Tyr507 phosphorylation of Lyn quickly recovered, and Tyr396 phosphorylation decreased with the removal of IL-6 synchronously to the exclusion of CD45RO from lipid rafts (Figure 6C). Therefore, these results suggested that CD45RO might confer some advantage to myeloma cells by its quick translocation in response to IL-6.
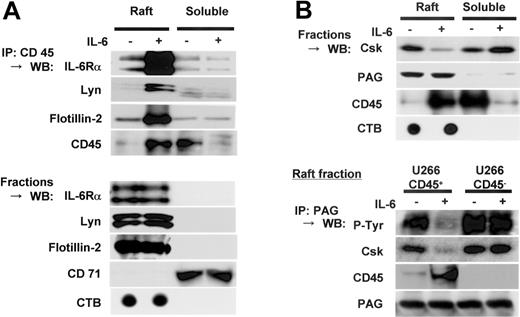
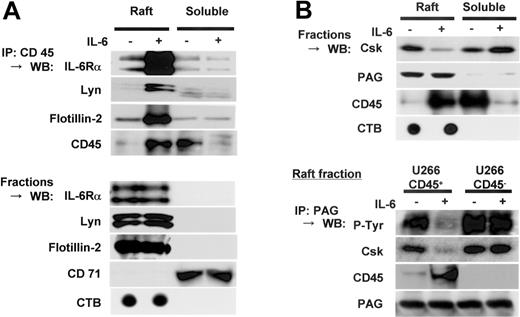
Translocated CD45 complexed with IL-6Rα, Lyn, Flotillin-2, and PAG in lipid rafts
Since our previous report indicated the formation of a complex of CD45 with Lyn,7 we examined if CD45 associated with IL-6Rα, Lyn, and flotillin-2 in lipid rafts in response to IL-6. IL-6Rα, Lyn, and flotiilin-2 were coprecipitated with CD45 only in the raft fractions (Figure 7A). Simple Western blotting data showed the constitutive localization of these proteins in lipid rafts. Thus, these results indicated that CD45 translocated to lipid rafts after IL-6 stimulation and then formed a complex with IL-6Rα, Lyn, and an integral raft protein, flotilin-2.
Translocated CD45 complexed with IL-6Rα and Lyn, and PAG in lipid rafts. (A) U266 CD45+ cells starved of IL-6 for 12 hours were treated (+) or not treated (-) with IL-6 (10 ng/mL) for 5 minutes and separated by sucrose fractionation. The fractions of lipid rafts or nonrafts were mixed and immunoprecipitated with anti-CD45 antibody after adding 1% octyl-glucoside. Precipitated protein was immunoblotted with antibodies against IL-6Rα, Lyn, flotillin-2, or CD45. Control blots were performed using the raft or nonraft mixture directly with the antibodies indicated. (B) Fractions of cell lysates prepared as in panel A were subjected to immunoblotting with antibodies against Csk, PAG, and CD45. Then, 1% octyl-glucoside-added lipid raft fractions were immunoprecipitated with anti-PAG antibody. Precipitated proteins were immunoblotted with antibodies against phosphotyrosine 4G10 (detects phosphorylated PAG according to the size of the band), Csk, CD45, and PAG. IP indicates immunoprecipitation; WB, Western blot.
Translocated CD45 complexed with IL-6Rα and Lyn, and PAG in lipid rafts. (A) U266 CD45+ cells starved of IL-6 for 12 hours were treated (+) or not treated (-) with IL-6 (10 ng/mL) for 5 minutes and separated by sucrose fractionation. The fractions of lipid rafts or nonrafts were mixed and immunoprecipitated with anti-CD45 antibody after adding 1% octyl-glucoside. Precipitated protein was immunoblotted with antibodies against IL-6Rα, Lyn, flotillin-2, or CD45. Control blots were performed using the raft or nonraft mixture directly with the antibodies indicated. (B) Fractions of cell lysates prepared as in panel A were subjected to immunoblotting with antibodies against Csk, PAG, and CD45. Then, 1% octyl-glucoside-added lipid raft fractions were immunoprecipitated with anti-PAG antibody. Precipitated proteins were immunoblotted with antibodies against phosphotyrosine 4G10 (detects phosphorylated PAG according to the size of the band), Csk, CD45, and PAG. IP indicates immunoprecipitation; WB, Western blot.
