Abstract
Antiapoptotic members of the bcl-2 family have recently been implicated in the pathogenesis of chronic myeloid leukemia (CML), a hematopoietic neoplasm associated with the BCR/ABL oncogene. We have examined expression of MCL-1 in primary CML cells and BCR/ABL-transformed cell lines. Independent of the phase of disease, isolated primary CML cells expressed myeloid cell leukemia-1 (mcl-1) mRNA and the MCL-1 protein in a constitutive manner. The BCR/ABL inhibitor imatinib (=STI571) decreased the expression of MCL-1 in these cells. Correspondingly, BCR/ABL enhanced mcl-1 promoter activity, mcl-1 mRNA expression, and the MCL-1 protein in Ba/F3 cells. BCR/ABL-dependent expression of MCL-1 in Ba/F3 cells was counteracted by the mitogen-activated protein-kinase/extracellular signal-regulated kinase (MEK) inhibitor, PD98059, but not by the phosphoinositide 3-kinase inhibitor, LY294002. Identical results were obtained for constitutive expression of MCL-1 in primary CML cells and the CML-derived cell lines K562 and KU812. To investigate the role of MCL-1 as a survival-related target in CML cells, mcl-1 siRNA and mcl-1 antisense oligonucleotides (ASOs) were applied. The resulting down-regulation of MCL-1 was found to be associated with a substantial decrease in viability of K562 cells. Moreover, the mcl-1 ASO was found to synergize with imatinib in producing growth inhibition in these cells. Together, our data identify MCL-1 as a BCR/ABL-dependent survival factor and interesting target in CML. (Blood. 2005;105:3303-3311)
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative disease in which leukemic cells exhibit the reciprocal translocation t(9;22) and the bcr/abl oncogene.1-3 The resulting oncoprotein, BCR/ABL, displays constitutive tyrosine kinase activity and activates a number of signaling molecules including RAS/RAF/MAP kinases, the phosphoinositide 3-kinase (PI3-kinase), and signal transducer and activator of transcription 5 (STAT5).4-8 In addition, BCR/ABL converts cytokine-dependent cell lines to growth factor independence and acts oncogenic in mice.9
Several different mechanisms have been implicated in BCR/ABL-dependent growth and accumulation of leukemic cells in CML.10-12 One important mechanism may be inhibition of apoptosis.10,13-16 Thus, a number of antiapoptotic molecules are expressed in CML cells and may contribute to enhanced survival of leukemic cells.10-17 Likewise, it has been shown that CML cells express several members of the BCL-2 family including BCL-2, BCL-xL, or A1.13-17 However, the relative contribution of each of these molecules to inhibition of apoptosis in CML cells has not been defined yet. In addition, some of these molecules may only be expressed in leukemic cells in a subgroup of patients.17
MCL-1 is a well-characterized member of the BCL-2 family that is considered to act antiapoptotic in various neoplastic cells including several leukemia-derived cell lines.18-21 Originally, MCL-1 was described as a survival-enhancing molecule that is expressed during 12-O-tetra decanoyl phorbol 13-acetate (TPA) induced differentiation of leukemic ML-1 cells.18 The CML-derived cell line K562 has also been described to express the MCL-1 protein.19,21-23 However, little is known so far about expression of MCL-1 in primary CML cells, the regulation of expression of MCL-1 in these cells, and the role that this antiapoptotic molecule may play in survival and accumulation of leukemic cells in patients with CML.
In the present study, we have investigated the role of BCR/ABL in expression of MCL-1 in leukemic cells and analyzed underlying signaling pathways. The results of our study show that primary CML cells express MCL-1 in a constitutive manner and that BCR/ABL promotes the expression of MCL-1 through activation of the RAS/RAF/MAP kinase pathway. Moreover, our data show that down-regulation of MCL-1 by antisense oligonucleotides (ASOs) counteracts growth and viability in CML cells. Finally, we show that mcl-1 ASO and STI571 synergize in producing growth inhibition in BCR/ABL-transformed cells.
Patients, materials, and methods
Constructs
Plasmids used in this study were pDCR-ras-G12V24,25 (kindly provided by Yoel Kloog, Hadassah Medical Center, Jerusalem, Israel), pMT-Ha-ras-N1726 (kindly provided by Mark Ewen, Dana Farber Cancer Institute, Boston, MA), and pBSK-mcl-127,28 (kindly provided by Stanley J. Korsmeyer, Dana Farber Cancer Institute). cDNAs were cloned into pcDNA3.1+ vector (Invitrogen, Carlsbad, CA). The mcl-1 reporter gene construct (mcl-1-Luc)29 was a kind gift from Steven W. Edwards (School of Biological Sciences, University of Liverpool, Liverpool, United Kingdom).
Cell lines and culture conditions
Ton.B210-X is an interleukin-3 (IL-3)-dependent Ba/F3 cell line in which the 210-kDa form of BCR/ABL can conditionally be induced through addition of doxycycline (1 μg/mL).9,30 Ton.B210-X cells were grown in RPMI 1640 medium (Mediatech Cellgrow, Herndon, VA) with 10% fetal calf serum (FCS; Gibco, Carlsbad, CA) and 10% WEHI-3B-conditioned medium (as source of murine IL-3) at 37°C and 5% CO2. For starvation, Ton.B210-X cells were cultured in the absence of IL-3 for up to 24 hours. In all promoter activity experiments, Ton.B210-X cells were cultured in serum-free UltraCulture medium (BioWhittaker, Walkersville, MD). The BCR/ABL+ Ba/F3 cell line C7.28,6 in which expression of a dominant-negative STAT5 mutant can be induced by addition of doxycycline, as well as the CML-derived cell lines K562 and KU812 were maintained in RPMI 1640 medium and 10% FCS. In select experiments, an STI571-resistant subclone of K56231 was used.
Isolation and culture of primary cells
After obtaining approval from the local institutional review board of the Department of Internal Medicine I at the Medical University of Vienna, primary leukemic cells were obtained from 4 patients with untreated chronic phase CML, 2 with accelerated phase, and 3 with blast phase of CML. Normal bone marrow cells were obtained from patients with lymphoma (routine staging) without bone marrow involvement (n = 4). Informed consent was obtained prior to bone marrow puncture or blood donation in each case. Peripheral blood mononuclear cells (PBMCs) and bone marrow mononuclear cells (BMMCs) were isolated by density centrifugation using Ficoll.
Treatment with inhibitors
Primary cells or cell lines were incubated with the PI3-kinase inhibitor LY294002 (20 μM), mitogen-activated protein kinase/extracellular signal-regulated kinase (MEK) inhibitor PD9059 (50 μM), rapamycin (20 nM; all from Calbiochem, San Diego, CA), STI571 (60 nM-1.0 μM; kindly provided by Novartis Pharma AG, Basel, Switzerland), or control medium and 10% FCS at 37°C for up to 24 hours. In experiments analyzing promoter activities, cells were kept in serum-free UltraCulture medium without FCS.
mcl-1 reporter gene assay
To determine mcl-1 promoter activity, the mcl-1-Luc construct (12.5 μg) was transfected together with a pCMV-βGAL construct (12.5 μg) by electroporation (0.35 kV, 960 μF, Gene Pulser, Bio-Rad, Hercules, CA) into Ton.B210-X cells (1 × 107 cells in 800 μL). Thereafter, cells were maintained in the presence or absence of doxycycline (1 μg/mL) in serum-free UltraCulture medium for 12 hours. Luciferase activity was determined as described.30 In brief, cells were resuspended in lysis buffer and lysates incubated with luciferase assay buffer and d-luciferin (PharMingen, San Diego, CA). Luciferase activity was determined by an automated luminometer (Wallac 1420 multilabel counter, Perkin Elmer, Turku, Finland). Plasmid pCMV-βGAL (Invitrogen) was used as a reporter for transfection efficiency. To determine β-galactosidase (βGAL) activity, we used the Invitrogen βGAL assay kit (Invitrogen). The mcl-1 reporter gene activity was given as a relative ratio of luciferase activity to βGAL activity. In a separate set of experiments, ras-G12V (8 μg) or ras-N17 (8 μg) was transfected together with mcl-1-Luc (8 μg) and pCMV-βGAL (8 μg) into Ton.B210-X cells.
