Abstract
In the search for a more effective adjuvant therapy to treat multiple myeloma (MM), we investigated the effects of the traditional Chinese herbal medicines Huang-Lian-Jie-Du-Tang (HLJDT), Gui-Zhi-Fu-Ling-Wan (GZFLW), and Huang-Lian-Tang (HLT) on the proliferation and apoptosis of myeloma cells. HLJDT inhibited the proliferation of myeloma cell lines and the survival of primary myeloma cells, especially MPC-1- immature myeloma cells, and induced apoptosis in myeloma cell lines via a mitochondria-mediated pathway by reducing mitochondrial membrane potential and activating caspase-9 and caspase-3. Further experiments confirmed that Scutellaria radix was responsible for the suppressive effect of HLJDT on myeloma cell proliferation, and the baicalein in Scutellaria radix showed strong growth inhibition and induction of apoptosis in comparison with baicalin or wogonin. Baicalein as well as baicalin suppressed the survival in vitro of MPC-1- immature myeloma cells rather than MPC-1+ myeloma cells from myeloma patients. Baicalein inhibited the phosphorylation of IkB-α, which was followed by decreased expression of the IL-6 and XIAP genes and activation of caspase-9 and caspase-3. Therefore, HLJDT and Scutellaria radix have an antiproliferative effect on myeloma cells, especially MPC-1- immature myeloma cells, and baicalein may be responsible for the suppressive effect of Scutellaria radix by blocking IkB-α degradation. (Blood. 2005;105:3312-3318)
Introduction
Multiple myeloma (MM) is an incurable plasma-cell malignancy and the second most common hematologic malignancy, with 14 000 new patients diagnosed in the United States annually.1,2 Although combination chemotherapy offers initial response rates of 40% to 70% in MM patients,3 refractoriness to these regimens eventually develops. High-dose chemotherapy with stem cell support has achieved higher response rates than conventional therapy, but few patients remain in long-term remission.4 Thus, the development of a more effective therapy to treat early and advanced MM has become a priority.
Many components from herbs have been identified as effective in the treatment of human disease. Curcumin, a major component of turmeric, is able to correct defects associated with the homozygous expression of delta F508 cystic fibrosis5 and to suppress the growth of myeloma cells.6 Arsenic trioxide, a compound of arsenic, is very effective in the treatment of patients with acute promyelocytic leukemia who have developed resistance to all-trans retinoic acid (ATRA).7 Artemisinins, extracted from sweet wormwood, are the most potent antimalarials available, rapidly killing Plasmodium falciparum at all asexual stages by inhibiting the sarcoplasmic or endoplasmic reticulum calcium ATPase (SERCA) ortholog (PfATP6) in Xenopus oocytes with a similar potency to thapsigargin.8 Consequently, they are widely used to treat multidrug-resistant malaria, a disease that claims 1 million lives annually.9
Inflammation and MM may be induced partly in the same way, as interleukin 6 (IL-6) is a potential mediator in these conditions.10,11 Many Kampo medicines have been used historically in anti-inflammatory therapy. By screening the effects of anti-inflammatory Kampo formulas on MM cells, we hoped to find one to treat MM. Huang-Lian-Jie-Du-Tang (HLJDT) contains Coptis rhizoma, Phellodendron bark, Scutellaria radix (root), and Gardenia fruit in 2.0, 1.5, 3.0, and 2.0 parts, respectively. It is recognized in Japan and China as an effective anti-inflammatory agent and has been widely used in the treatment of various inflammatory diseases such as gastritis, dermatitis, aphthous stomatitis, and hypertension. HLJDT exhibited anti-inflammatory activity in experimental colitis induced by dextran sulfate sodium,12 and in animal experiments it inhibited the proliferation of lymphocytes under inflammatory conditions by suppressing the secretion of proinflammatory cytokines including interferon α (IFN-α) and IFN-γ.13 The secretion of these cytokines was also reported to be suppressed by HLJDT in vitro.14 As IL-6 is one of the most important growth factors for myeloma cell proliferation,11 we examined whether Kampo medicines with anti-inflammatory properties are of a potential therapeutic value for MM patients. In this study, we examined the effects of the anti-inflammatory formulas HLJDT and Huang-Lian-Tang (HLT) and the non-anti-inflammatory formula Gui-Zhi-Fu-Ling-Wan (GZFLW) on myeloma cell proliferation. We found that HLJDT had the strongest inhibitory effect. Among the constituents of HLJDT, we found that Scutellaria radix contributed most to the inhibitory activity in myeloma cells. Since Scutellaria radix contains baicalein, baicalin, and wogonin, we examined the effect of these 3 components on myeloma cell proliferation and also investigated the mechanism of suppression.