Then we assessed the movement of Csk and PAG, because Csk is considered to be recruited to lipid rafts and activated by binding to the transmembrane adaptor protein PAG.37,38 In human resting T cells, PAG is reported to be constitutively phosphorylated at Tyr314, which causes Csk binding. Upon T-cell activation, rapid dephosphorylation of PAG induces the dissociation of Csk,38 followed by activation of src. Following these findings, we hypothesized that CD45 attributes to the dephosphorylation of PAG and dissociation of Csk from lipid rafts in myeloma cells. In accordance with previous reports in T cells,37 we confirmed by immunoblotting that almost all PAG was present in lipid rafts of U266 CD45+ cells, regardless of IL-6 stimulation (Figure 7B, upper panel). Importantly, a significant amount of Csk was found in lipid rafts in the absence of IL-6, but IL-6 stimulation caused Csk to be extruded from lipid rafts, in contrast to the movement of CD45. Because CD45 bound to its substrate Lyn in myeloma cells, we further checked if CD45 would also bind to PAG in lipid rafts. Anti-PAG antibody immunoprecipitation of raft fractions followed by immunoblotting showed that PAG was tyrosine phosphorylated and Csk was coprecipitated with PAG in both CD45+ and CD45- cells before IL-6 treatment. However, PAG was coprecipitated with CD45 accompanied by decreased PAG tyrosine phosphorylation and decreased coprecipitation with Csk only in CD45+ cells after IL-6 stimulation (Figure 7B, lower panel). These results suggested that CD45 acted as a PAG phosphatase for translocation to lipid rafts and the formation of a complex in response to IL-6.
Discussion
In this paper, we tried to clarify the meaning of the presence of CD45 and difference in the expression of CD45 isoforms in human myeloma cells. We found that in response to IL-6, CD45RO as well as CD45RB, but not CD45RA, was translocated to lipid rafts in human myeloma cell lines and an IL-6Rα-transfected B-cell line by immunostaining and sucrose density ultracentrifugation. Furthermore, IL-6 induced a more rapid translocation of CD45RO to lipid rafts than CD45RB, and the translocated CD45 functioned as a positive regulator of proliferation signaling by triggering dephosphorylation of Lyn Tyr507 on lipid rafts, where IL-6Rα, gp130, and Lyn were constitutively located. In addition, removal of IL-6 induced the movement of CD45 from inside to outside lipid rafts. This is the first report that IL-6 treatment induced the translocation of CD45 molecules in B-lineage cells, and this movement was quite different from that on BCR stimulation of B cells (data not shown; Cheng et al24 ). The BCR complex is reported to localize outside lipid rafts without stimulation, and ligand (antigen)-to-receptor interaction first occurs outside lipid rafts where CD45 exists, followed by a rapid translocation of BCR to lipid rafts without CD45. By contrast, both IL-6Rα and gp130 constitutively localize in lipid rafts even before IL-6 stimulation, and ligand (IL-6)-to-receptor interaction first occurs inside lipid rafts where CD45 is absent, and then induces the translocation of CD45 to lipid rafts. These findings suggest that the translocation occurs to receive the signal from the receptor complex in response to IL-6. In order to provide data supporting such speculation, we are now investigating the mechanism by which CD45 is translocated to lipid rafts in response to IL-6 but not anti-IgM in B-lineage cells, although many issues remain to be clarified.