Northern blot analysis
Total RNA was isolated using Trizol (Invitrogen) according to the manufacturer's instructions. Northern blotting was performed essentially as described.30 In brief, 15 μg total RNA was size-fractionated on 1.0% formaldehyde-agarose gels and transferred to nylon membranes (Hybond N, Amersham, Buckinghamshire, United Kingdom) as described by Chomczynski.32 Membranes were hybridized in rapid-hyb buffer (Amersham). Hybridization was performed with 32P-labeled cDNAs specific for c-abl, mcl-1, or β-actin. Primers for polymerase chain reaction (PCR) amplification of probes were as follows: c-abl: 5′-TGTGTCCCCCAACTACGACAA-3′ (forward) and 5′-CATTGTTTCAAAGGCTTGGTGG-3′ (reverse); murine β-actin: 5′-GACGGCCAGGTCATCACTAT-3′ (forward) and 5′-AGGGAGACCAAAGCCTTC→ ←AT-3′ (reverse); and human β-actin: 5′-ATGGATGATGATATCGCCGCG-3′ (forward) and 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGGGCC-3′ (reverse). The mcl-1 probe was generated by excising the full-length cDNA of mcl-1 from pBSK-mcl-1.27,28 Labeling was performed using the Megaprime kit (Amersham). mRNA expression levels were quantified by densitometry of autoradiograms using E.A.S.Y. Win32 software (Herolab, Wiesloch, Germany).
Western blot analysis and immunocytochemistry
Western blotting was performed (on primary CML cells, K562, KU812, and Ton.B210-X) as described33 using a polyclonal rabbit anti-MCL-1 antibody (S19; Santa Cruz Biotechnology, Santa Cruz, CA), an antiactin antibody (Sigma, St Louis, MO), or anti-BAK antibody (Santa Cruz Biotechnology). Antibody reactivities were made visible by anti-mouse/anti-rabbit IgG antibodies (BM chemiluminescence Western blotting kit, Roche, Penzberg, Germany) and Biomax MS film (Kodak, Rochester, NY). In a separate set of experiments, Western blotting was performed on Ton.B210-X cells and K562 cells using polyclonal rabbit antibodies against phosphorylated MAPK (anti-p44/42 MAP kinase), phosphorylated p70S6 kinase, and phosphorylated AKT (all from Cell Signaling Technology, Beverly, MA), and polyclonal rabbit antibodies against the respective nonphosphorylated proteins—that is, anti-MAPK (Santa Cruz Biotechnology), anti-p70S6-kinase, and anti-AKT (both from Cell Signaling Technology). Prior to Western blotting, cells were kept in RPMI 1640 medium without FCS for 24 hours. Cells were then exposed to control medium or pharmacologic inhibitors, that is, PD98059 (50 μM), LY294002 (20 μM), or rapamycin (20 nM) for 45 minutes. In case of primary cells and K562, cells were also exposed to STI571 (1 μM, 16 hours).
Immunocytochemistry was conducted on cytospin preparations of untreated and doxycycline-treated Ton.B210-X cells as well as primary BMMCs obtained from 4 patients with CML and 4 with normal bone marrow. Immunocytochemical staining was performed as described33 using an antibody against MCL-1 (work dilution 1:200; Santa Cruz Biotechnology) and biotinylated goat ant-rabbit IgG (Biocarta, San Diego, CA). In select experiments, an MCL-1-blocking peptide (Santa Cruz Biotechnology) was applied. Antibody reactivity was made visible using neofuchsin (Nichirei, Tokyo, Japan). In primary CML cells, antibody reactivity was determined by 2 independent observers using the following semiquantitative score: negative (-), positive (+), and strongly reactive (++).
Mcl-1 ASOs and siRNA
K562 cells (STI571-sensitive as well as an STI571-resistant K562 subclone31 ) were transfected with mcl-1 ASO (ISIS 20408; 5′-TTGGCTTTGTGTCCTTGGCG-3′) or with a universal (scramble) control oligonucleotide pool (ISIS 29848). 2′-O-methoxyethyl/2′-deoxynucleotide chimeric phosphorothioate ASOs were provided by ISIS Pharmaceuticals (Carlsbad, CA). Scramble control represents a mixture of A (adenine), G (guanine), T (thymine), and C (cytosine) bases, the resulting preparation containing an equimolar mixture of oligonucleotides. The oligonucleotide chemistry of ISIS 29848 is identical to that of ISIS 20408.34
In separate experiments, an annealed, purified, and desalted double-stranded mcl-1 siRNA (AAGAAACGCGGUAAUCGGACU)35 and a control siRNA against luciferase (CUUACGCUGAGUACUUCGA), both obtained from Dharmacon (Lafayette, CO), were applied.
For transfection, 500 000 cells were seeded in 75-cm2 culture plates at 37°C for 24 hours. ASO and siRNA were complexed with lipofectin reagent (Invitrogen) in RPMI 1640 medium as described by the supplier. K562 cells were incubated with various concentrations of mcl-1 ASO (50-250 nM) or 200 nM siRNA at 37°C for 4 hours. After exposure to mcl-1-targeting compounds, cells were washed and cultured in RPMI 1640 medium with 10% FCS for another 20 hours before being analyzed.
Determination of cell viability and 3H-thymidine uptake
To show that the mcl-ASO leads to induction of apoptosis in CML cells, K562 cells were transfected with mcl-1 ASO or scramble control, or were incubated with lipofectin alone for 24 hours. Cell viability was analyzed by combined annexin V/propidium iodide staining on a flow cytometer (FACSCalibur, Becton Dickinson, Heidelberg, Germany) as described.33 Cell viability was also determined by the trypan blue exclusion test.
To determine potential synergistic inhibitory effects of STI571 and mcl-1 ASO, K562 cells were transfected with mcl-1 ASO (100 nM or 200 nM) or scramble control and were then cultured for 12 hours. Thereafter, cells were exposed to various concentrations of STI571 (50 nM, 100 nM, 200 nM) or control medium for 24 hours. The percentage of apoptotic cells was quantified on Wright-Giemsa-stained cytospin preparations. Apoptosis was defined according to conventional cytomorphologic criteria.36
To investigate antiproliferative effects of mcl-1 ASO and STI571, 3H-thymidine incorporation was determined. For this purpose, K562 cells (untransfected or transfected with mcl-1 ASO or scramble control) were cultured in 96-well microtiter plates (5 × 104 cells/well) in the absence or presence of various concentrations of STI571 (60 nM to 1 μM) for 24 hours. Thereafter, 1 μCi (0.037 MBq) 3H-thymidine was added. Twelve hours later, cells were harvested on filter membranes (Packard Bioscience, Meriden, CT) in a Filtermate 196 harvester (Packard Bioscience). Bound radioactivity was measured in a β-counter (Top-Count NXT, Packard Bioscience). All experiments were performed in triplicate.