Patients, materials, and methods
Preparation of traditional Chinese medicines
Extracts of HLJDT, HLT, GZFLW, HLJDT free of Scutellaria radix, and Scutellaria radix were provided by Tsumura (Tokyo, Japan). Water extracts were prepared as follows. Five grams of each powder was dissolved in distilled water to 50 mL and the fluid was stirred at 4°C overnight. After centrifugation, the supernatant was sterilized by filtration through a 0.2-μm Millipore filter (Bedford, MA) and stored at 4°C prior to use.
Cell lines and reagents
The human myeloma cell lines U266 (obtained from America Type Culture Collection; ATCC, Rockville, MD) and NOP-2, AMO1, and ILKM214 (a gift from Dr S. Shimizu, Shimane Prefectural Hospital, Matsue, Japan) were maintained in RPMI-1640 medium (Nissui, Tokyo, Japan) supplemented with 10% fetal bovine serum (FBS; MA Bioproducts, Walkersville, MD) in the absence or presence of 2 ng/mL recombinant human IL-6 (Sawady Technology, Tokyo, Japan). Cells were grown at 37°C in a humidified 5% CO2 atmosphere. Baicalein, baicalin, and wogonin were purchased from WAKO (Tokyo, Japan) and were first dissolved in dimethyl sulfoxide (DMSO) and then serially diluted in RPMI-1640 medium immediately prior to experiments.
Isolation of bone marrow mononuclear cells from myeloma patients and culture in vitro
Bone marrow (BM) aspirates were obtained from patients with newly diagnosed MM. All studies were performed with the patients' informed consent and approved by the Institutional Review Board of Yamaguchi University School of Medicine. Bone marrow monoclonal cells (BMMNCs) from BM aspirates were separated by Ficoll-Hypaque centrifugation. BMMNCs were suspended at a concentration of 0.5 × 106 to 1 × 106 cells/mL and cultured in RPMI-1640 medium supplemented with 10% FBS. Cells were treated with the Kampo medicines at 25 to 200 ng/mL for 2 to 5 days. Cellular phenotypic analyses were done by flow cytometry. The antibodies used for myeloma cell surface staining included fluorescein isothiocyanate (FITC)-labeled anti-CD38 (Beckman Coulter, Tokyo, Japan), phycoerythrin (PE)-labeled anti-mature plasma cell-1 (MPC-1) (Japan Immunoresearch Laboratories, Takasaki, Japan), and positive cofactor (PC5)-labeled anti-CD45 (Beckman Coulter) antibody. The stained cells were incubated for 30 minutes at 4°C and analyzed with a flow cytometer (Epics Elite ESP; Coulter, Tokyo, Japan).
Assessment of viable and nonviable cell numbers by flow cytometry
Cells were cultured with traditional Chinese medicines or their constituents, and viable and nonviable cell numbers were assessed by harvesting a constant volume of the cell suspension and applying it to a flow cytometer (Epics Elite ESP). From the profile of forward light scatter (FS) and side light scatter (SS), we evaluated the number of viable and nonviable cells in the sample as described previously.15
Measurement of mitochondrial membrane potential using DiOC6 staining
Cells were initially treated with various concentrations of HLJDT for 12 to 24 hours. Treated or untreated cells were collected and suspended in 0.5 mL of phosphate-buffered saline (PBS) containing 100 nM DiOC6 (3′,3′-dihexyloxacarbo-cyanine iodide; Molecular Probes, Eugene, OR) and incubated at 37°C for 20 minutes. DiOC6-stained cells were subjected to analysis using a flow cytometer (Epics Elite ESP); DiOC6 was excited at 488 nM and detected at 525 nM.