Another important finding in this paper is that different patterns of translocation among CD45 isoforms were found following IL-6 stimulation. Both CD45RO and CD45RB could translocate to lipid rafts, but CD45RA could not in response to IL-6 in myeloma cell lines and B-cell lines. With regard to the structure of CD45 isoforms, there is a difference in the length and amount of glycosylation within the extracellular stretch of these isoforms, while all these isoforms have the same transmembrane and intracellular domains. CD45 exons 4, 5, and 6 code for multiple glycosylation sites; CD45RA is the longest isoform with all the glycosylated stretch. It is likely that glycosylation of the extracellular domain determines the ability for lipid raft translocation and changes the phosphatase activity of the intracellular domain as a consequence. Glycosylation of CD45 is reported to regulate the formation of clusters induced by galectin-1 binding and resultant phosphatase activity.39 Also, we cannot exclude the possibility of a different complex with other proteins, different substrate binding, or different type of dimeric formation. Recent reports showed that CD45RO dimerized more efficiently and rapidly than CD45RA in T cells,31,40,41 but dimerization of CD45 molecules was supposed to lose phosphatase activity because of the covertures of phosphatase domain by the inhibitory edge of its counterpart. In our IL-6 stimulation system, the dimeric formation of CD45RO followed by a resultant loss of phosphatase activity is not likely to occur, because the translocated CD45RO functions as active phosphatase inside lipid rafts. On the other hand, expression of CD45RO is found on myeloma cells but not on normal plasma cells. Recently, the regulatory factors responsible for the alternative splicing of CD45 have been identified; serine/arginine-rich splicing factors (SR protein) such as SRp20, SC35, SRp30c, SRp40, and SRp55 are strong candidates.42,43 Further studies of these SR proteins will show how CD45RO expression is obtained in myeloma cells by alternative splicing of the CD45 transcript. On the basis of these reports, we speculate that the IL-6-induced alteration from CD45RA to CD45RO may be related to myeloma oncogenesis, which requires IL-6-induced proliferation of myeloma cells with expression of CD45RO but not CD45RA.
As for the functional role of the translocated CD45 in IL-6 stimulation of myeloma cells, we confirmed the formation of a complex between CD45 and Lyn, decreased phosphorylation of Lyn Tyr507 at the carboxy terminal, and subsequent kinase domain Tyr396 phosphorylation by IL-6. Lyn Tyr507 kinase, Csk, is recruited by PAG, which targets lipid rafts by palmitoylation.37,38 The association of PAG with Csk is controlled by dephosphorylation of PAG. Our data also suggest that PAG dephosphorylation and the resultant release of Csk from lipid rafts occur after IL-6 stimulation in accordance with CD45 translocation as shown in Figure 7. Davidson et al44 reported that CD45 might be a candidate for the tyrosine phosphatase of PAG, and dephosphorylation of PAG allows Csk to be released from PAG. We confirmed that CD45 was directly associated with PAG in lipid rafts after IL-6 stimulation. Thus, our data suggest that IL-6 treatment induces the translocation of CD45 to lipid rafts sequentially, followed by the association of CD45 with Lyn and PAG; dephosphorylation of Lyn Tyr507 and PAG Tyr314; Lyn activation; and Csk release from lipid rafts, where CD45 functions as a positive regulator of signal transduction in myeloma cells, although CD45 is also reported to negatively regulate cytokine activation.45 The exact mechanism by which CD45 translocates to lipid rafts in response to IL-6 remains to be clarified. In this paper, we found that the translocated CD45 bound to flotillin 2 (Figure 7A), which possibly organizes the network of lipid rafts. The function of flotillin and the significance of the complex with CD45 is not clear; however, the report that flotillins form preassembled platforms in hematopoietic cells and these platforms recruit signaling molecules upon activation through lipid rafts46 raises the possibility that flotillins might participate in CD45-recruiting mechanisms to lipid rafts to transduce IL-6 signal from IL-6Rα.
Finally, in view of the therapeutic strategy in multiple myeloma, it may be useful to prevent translocation of CD45, especially CD45RO, to lipid rafts during IL-6 treatment of myeloma cells, as CD45RO is specifically expressed on myeloma cells but not on normal plasma cells, and CD45 is essentially required for IL-6-induced proliferation of myeloma cells. Simply, anti-CD45RO antibody treatment may be effective in the cytoreduction of myeloma cells as shown in a murine model, where a lytic anti-CD45 monoclonal antibody was systemically administered to mice, and marked cytoreduction was observed.47 Thus, we believe that further approaches to CD45RO-targeted therapy will shed light on the incurable nature of multiple myeloma.