STAT5-DNA-binding assay and analysis of dominant-negative STAT5 in C7.28 cells
DNA binding of STAT5 in leukemic cells was analyzed as described37 using extracts of BMMCs obtained from a patient with CML blast crisis and normal BMMCs. Cells were incubated with double-stranded blunt-ended annealed oligonucleotides corresponding to the STAT5-binding site of the bovine β-casein promoter. Oligonucleotides were end-labeled using 32P-γ-adenosine triphosphate (ATP) through T4 polynucleotide kinase to a specific activity of 10 000 cpm/fmol. Normal BMMCs were cultured in control medium, recombinant human (rh) IL-3 (100 ng/mL), or rh granulocyte-macrophage colony-stimulating factor (rhGM-CSF; 100 ng/mL, both from PeproTech (Rocky Hill, NJ) for 30 minutes. For band shift assays, 20 μg BMMC extract was incubated with double-stranded α-32P-labeled DNA probes. The role of STAT5 in BCR/ABL-induced expression of MCL-1 was analyzed in C7.28 cells.6 The mcl-1 promoter activity was determined in these cells as described (see “mcl-1 reporter gene assay”).
Statistical analysis
To determine the level of significance in differences found in the evaluation of data, the paired Student t test was applied. Results were considered to be significantly different with P < .05. To determine synergistic effects of mcl-1 ASO and STI571, combination index values were calculated according to published guidelines38 using commercially available software (Calcusyn; Biosoft, Ferguson, MO).39
Results
Primary CML cells express MCL-1 in a constitutive manner
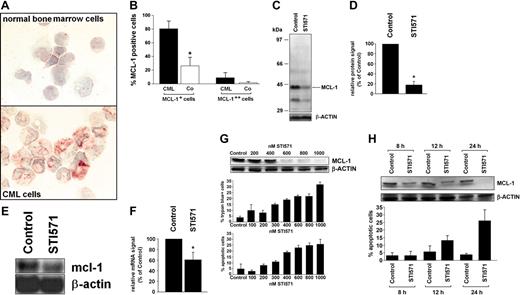
As assessed by immunocytochemistry and Western blotting, isolated primary (unstimulated) CML cells expressed the MCL-1 protein in all patients examined independent of the phase of disease. The levels of immunoreactive MCL-1 were higher in BMMCs in patients with CML compared to normal bone marrow (Figure 1A,B). The CML cell line K562 also expressed immunoreactive MCL-1 by immunocytochemistry. The specificity of the antibody reactivity was demonstrable by applying mcl-1 ASO and an MCL-1-specific blocking peptide. In fact, when treated with mcl-1 ASO, K562 cells showed only weak or no staining with the anti-MCL-1 antibody, and the same was found when the anti-MCL-1 antibody was preincubated with the MCL-1-specific blocking peptide (not shown). In line with our Western blot and immunostaining experiments, isolated primary CML PBMCs expressed mcl-1 mRNA in all patients examined (chronic phase, n = 3; accelerated phase, n = 2; blast phase, n = 1). Exposure of primary CML cells to STI571 (1 μM) resulted in a substantial decrease (to 18% ± 7% of control) in expression of the MCL-1 protein (Figure 1C,D) as well as a decrease in mcl-1 mRNA expression (Figure 1E,F). Corresponding results were obtained with K562 cells (Figure 1G,H). In the CML-derived (Philadelphia chromosome-positive [Ph+]) cell line KU812, STI571 also down-regulated expression of MCL-1, whereas no effect of STI571 on MCL-1 expression was seen in the BCR/ABL- cell lines HL60, KG1, and U937 (data not shown). The effect of STI571 on expression of MCL-1 in K562 cells was dose dependent and associated with a decrease in cell viability and an increased number (percentage) of apoptotic cells (Figure 1G). In time-course experiments, the STI571-induced down-regulation of MCL-1 preceded STI571-induced apoptosis in K562 cells (Figure 1H).
Expression of MCL-1 in primary CML cells and CML-derived cell lines. (A,B) Immunocytochemistry performed with bone marrow mononuclear cells (BMMCs) and an antibody against MCL-1. (A) A typical staining result in normal BMMCs (top) and BMMCs in a patient with CML (bottom). An Olympus BX50 microscope (Olympus, Tokyo, Japan) was used; magnification, × 400; camera, Olympus DP11 (Olympus); software, Adobe Photoshop (Adobe Systems, San Jose, CA). (B) Semiquantitative analysis of immunostaining data (percentage of MCL-1++ cells and of MCL-1+ cells) in patients with CML (n = 4, ▪) and normal BMMCs (n = 4, Co; □); results represent the mean ± SD from 4 donors; *P is less than .05. (C,D) Western blot analysis of expression of MCL-1 in primary CML cells after exposure to STI571 (1 μM) or control medium (control) for 24 hours. Panel C shows a representative experiment with molecular weight markers (kDa) and panel D a densitometric evaluation of MCL-1 expression (relative to β-actin) in primary CML cells with results (given as percent of control) representing the mean ± SD from 3 donors. (E,F) Northern blot analysis of expression of mcl-1 mRNA in primary CML cells exposed to control medium (control) or STI571 (1 μM) for 12 hours. Panel E shows a typical experiment in one donor and panel F a densitometric evaluation of data with results (given as percent of control) representing the mean ± SD from 3 donors; *P < .05. (G,H) Dose-dependent (G) and time-dependent (H) effects of STI571 on expression of the MCL-1 protein (top), cell viability (percent trypan blue-positive cells), and percentage of apoptotic cells (bottom) in K562 cells. Results represent the mean ± SD from 3 independent experiments. MCL-1 protein expression was determined by Western blotting; β-actin served as a loading control.
Expression of MCL-1 in primary CML cells and CML-derived cell lines. (A,B) Immunocytochemistry performed with bone marrow mononuclear cells (BMMCs) and an antibody against MCL-1. (A) A typical staining result in normal BMMCs (top) and BMMCs in a patient with CML (bottom). An Olympus BX50 microscope (Olympus, Tokyo, Japan) was used; magnification, × 400; camera, Olympus DP11 (Olympus); software, Adobe Photoshop (Adobe Systems, San Jose, CA). (B) Semiquantitative analysis of immunostaining data (percentage of MCL-1++ cells and of MCL-1+ cells) in patients with CML (n = 4, ▪) and normal BMMCs (n = 4, Co; □); results represent the mean ± SD from 4 donors; *P is less than .05. (C,D) Western blot analysis of expression of MCL-1 in primary CML cells after exposure to STI571 (1 μM) or control medium (control) for 24 hours. Panel C shows a representative experiment with molecular weight markers (kDa) and panel D a densitometric evaluation of MCL-1 expression (relative to β-actin) in primary CML cells with results (given as percent of control) representing the mean ± SD from 3 donors. (E,F) Northern blot analysis of expression of mcl-1 mRNA in primary CML cells exposed to control medium (control) or STI571 (1 μM) for 12 hours. Panel E shows a typical experiment in one donor and panel F a densitometric evaluation of data with results (given as percent of control) representing the mean ± SD from 3 donors; *P < .05. (G,H) Dose-dependent (G) and time-dependent (H) effects of STI571 on expression of the MCL-1 protein (top), cell viability (percent trypan blue-positive cells), and percentage of apoptotic cells (bottom) in K562 cells. Results represent the mean ± SD from 3 independent experiments. MCL-1 protein expression was determined by Western blotting; β-actin served as a loading control.