Western blotting
Cells (1 × 106) were treated with reagents at various concentrations for specific periods. The treated cells were washed twice with PBS and lysed in ice-cold cell lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1 mM ethyleneglycotetraacetic acid, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM glycerolphosphate, 1 mM sodium orthovanadate, and 1 μg/mL leupeptin) containing 1 mM phenylmethylsulfonyl fluoride. Equivalent amounts of protein (10 μg) were boiled in Laemmli sample buffers and fractionated by 10% to 15% polyacrylamide gel electrophoresis (PAGE) with sodium dodecyl sulfate (SDS). Mitochondrial and cytosolic extracts were prepared using an ApoAlert cell fractionation kit (Clontech, Palo Alto, CA) according to the manufacturer's instructions. Separated proteins in the gel were transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA) by an electroblot apparatus (Bio-Rad). Immunoassaying of the membranes using specific antibodies for phosphorylated IkB-α (Cell Signaling, Beverly, CA), IkB-α (Cell Signaling), β-actin (Sigma, St Louis, MO), horseradish peroxidase-labeled antibodies, and a chemiluminescent substrate (KPL, Gaithersburg, MD) was performed according to the manufacturers' directions.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total cellular RNA was extracted from U266 with Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. RNA (10 μg) was reverse transcribed to cDNA using random hexamers by Superscript II as recommended by the manufacturer (GIBCO-BRL, Gaithersburg, MD).
For the evaluation of gene expression, RT-PCR was performed with an increasingly large number of cycles (25, 28, 30, 33, 35 cycles). Each cycle comprised denaturation at 94°C for 1 minute, primer annealing for 1 minute at 65°C, and primer extension at 72°C for 1 minute. All genes, including a housekeeping standard (glyceraldehyde-3-phosphate dehydrogenase [G3PDH]), were amplified within the logarithmic phase after 25 to 35 cycles. Amplified products were visualized on 2% agarose gel containing ethidium bromide. The oligonucleotide primers used were as follows: G3PDH forward: 5′-ACC ACA GTC CAT GCC ATC AC-3′, reverse: 5′-TCC ACC ACC CTG TTG CTG TA-3′; IL-6 forward: 5′-ATG AAC TCC TTC TCC ACA AGC GC-3′, reverse: 5′-GAA GAG CCC TCA GGC TGG ACT G-3′; X-linked inhibitor of apoptosis protein (XIAP) forward: 5′-GAA GAC CCT TGG GAA CAA CA-3′, reverse: 5′-TGT CCT TGAAAC TGAACC CC-3′.
Flow cytometric analysis of NF-kB translocation in nuclei preparations
Nucleic preparations were obtained by incubating the cells with 200 μL of piperazine diethanesulfonic acid (PIPES)-Triton buffers (10 mM PIPES, 0.1 M NaCl, 2 mM MgCl2, and 0.1% Triton X 100) for 30 minutes at 4°C. The nuclei were incubated with PE-labeled anti-nuclear factor-kB (anti-NF-kB; p65) antibody (sc-8008; Santa Cruz Biotechnology, Santa Cruz, CA) or PE-labeled control mouse immunoglobulin G1 (IgG1; IM-0670; Coulter) for 30 minutes at 4°C and analyzed with a flow cytometer.
Statistical analysis
Statistical analysis was conducted using the Student t test and statistical significance was defined as P less than .05 (*) or P less than .01 (**).