Prepublished online as Blood First Edition Paper, December 30, 2004; DOI 10.1182/blood-2004-10-4083.
Supported in part by grants from the Ministry of Education, Science, Sports and Culture of Japan; the Ministry of Health, Labour and Welfare of Japan; the Japan Society for the Promotion of Science; and the Public Trust Japan Leukaemia Research Fund; S. Abroun is a recipient of the Postdoctoral Fellowship Award for Foreign Researchers (PO4500) from JSPS.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Miss Riho Sato for her excellent secretarial assistance and the staff of Science Research Center, Yamaguchi University, for their support.

![Figure 1. CD45RO+ myeloma cells responded to IL-6 by proliferating. (A) Expression profiles of CD45 isoforms in myeloma and normal plasma cells from bone marrow aspirate of a representative myeloma patient and healthy donor were analyzed by flow cytometry. CD38++ CD45+ cells (regions a and b) were analyzed with CD45RA, CD45RB, or CD45RO antibody. (B) Incorporation of [3H]-TdR into normal plasma cells (region a in panel A), and CD45+ and CD45- immature myeloma cells (regions b and c) isolated from a healthy donor and myeloma patient, respectively, by the cell sorter in the presence (▪) or absence (□) of IL-6 (n = 5). FS indicates forward scatter; SS, side scatter.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/8/10.1182_blood-2004-10-4083/6/m_zh80080576950001.jpeg?Expires=1769134475&Signature=JLRL8CP2UV7gwHIFBgF2lkHUU89eMdsfnU69xvxRQkNCTACNBJrfD9PSfZyjk1ytwjmjCU3i3mIRtcGi7JOyaAGK37V3Wb1R0KWuYlyOM-C0TcyCOHLU~ip8vF6yvgAOZluxIgzXkkhb-V9wG8croB7TwSBEJbe7L5fmlSrTN16vETDgMlKsZ8SgsGpUdpuIdOX7JgpdohFsTTsGzW28mVB85TbAgaZCFA4LQbcP0hE4TyZVD2C8mrm2tEIC9eH2CvKsjQV6SJQmGcmzgsXItHfcxpPxtEgWJ1sr33VyVHvfxGxIq7JhgoMKBpFgh7A-clEwvjMKAVuSzbXOSNSQwg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. CD45RO+ myeloma cells responded to IL-6 by proliferating. (A) Expression profiles of CD45 isoforms in myeloma and normal plasma cells from bone marrow aspirate of a representative myeloma patient and healthy donor were analyzed by flow cytometry. CD38++ CD45+ cells (regions a and b) were analyzed with CD45RA, CD45RB, or CD45RO antibody. (B) Incorporation of [3H]-TdR into normal plasma cells (region a in panel A), and CD45+ and CD45- immature myeloma cells (regions b and c) isolated from a healthy donor and myeloma patient, respectively, by the cell sorter in the presence (▪) or absence (□) of IL-6 (n = 5). FS indicates forward scatter; SS, side scatter.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/8/10.1182_blood-2004-10-4083/6/m_zh80080576950001.jpeg?Expires=1769134476&Signature=GTNUziKpRg6vYAZX5hMFPNw5BOw4wTsM2qwV9N21ivBrwLljNzVrEde3pGa9jveELrY583vXulTfSSUJJCthKOC66JpYi5togb0oyBL4TUW7VRvuHFUrLL0KEbJnBbIrA1CO0QhwxEQs8NfSAe6ypkMmjZWKwUNVrFTu4zpgfWrZJUR3ISVo2IP82F9BZw5T0aepoSETY1kej~LuWde1l0YRx-ffJG5IXOuANDJDg8MqHHANz-KG6acxfmtanXY69smKjcDLjoDD45Ja4ospLpZQDeDqdivFIPhEXwJ2AsmuFRWEzpiNL6ar9SAMTo9T4TTQms5ydHHckuI5r~H~gg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)