BCR/ABL promotes MCL-1 expression in Ton.B210-X cells
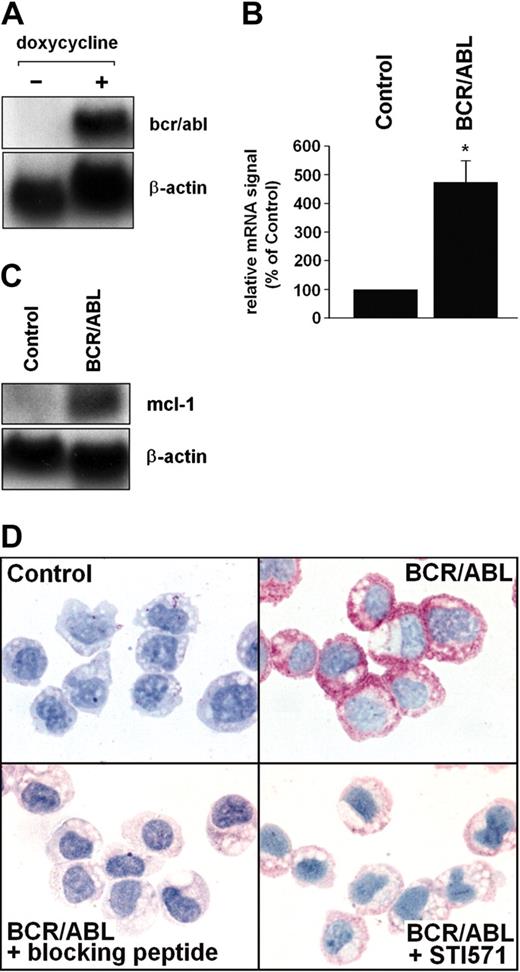
To investigate the role of BCR/ABL in MCL-1 expression, Ton.B210-X cells were examined. In this Ba/F3 cell line, expression of BCR/ABL can be induced by addition of doxycycline (Figure 2A). The doxycycline-induced expression of BCR/ABL in these cells was associated with increased expression of mcl-1 promoter activity (see “BCR/ABL-dependent expression of MCL-1 involves the RAS/RAF/MEK/ERK pathway”) and increased expression of mcl-1 mRNA (Figure 2B,C). Moreover, induction of BCR/ABL in Ton.B210-X cells was associated with enhanced expression of the MCL-1 protein determined by immunocytochemistry (Figure 2D). The BCR/ABL-induced expression of MCL-1 in Ton.B210-X cells was almost completely abrogated by addition of STI571 (1 μM; Figure 2D). The specificity of the antibody-reactivity was demonstrable using an MCL-1-specific blocking peptide (Figure 2D). In control experiments, doxycycline per se did not up-regulate mcl-1 promoter activity or MCL-1 protein expression in (BCR/ABL-) Ba/F3 cells (not shown).
BCR/ABL-dependent expression of MCL-1 in Ton.B210-X cells. (A) Ton.B210-X cells were induced to express BCR/ABL by addition of doxycycline (1 μg/mL, 16 hours). Thereafter, RNA was subjected to Northern blotting. Expression of bcr/abl mRNA was determined using a c-abl-specific cDNA probe. The β-actin loading control is also shown. (B,C) Analysis of mcl-1 mRNA expression in Ton.B210-X cells after induction of BCR/ABL. Panel B shows a typical experiment and panel C a densitometric evaluation of data (corrected for β-actin) with results given as percent of control and representing the mean ± SD of 3 independent experiments; *P < .05. (D) Immunocytochemical detection of MCL-1 in unstimulated Ton.B210-X cells (control), Ton.B210-X cells after exposure to doxycycline (BCR/ABL), BCR/ABL+ Ton.B210-X cells stained with anti-MCL-1 antibody that had been preincubated with MCL-1-blocking peptide (BCR/ABL+ blocking peptide), and BCR/ABL+ Ton.B210-X cells preincubated with STI571 (1 μM) for 24 hours prior to staining with the anti-MCL-1 antibody (BCR/ABL+ STI571). An Olympus BX50 microscope (Olympus, Tokyo, Japan) was used; magnification, × 400; camera, Olympus DP11 (Olympus); software, Adobe Photoshop.
BCR/ABL-dependent expression of MCL-1 in Ton.B210-X cells. (A) Ton.B210-X cells were induced to express BCR/ABL by addition of doxycycline (1 μg/mL, 16 hours). Thereafter, RNA was subjected to Northern blotting. Expression of bcr/abl mRNA was determined using a c-abl-specific cDNA probe. The β-actin loading control is also shown. (B,C) Analysis of mcl-1 mRNA expression in Ton.B210-X cells after induction of BCR/ABL. Panel B shows a typical experiment and panel C a densitometric evaluation of data (corrected for β-actin) with results given as percent of control and representing the mean ± SD of 3 independent experiments; *P < .05. (D) Immunocytochemical detection of MCL-1 in unstimulated Ton.B210-X cells (control), Ton.B210-X cells after exposure to doxycycline (BCR/ABL), BCR/ABL+ Ton.B210-X cells stained with anti-MCL-1 antibody that had been preincubated with MCL-1-blocking peptide (BCR/ABL+ blocking peptide), and BCR/ABL+ Ton.B210-X cells preincubated with STI571 (1 μM) for 24 hours prior to staining with the anti-MCL-1 antibody (BCR/ABL+ STI571). An Olympus BX50 microscope (Olympus, Tokyo, Japan) was used; magnification, × 400; camera, Olympus DP11 (Olympus); software, Adobe Photoshop.
BCR/ABL-dependent expression of MCL-1 involves the RAS/RAF/MEK/ERK pathway
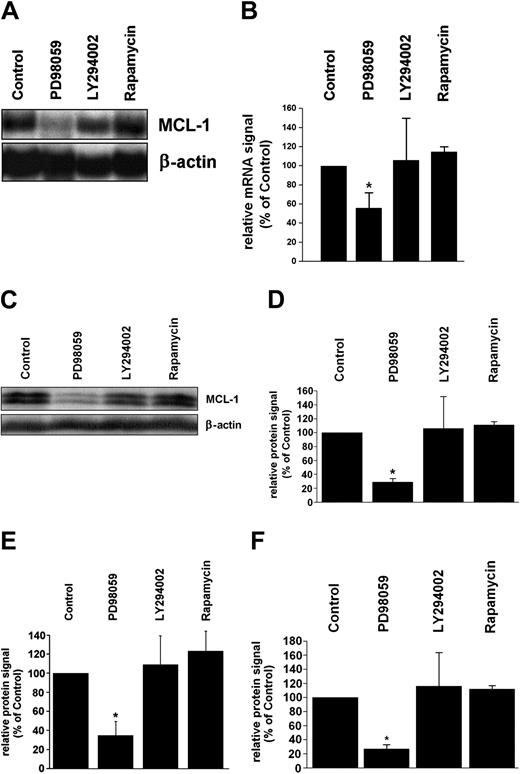
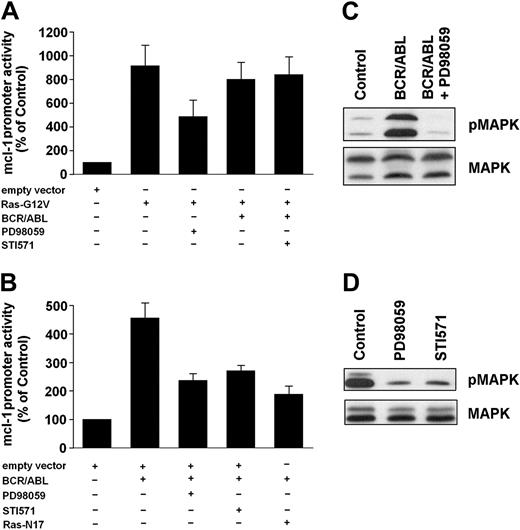
To characterize signal transduction pathways contributing to expression of MCL-1 in leukemic cells, pharmacologic inhibitors of MEK (PD98059), PI3-kinase (LY294002), and of the mammalian target of rapamycin (mTOR), that is, rapamycin, were applied. In primary CML cells, PD98059 down-regulated constitutive expression of mcl-1 mRNA (Figure 3A,B) as well as expression of the MCL-1 protein (Figure 3C,D), whereas no effects were seen with LY294002 or rapamycin. Corresponding results were obtained in K562 cells and KU812 cells. In fact, only PD98059, but not other compounds applied (LY294002, rapamycin), decreased the expression of mcl-1 mRNA (not shown) and expression of the MCL-1 protein in these cells (Figure 3E,F). In a next step, we analyzed the effects of PD98059 on BCR/ABL-induced expression of MCL-1 in Ton.B210-X cells and examined the role of RAS and of the MAP kinases. As expected, PD98059 counteracted BCR/ABL-induced expression of mcl-1 mRNA (not shown) as well as BCR/ABL-induced activation of the mcl-1 promoter in Ton.B210-X cells (Figure 4A). The same effect was seen when cells were incubated with STI571 or were transfected with a dominant-negative ras mutant, ras-N17 (Figure 4A). We next applied a constitutively active ras mutant, ras-G12V. Transient transfection of Ton.B210-X cells with this ras mutant led to a substantial (approximately 8-fold) increase in mcl-1 promoter activity compared to the empty vector (Figure 4B). As expected, the MEK-inhibitor PD98059 suppressed this ras-G12V-induced up-regulation of mcl-1 promoter activity (Figure 4B). Induction of BCR/ABL in ras-G12V-transfected cells did not further increase mcl-1 promoter activity compared to ras-G12V alone (Figure 4B). Interestingly, STI571 failed to counteract mcl-1 promoter activity in BCR/ABL-expressing (doxycycline-induced) Ton.B210X cells in the presence of ras-G12V (Figure 4B), but showed a clear inhibitory effect on BCR/ABL-induced activation of the mcl-1 promoter in Ton.B210-X cells in the absence of ras-G12V (Figure 4A). These data suggest that BCR/ABL signals through RAS to induce MCL-1 expression in Ton.B210-X cells. Finally, we were able to show that doxycycline-induced expression of BCR/ABL in Ton.B210-X cells is associated with phosphorylation of ERK, and that PD98059 counteracts ERK phosphorylation in these cells (Figure 4C) as well as the constitutive phosphorylation of ERK in K562 cells (Figure 4D). By contrast, LY294002 and rapamycin did not inhibit ERK phosphorylation but counteracted phosphorylation of AKT and mTOR, respectively (not shown).