Results
HLJDT inhibited proliferation of myeloma cell lines
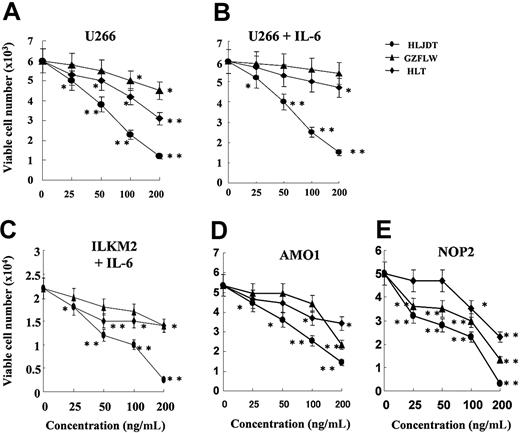
We examined the effect of 3 traditional Chinese medicines, HLJDT, GZFLW, and HLT, on the proliferation of myeloma cell lines U266, NOP-2, AMO1, and ILKM2. The IL-6-independent myeloma cell lines U266, NOP-2, and AMO1 were cultured with different doses (25, 50, 100, or 200 ng/mL) of HLJDT, GZFLW, or HLT for 4 days. HLJDT inhibited the proliferation of U266 cells in a dose-dependent manner more than GZFLW with and without 2 ng/mL IL-6 (Figure 1A-B). The IC50 (concentration needed for 50% growth inhibition) of HLJDT was about 70 ng/mL in both conditions whereas that of both GZFLW and HLT was greater than 200 ng/mL, as shown in Figure 1A-B. HLJDT also inhibited the proliferation of NOP-2 and AMO1 cells (Figure 1D-E). Next, we treated ILKM2,13 an IL-6-dependent myeloma cell line, with HLJDT, GZFLW, or HLT. HLDJT also inhibited the proliferation of ILKM2 cells in the presence of IL-6 (Figure 1C). The IC50 of HLDJT in ILKM2 cells was 55 ng/mL whereas that of both GZFLW and HLT was again greater than 200 ng/mL. These results showed that only HLDJT out of 3 traditional Chinese medicines could inhibit the proliferation of both the IL-6-independent myeloma cell lines (U266, NOP-2, AMO1) and the IL-6-dependent myeloma cell line (ILKM2).
Effect of HLJDT, GZFLW, or HLT on proliferation of myeloma cell lines. U266 cells were cultured without IL-6 (A) or with IL-6 at 2 ng/mL (B) in the presence of HLJDT (•), GZFLW (▴), or HLT (♦) at the indicated concentration for 4 days. Cell viability was assessed by flow cytometric detection of FS/SS as described in “Patients, materials, and methods.” (C) ILKM-2, an IL-6-dependent myeloma cell line, was cultured with IL-6 at 2 ng/mL in the presence of HLJDT, GZFLW, or HLT at the indicated concentration for 4 days. AMO1 (D) or NOP-2 cells (E), IL-6-independent myeloma cell lines, were also cultured. Viable cell number was assessed by flow cytometric detection of FS/SS. Values shown in these figures are the mean ± 1 SD of 3 independent experiments. *P < .05, **P < .01.
Effect of HLJDT, GZFLW, or HLT on proliferation of myeloma cell lines. U266 cells were cultured without IL-6 (A) or with IL-6 at 2 ng/mL (B) in the presence of HLJDT (•), GZFLW (▴), or HLT (♦) at the indicated concentration for 4 days. Cell viability was assessed by flow cytometric detection of FS/SS as described in “Patients, materials, and methods.” (C) ILKM-2, an IL-6-dependent myeloma cell line, was cultured with IL-6 at 2 ng/mL in the presence of HLJDT, GZFLW, or HLT at the indicated concentration for 4 days. AMO1 (D) or NOP-2 cells (E), IL-6-independent myeloma cell lines, were also cultured. Viable cell number was assessed by flow cytometric detection of FS/SS. Values shown in these figures are the mean ± 1 SD of 3 independent experiments. *P < .05, **P < .01.