Effects of pharmacologic inhibitors on MCL-1 expression in CML cells. (A,B) Primary CML cells (n = 3) were incubated with PD98059 (50 μM), LY294002 (20 μM), rapamycin (20 nM), or control medium (control) for 12 hours. After incubation, RNA was isolated and subjected to Northern blot analysis using an mcl-1-specific cDNA probe. Panel A shows a representative experiment and panel B a densitometric evaluation of mcl-1 mRNA expression (relative to β-actin loading control); results are given as percent of control (untreated cells). (C,D) Western blot analysis of primary CML cells after incubation with inhibitors (same type and dose as in panel A) for 24 hours. Panel C shows a representative experiment and panel D a densitometric evaluation of MCL-1 protein expression (relative to β-actin). Results represent the mean ± SD from the 3 donors and are given as percent of control (ie, untreated cells). (E,F) Western blot analysis (densitometry corrected for β-actin) of MCL-1 expression in K562 cells (E) and KU812 cells (F) after incubation with inhibitors (PD98059, 50 μM; LY294002, 20 μM; rapamycin, 20 nM) or control medium. Results are given as percent of control (ie, untreated cells) and represent the mean ± SD from 3 independent experiments in each cell line; *P < .05.
Effects of pharmacologic inhibitors on MCL-1 expression in CML cells. (A,B) Primary CML cells (n = 3) were incubated with PD98059 (50 μM), LY294002 (20 μM), rapamycin (20 nM), or control medium (control) for 12 hours. After incubation, RNA was isolated and subjected to Northern blot analysis using an mcl-1-specific cDNA probe. Panel A shows a representative experiment and panel B a densitometric evaluation of mcl-1 mRNA expression (relative to β-actin loading control); results are given as percent of control (untreated cells). (C,D) Western blot analysis of primary CML cells after incubation with inhibitors (same type and dose as in panel A) for 24 hours. Panel C shows a representative experiment and panel D a densitometric evaluation of MCL-1 protein expression (relative to β-actin). Results represent the mean ± SD from the 3 donors and are given as percent of control (ie, untreated cells). (E,F) Western blot analysis (densitometry corrected for β-actin) of MCL-1 expression in K562 cells (E) and KU812 cells (F) after incubation with inhibitors (PD98059, 50 μM; LY294002, 20 μM; rapamycin, 20 nM) or control medium. Results are given as percent of control (ie, untreated cells) and represent the mean ± SD from 3 independent experiments in each cell line; *P < .05.
Role of RAS and MAP kinases in MCL-1 expression in BCR/ABL-transformed cells. (A,B) Ton.B210-X cells were transfected with mcl-1-luc/pCMV-βGal, and in addition with either pcDNA3-ras-N17, pcDNA3-ras-G12V, or empty (pcDNA3) vector. Cells (nontransfected or transfected) were incubated with doxycycline (1 μg/mL) to induce BCR/ABL in the presence or absence of inhibitors, that is, PD98059 (50 μM) or STI571 (1 μM) as indicated, for 16 hours at 37°C in serum-free UltraCulture medium. After incubation, cells were harvested and assayed for luciferase and βGal activities. Luciferase activity was reported as the ratio mcl-1-luc/pCMV-βGal. Results are given as percent of control (ie, empty vector) and represent the mean ± SD of 3 independent experiments. (C) Ton.B210-X cells were grown in serum-free UltraCulture medium in the absence of doxycycline (control) or were induced to express BCR/ABL (BCR/ABL) by addition of doxycycline. After 15 hours, cells were exposed to PD98059, 50 μM (BCR/ABL + PD98059) for 45 minutes. Then, cells were harvested and subjected to Western blotting. Expression of phosphorylated ERK was determined using a pMAPK antibody (top row). After stripping, the membrane was reprobed with a MAPK antibody (bottom row). (D) K562 cells were cultured in serum-free UltraCulture medium containing STI571 (1 μM) for 16 hours, or in UltraCulture medium for 15 hours followed by incubation in PD98059 (50 μM) for 45 minutes. After incubation, cells were harvested and subjected to Western blotting. Expression of phosphorylated ERK (top row) and total ERK (bottom row) was determined using a pMAPK and a MAPK antibody, respectively.
Role of RAS and MAP kinases in MCL-1 expression in BCR/ABL-transformed cells. (A,B) Ton.B210-X cells were transfected with mcl-1-luc/pCMV-βGal, and in addition with either pcDNA3-ras-N17, pcDNA3-ras-G12V, or empty (pcDNA3) vector. Cells (nontransfected or transfected) were incubated with doxycycline (1 μg/mL) to induce BCR/ABL in the presence or absence of inhibitors, that is, PD98059 (50 μM) or STI571 (1 μM) as indicated, for 16 hours at 37°C in serum-free UltraCulture medium. After incubation, cells were harvested and assayed for luciferase and βGal activities. Luciferase activity was reported as the ratio mcl-1-luc/pCMV-βGal. Results are given as percent of control (ie, empty vector) and represent the mean ± SD of 3 independent experiments. (C) Ton.B210-X cells were grown in serum-free UltraCulture medium in the absence of doxycycline (control) or were induced to express BCR/ABL (BCR/ABL) by addition of doxycycline. After 15 hours, cells were exposed to PD98059, 50 μM (BCR/ABL + PD98059) for 45 minutes. Then, cells were harvested and subjected to Western blotting. Expression of phosphorylated ERK was determined using a pMAPK antibody (top row). After stripping, the membrane was reprobed with a MAPK antibody (bottom row). (D) K562 cells were cultured in serum-free UltraCulture medium containing STI571 (1 μM) for 16 hours, or in UltraCulture medium for 15 hours followed by incubation in PD98059 (50 μM) for 45 minutes. After incubation, cells were harvested and subjected to Western blotting. Expression of phosphorylated ERK (top row) and total ERK (bottom row) was determined using a pMAPK and a MAPK antibody, respectively.
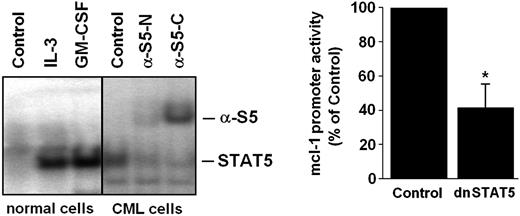
Role of STAT5 in expression of MCL-1 in BCR/ABL+ cells
Recent data suggest that STAT5 is an important signaling molecule in BCR/ABL-transformed cells.6,7 We therefore asked whether STAT5 activation can be detected in primary CML cells and would contribute to MCL-1 expression in leukemic cells. As shown in Figure 5A, normal bone marrow cells displayed active STAT5 in the presence but not absence of IL-3 or GM-CSF, whereas primary CML cells expressed active STAT5 in a constitutive manner. The presence of activated STAT5 in primary CML cells could be reconfirmed by band shift assay (Figure 5A). In a next step, we attempted to define a role for STAT5 activation in MCL-1 expression. As shown in Figure 5B, silencing of STAT5 by a dominant-negative STAT5 mutant (dnSTAT5) resulted in a substantial decrease in mcl-1 promoter activity in C7.28 cells. These data suggest that apart from the RAS/RAF/MEK/ERK pathway, STAT5 activation may also contribute to BCR/ABL-dependent expression of MCL-1 in leukemic cells.
Role of STAT5 in BCR/ABL-dependent expression of MCL-1. (A) Detection of active STAT5 in normal bone marrow cells (left) and primary leukemic cells in a patient with CML by electromobility shift assay (EMSA; right). Normal bone marrow cells were incubated with control medium (control), rhIL-3 (100 ng/mL), or rhGM-CSF (100 ng/mL) for 30 minutes at 37°C before being analyzed. Whole-cell extracts were subjected to EMSA using the STAT5 β-casein response element. Two different antisera for supershift analysis were used (α-S5N, N-terminal STAT5 epitope; α-S5C, C-terminal STAT5 epitope). Normal bone marrow cells displayed active STAT5 only in the presence but not in the absence of IL-3, whereas primary CML cells expressed active STAT5 in a constitutive manner. (B) Expression of a dnSTAT5 mutant in BCR/ABL-expressing C7.28 cells resulted in a substantial decrease in mcl-1 promoter activity. Results represent the mean ± SD from 3 independent experiments; *P < .05.
Role of STAT5 in BCR/ABL-dependent expression of MCL-1. (A) Detection of active STAT5 in normal bone marrow cells (left) and primary leukemic cells in a patient with CML by electromobility shift assay (EMSA; right). Normal bone marrow cells were incubated with control medium (control), rhIL-3 (100 ng/mL), or rhGM-CSF (100 ng/mL) for 30 minutes at 37°C before being analyzed. Whole-cell extracts were subjected to EMSA using the STAT5 β-casein response element. Two different antisera for supershift analysis were used (α-S5N, N-terminal STAT5 epitope; α-S5C, C-terminal STAT5 epitope). Normal bone marrow cells displayed active STAT5 only in the presence but not in the absence of IL-3, whereas primary CML cells expressed active STAT5 in a constitutive manner. (B) Expression of a dnSTAT5 mutant in BCR/ABL-expressing C7.28 cells resulted in a substantial decrease in mcl-1 promoter activity. Results represent the mean ± SD from 3 independent experiments; *P < .05.
Down-regulation of MCL-1 expression counteracts viability in CML cells
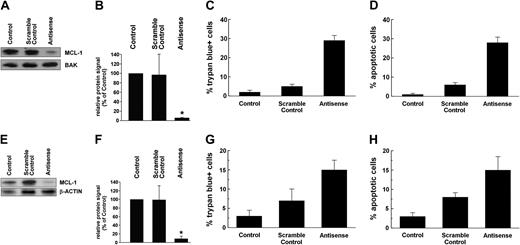
To investigate the role of MCL-1 as a survival molecule and potential target in CML cells, expression of MCL-1 was inhibited in K562 cells by an ASO approach. As determined by Western blotting, transfection of K562 cells with mcl-1 ASO (250 nM) virtually abolished expression of the MCL-1 protein when compared with the scramble control or untransfected cells (Figure 6A,B). The same result was obtained when analyzing K562 cells by immunocytochemistry (not shown). As expected, transfection with mcl-1 ASO also led to a substantial increase in trypan blue-positive cells (Figure 6C) and to an increase in the percentage of apoptotic cells (Figure 6D).
Effects of mcl-1 ASOs on MCL-1 protein expression and cell viability in STI571-sensitive and STI571-resistant K562 cells. STI571-sensitive K562 cells (A-D) and STI571-resistant K562 cells (E-H) were transfected with an mcl-1 ASO at 250 nM (antisense) or a scramble control or were left untransfected (control). After 24 hours, cells were subjected to Western blot analysis (A-B,E-F), trypan blue exclusion test (C,G), and evaluation of numbers (%) of apoptotic cells (D,H). Western blot analysis was performed using an MCL-1 antibody; BAK or β-actin served as loading control. Representative Western blot experiments for STI571-sensitive K562 cells (A) and STI571-resistant K562 cells (E) as well as a densitometric evaluation of Western blot data (B,F) are shown. Results represent the mean ± SD from 3 independent experiments; *P < .05.
Effects of mcl-1 ASOs on MCL-1 protein expression and cell viability in STI571-sensitive and STI571-resistant K562 cells. STI571-sensitive K562 cells (A-D) and STI571-resistant K562 cells (E-H) were transfected with an mcl-1 ASO at 250 nM (antisense) or a scramble control or were left untransfected (control). After 24 hours, cells were subjected to Western blot analysis (A-B,E-F), trypan blue exclusion test (C,G), and evaluation of numbers (%) of apoptotic cells (D,H). Western blot analysis was performed using an MCL-1 antibody; BAK or β-actin served as loading control. Representative Western blot experiments for STI571-sensitive K562 cells (A) and STI571-resistant K562 cells (E) as well as a densitometric evaluation of Western blot data (B,F) are shown. Results represent the mean ± SD from 3 independent experiments; *P < .05.
Role of MCL-1 for survival of STI571-resistant CML cells
The BCR/ABL tyrosine kinase inhibitor STI571 effectively counteracts growth of CML cells in vitro as well as in vivo.40-42 However, resistance to STI571 can occur.43-46 We therefore asked whether MCL-1 would serve as an antiapoptotic factor and target in STI571-resistant K562 cells. As shown in Figure 6E,F, mcl-1 ASO decreased expression of the MCL-1 protein in STI571-resistant K562 cells in the same way as in STI571-sensitive K562 cells. In addition, the mcl-1 ASO-induced down-regulation of MCL-1 in STI571-resistant K562 cells was associated with a decrease in viability and increased percentage of apoptotic cells (Figure 6G,H).
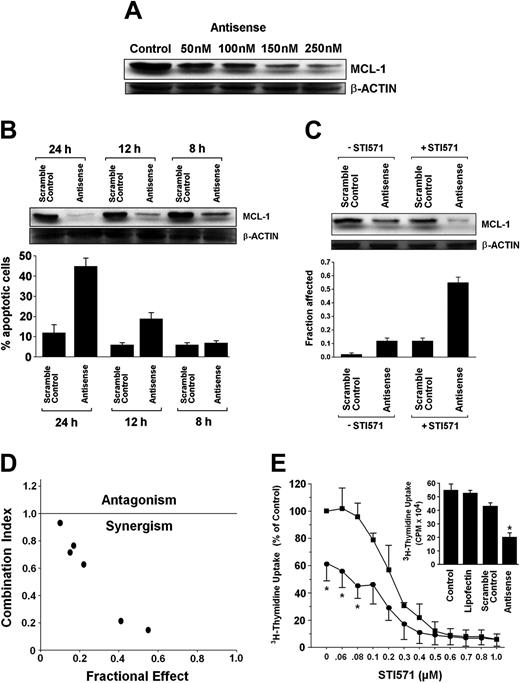
Mcl-1 ASO cooperate with STI571 in inhibition of growth of CML cells
Recently, targeting of MCL-1 has been shown to sensitize neoplastic cells for chemotherapeutic agents.34,47,48 We therefore asked whether treatment of CML cells with mcl-1 ASO would enhance the antileukemic effects of STI571. To define an optimal condition for cooperating drug effects, we first established the dose dependence and a time course for mcl-1 ASO effects in K562 cells. Respective results are shown in Figure 7A,B. In a next step, suboptimal concentrations of STI571 and of mcl-1 ASO (concerning MCL-1 down-regulation and induction of apoptosis) were applied. As shown in Figure 7C, a combination of mcl-1 ASO (200 nM) and STI571 (200 nM) produced a substantial inhibitory effect on expression of the MCL-1 protein in K562 cells, whereas exposure to a single agent alone (either mcl-1 ASO or STI571) showed only minor effects. Exposure to the combination of STI571 and mcl-1 ASO exerted synergistic apoptotic effects on K562 cells as determined by the median dose-effect analysis described by Chou and Talalay38 with combination index values of less than 1.0 indicating synergism (Figure 7D). Corresponding results were obtained when analyzing the effects of STI571 and mcl-1 ASO on 3H-thymidine uptake (Figure 7E).
Effects of mcl-1 ASO alone and in combination with STI571 on growth and viability of K562 cells. (A) Serial dilution of mcl-1 ASO and its effect on MCL-1 protein expression determined by Western blotting. β-actin served as loading control. (B) Time-dependent effect of mcl-1 ASO (250 nM) and scramble control on expression of MCL-1 protein (determined by Western blotting, top) and on viability, that is, the percentage of apoptotic K562 cells (bottom). Results represent the mean ± SD from 3 independent experiments. (C) Effects of mcl-1 ASO or scramble control (each 200 nM) applied with (+STI571, 200 nM) or without STI571 (-STI571), on expression of MCL-1 (determined by Western blotting, top) and on cell viability, that is, percentage of apoptotic cells (bottom); results represent the mean ± SD from 3 independent experiments. (D) Using CalcuSyn software, analyses of dose-effect relationships of STI571- and mcl-1 ASO-induced apoptosis in K562 cells were calculated according to the median effect method of Chou and Talalay.38 A combination index (CI) less than 1 indicates synergism. (E) Effects of various concentrations of STI571 on 3H-thymidine uptake by K562 cells transfected with scramble control (▪) or mcl-1 antisense, 250 nM (•). Results are expressed as percent of control (ie, scramble control without STI571) and represent the mean ± SD of 3 independent experiments. Neither the scramble control nor lipofectin (used for transfection) produced a substantial decrease in 3H-thymidine uptake compared to untransfected cells (ie, control; see insert). CPM indicates counts per minute.
Effects of mcl-1 ASO alone and in combination with STI571 on growth and viability of K562 cells. (A) Serial dilution of mcl-1 ASO and its effect on MCL-1 protein expression determined by Western blotting. β-actin served as loading control. (B) Time-dependent effect of mcl-1 ASO (250 nM) and scramble control on expression of MCL-1 protein (determined by Western blotting, top) and on viability, that is, the percentage of apoptotic K562 cells (bottom). Results represent the mean ± SD from 3 independent experiments. (C) Effects of mcl-1 ASO or scramble control (each 200 nM) applied with (+STI571, 200 nM) or without STI571 (-STI571), on expression of MCL-1 (determined by Western blotting, top) and on cell viability, that is, percentage of apoptotic cells (bottom); results represent the mean ± SD from 3 independent experiments. (D) Using CalcuSyn software, analyses of dose-effect relationships of STI571- and mcl-1 ASO-induced apoptosis in K562 cells were calculated according to the median effect method of Chou and Talalay.38 A combination index (CI) less than 1 indicates synergism. (E) Effects of various concentrations of STI571 on 3H-thymidine uptake by K562 cells transfected with scramble control (▪) or mcl-1 antisense, 250 nM (•). Results are expressed as percent of control (ie, scramble control without STI571) and represent the mean ± SD of 3 independent experiments. Neither the scramble control nor lipofectin (used for transfection) produced a substantial decrease in 3H-thymidine uptake compared to untransfected cells (ie, control; see insert). CPM indicates counts per minute.
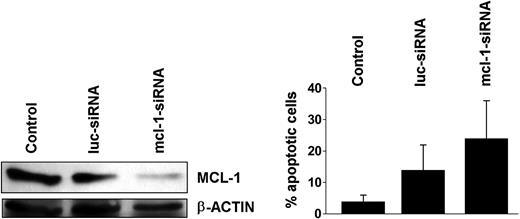
Effects of mcl-1 siRNA on K562 cells
To further demonstrate the role of MCL-1 as a survival molecule and potential target in BCR/ABL+ cells, we applied an mcl-1-specific siRNA.35 In these experiments, the mcl-1 siRNA down-regulated expression of the MCL-1 protein and induced apoptosis in K562 cells (Figure 8A,B). These data provide further evidence that specific targeting of mcl-1 in BCR/ABL-transformed cells is associated with a loss of cell viability.
Effects of mcl-1-specific siRNA on K562 cells. K562 cells were kept untransfected (control) or were transfected with an mcl-1-specific siRNA (mcl-1-siRNA; 200 nM). An siRNA against luciferase (luc-siRNA) was used as control. (A) Effect of mcl-1-siRNA on expression of MCL-1 protein in K562 cells determined by Western blotting. Equal loading was confirmed by probing for β-actin. (B) Effects of luc-siRNA and mcl-1-siRNA on cell viability determined by recording the percentage (%) of apoptotic cells. Results represent the mean ± SD from 3 independent experiments.
Effects of mcl-1-specific siRNA on K562 cells. K562 cells were kept untransfected (control) or were transfected with an mcl-1-specific siRNA (mcl-1-siRNA; 200 nM). An siRNA against luciferase (luc-siRNA) was used as control. (A) Effect of mcl-1-siRNA on expression of MCL-1 protein in K562 cells determined by Western blotting. Equal loading was confirmed by probing for β-actin. (B) Effects of luc-siRNA and mcl-1-siRNA on cell viability determined by recording the percentage (%) of apoptotic cells. Results represent the mean ± SD from 3 independent experiments.
Discussion
MCL-1 is a well-characterized antiapoptotic member of the bcl-2 family that has recently been implicated in the pathogenesis of various myeloid neoplasms.18-23 In the present study, we show that MCL-1 is expressed in primary CML cells in a constitutive manner at the mRNA and protein level and that the disease-related oncogene, BCR/ABL, promotes expression of MCL-1 in leukemic cells. Moreover, our data show that MCL-1 acts as an antiapoptotic molecule in CML cells and that targeting of mcl-1 by an antisense oligonucleotide is associated with increased sensitivity of leukemic cells against the BCR/ABL tyrosine kinase inhibitor STI571. All in all, these data suggest that MCL-1 is an important survival factor and interesting target in CML.
So far, MCL-1 has been described to be expressed in various cell lines of myeloid origin including the CML-derived erythroleukemia cell line K562 and the basophil cell line KU812.19,22,23 In the present study, we were able to confirm expression of MCL-1 in these cell lines and show that primary CML cells also express mcl-1 mRNA and the MCL-1 protein in a constitutive manner. The constitutive expression of MCL-1 in primary leukemic cells is of particular interest because this survival factor was initially characterized as an “early” activation-related gene in myeloid (leukemic) cells.18,19 Another interesting aspect of our study was that MCL-1 is expressed in CML cells independent of the phase of disease. Thus, MCL-1 may be up-regulated already in an initial phase of leukemogenesis in the CML clone.
We therefore were interested to know whether the disease-related oncogene BCR/ABL plays a role in expression of MCL-1 in leukemic cells. In a first step, we found that STI571 down-regulates expression of MCL-1 in primary CML cells. The same result was obtained with KU812 and K562 cells, confirming the data of Yu et al22,23 and Mow et al.49 We next examined Ba/F3 cells with doxycycline-inducible expression of BCR/ABL. Using these cells, we were able to show that BCR/ABL directly induces expression of MCL-1. In fact, induction of BCR/ABL by doxycycline resulted in a substantial increase in mcl-1 promoter activity and expression of mcl-1 mRNA as well as expression of the MCL-1 protein in Ton.B210-X cells. All in all, our data show that BCR/ABL promotes expression of MCL-1 in leukemic cells.
However, apart from BCR/ABL, other factors may also play a role in MCL-1 expression in neoplastic cells. Likewise, it has been shown that activin A induces expression of MCL-1 in K562 cells and KU812 cells,19 and both IL-3 and GM-CSF reportedly up-regulate expression of Mcl-1 in Ba/F3 cells and TF-1 cells.50-53 Whether such additional factors can also regulate expression of MCL-1 in primary CML cells in vivo remains at present unknown. Such “additional” up-regulation may indeed occur because CML cells express functional receptors for activin A, IL-3, and GM-CSF19,54,55 and because the signaling pathways that underlie cytokine-induced up-regulation of MCL-1 in myeloid (progenitor) cells may in part differ from those contributing to BCR/ABL-induced expression of MCL-1 in leukemic cells.
A number of different signaling pathways are involved in BCR/ABL-dependent gene regulation and BCR/ABL-dependent growth and survival of leukemic cells.4-8 In the present study, we attempted to identify signaling pathways underlying BCR/ABL-induced expression of MCL-1 using various signal transduction inhibitors. In respective experiments, we found that the MEK inhibitor PD98059 counteracts BCR/ABL-dependent expression of MCL-1 in Ton.B210-X cells in the same way as STI571 did, whereas inhibitors of PI3-kinase (LY294002) or mTOR (rapamycin) showed no effects. The same result was obtained with primary CML cells. In consecutive experiments we were able to show that a constitutively active RAS mutant, ras-G12V, enhances mcl-1 promoter activity in Ba/F3 cells, and that this RAS-induced up-regulation of MCL-1 is counteracted by PD98059. Furthermore, we found that a dominant-negative RAS mutant, ras-N17, counteracts BCR/ABL-dependent mcl-1 promoter activity in Ba/F3 cells. These data provide evidence that the RAS/RAF/MEK/ERK pathway contributes to BCR/ABL-induced expression of MCL-1 in leukemic cells.
The involvement of the RAS/RAF/MEK/ERK pathway in BCR/ABL-induced expression of MCL-1 in leukemic cells is of particular interest. First, it is noteworthy that this signaling pathway has also been implicated in expression of MCL-1 in the ML-1 myeloblastic leukemia cell line.23,56 However, depending on the cell type and stimulator (cytokine) applied, other signaling pathways (apart from the RAS/RAF/MEK/ERK pathway) have also been reported to be involved in MCL-1 expression.50-53 Likewise, the PI3-kinase pathway was found to contribute to GM-CSF- and IL-3-induced expression of MCL-1 in Ba/F3 cells and TF-1 cells.52-56 Other studies have shown that both the PI3-kinase pathway and the RAS/RAF/MEK/ERK pathway are important for stem cell factor (SCF)- and IL-5-induced expression of MCL-1 in JYTF-1 cells,57 and GM-CSF-induced up-regulation of MCL-1 in TF-1 cells.52 Finally, STAT5 has also been implicated in MCL-1 expression in leukemic cells.58-60
Because BCR/ABL reportedly signals through STAT5,6,7 we were interested as to whether, apart from MEK, STAT5 also plays a role in BCR/ABL-dependent expression of MCL-1. In respective experiments, we found that a dnSTAT5 mutant down-regulates expression of mcl-1 promoter activity in the BCR/ABL+ cell line C7.28. Thus, both MEK- and STAT5-dependent signaling may contribute to BCR/ABL-dependent expression of MCL-1 in leukemic cells. These data are in line with the observation that STAT5 and RAS cooperate in promoting growth of BCR/ABL-transformed cells.61,62
A number of previous studies have shown that MCL-1 serves as an antiapoptotic molecule in various neoplastic cells.19-21,49-53,60 We were therefore interested to know whether MCL-1 would also act antiapoptotic in CML cells and would thus serve as a potential therapeutic target. To address this question, we transfected K562 cells with mcl-1 ASOs, which resulted in an almost complete inhibition of MCL-1 protein expression. The mcl-1 ASO-transfected cells were less viable and underwent apoptosis at higher rates compared to K562 cells transfected with a control oligonucleotide. These data suggest that MCL-1 is an antiapoptotic molecule and interesting target in CML cells.
Because resistance to STI571 (imatinib) has become a major clinical problem in patients with CML,43-46 we next asked whether Mcl-1 ASO would also lead to apoptosis in STI571-resistant CML cells. To address this question, we examined an STI571-resistant subclone of K562.31 Using this cell line, we were indeed able to show that mcl-1 ASO can inhibit the survival of STI571-resistant K562 cells.
A number of different strategies have been proposed to prevent the development of STI571 (imatinib) resistance in patients with CML.63-66 One strategy is to combine STI571 with other (conventional or targeted) drugs.63-66 Because mcl-1 antisense reportedly promotes the effects of other antineoplastic drugs on various neoplastic cells,34,47,48 we were interested to know whether mcl-1 ASO and STI571 would show synergistic antiproliferative effects on CML cells. To address this question, K562 cells were transfected with the mcl-1 ASO and then exposed to various concentrations of STI571. In these experiments, pretreatment with mcl-1 ASO resulted in a substantial increase in sensitivity of leukemic cells against STI571 compared with a control oligonucleotide.
In aggregate, our data suggest that targeting of MCL-1 in CML cells may be a novel promising approach to counteract leukemia cell growth in patients with CML. However, several aspects have to be taken into account when considering antisense-based therapy in such patients. First, to date, the clinical experience with antisense probes in leukemia patients is limited to a few trials67-69 and no reports on the in vivo effects of mcl-1 ASO in clinical studies are available. Second, although mcl-1 ASO produced a clear antileukemic effect in STI571-resistant K562 cells in vitro, it remains unknown whether the same effect can be expected to occur in all patients with STI571-resistant CML. In fact, as mentioned, a number of different mechanisms and molecular defects underlie STI571 resistance.43-46,63-66
In summary, we have identified MCL-1 as a BCR/ABL-dependent survival factor and potential target in CML. Whether this concept can indeed be developed far enough to reach clinical application in patients with CML remains at present unknown.
Prepublished online as Blood First Edition Paper, December 30, 2004; DOI 10.1182/blood-2004-02-0749.
Supported by the Hans and Blanca Moser-Stiftung and by the Fonds zur Förderung der Wissenschaftlichen Forschung in Österreich-FWF grant P-16412. S.D. and W.F.P. were supported by grant 20030 from CeMM Center of Molecular Medicine, Austrian Academy of Sciences.
B.P.M. is employed at ISIS Pharmaceuticals Inc, whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Regina Haslinger and Hans Semper for skillful technical assistance.