HLJDT selectively inhibited survival of MPC-1- immature myeloma cells
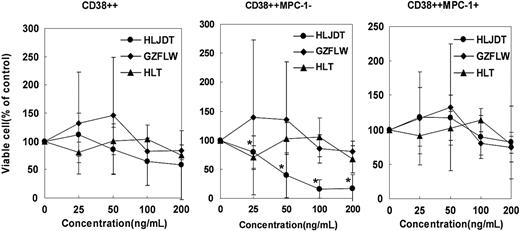
Next, we examined the effect of these 3 formulas on the survival of freshly isolated primary myeloma cells in vitro. We isolated BMMNCs from BM aspirates of 5 patients with overt myeloma and cultured them with HLJDT, GZFLW, or HLT for 5 days. Then, we performed a phenotypic analysis with a flow cytometer and evaluated the viable cell number in CD38++ total myeloma cell, MPC-1- immature myeloma cell, and MPC-1+ intermediate/mature myeloma cell fractions.16 HLJDT reduced the number of viable cells markedly in MPC-1- immature myeloma cells (IC50 = 44 ng/mL), slightly in total myeloma cells (CD38++; IC50 = 200 ng/mL), and not significantly in MPC-1+ myeloma cells (IC50 = greater than 400 ng/mL), whereas GZFLW decreased the viable cell number slightly in CD38++ total myeloma cells, MPC-1- immature myeloma cells, and MPC-1+ myeloma cells. The IC50 of GZFLW in all these cells was greater than 400 ng/mL, as shown in Figure 2. Thus, we confirmed that HLDJT also inhibited the survival of primary myeloma cells in vitro, especially MPC-1- immature myeloma cells.
Effect of HLJDT, GZFLW, or HLT on proliferation of primary myeloma cells. Bone marrow mononuclear cells obtained from 5 untreated myeloma patients were cultured with 5 ng/mL of IL-6 in the presence of HLJDT (•), GZFLW (♦), or HLT (▴) at the indicated concentration for 5 days. After the culture, the cells were harvested and stained with FITC-anti-CD38, PE-anti-MPC-1, and PC5-anti-CD45 antibody and applied to a flow cytometer. Viable cell numbers in the CD38++, CD38++MPC-1-, and CD38++MPC-1+ fractions were examined and compared with those in medium alone (100%). Values shown in these figures are the mean ± 1SD of 5 cases. *P < .05.
Effect of HLJDT, GZFLW, or HLT on proliferation of primary myeloma cells. Bone marrow mononuclear cells obtained from 5 untreated myeloma patients were cultured with 5 ng/mL of IL-6 in the presence of HLJDT (•), GZFLW (♦), or HLT (▴) at the indicated concentration for 5 days. After the culture, the cells were harvested and stained with FITC-anti-CD38, PE-anti-MPC-1, and PC5-anti-CD45 antibody and applied to a flow cytometer. Viable cell numbers in the CD38++, CD38++MPC-1-, and CD38++MPC-1+ fractions were examined and compared with those in medium alone (100%). Values shown in these figures are the mean ± 1SD of 5 cases. *P < .05.
Treatment with HLJDT resulted in apoptosis of U266
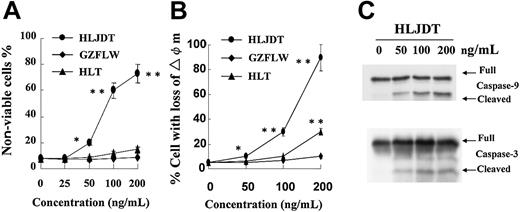
Following the treatment with HLDJT in U266 cells, we observed not only a decrease in the viable cell number but also an increase in the nonviable cell number by flow cytometry. As shown in Figure 3A, at higher concentrations (greater than 50 ng/mL) HLDJT but not GZFLW or HLT induced an increase in the nonviable cell number as evaluated from FS/SS histograms of flow cytometry reported previously.15,16 The morphologic examination was also compatible with apoptotic features under a Nikon OptiPhot microscope (Nikon, Tokyo, Japan). A dose of 100 ng/mL of HLJDT induced approximately 30% of the U266 cells to undergo apoptosis even after 12 hours and 60% after 24 hours of treatment (Figure 3A). To examine whether the accumulation of apoptotic cells is accompanied by a loss of mitochondrial membrane potential, we stained viable cells with DiOC6. After treatment with 200 ng/mL of HLJDT for 12 hours, up to 80% of the cells lost their mitochondrial transmembrane potential (Figure 3B). U266 cells treated with HLJDT for 12 hours also demonstrated cleavage of caspase-9 and caspase-317 on Western blotting at a concentration of 50 ng/mL (Figure 3C). These results indicated that mitochondria-mediated apoptosis may be induced by a high concentration of HLJDT in U266 cells.
HLJDT induced apoptosis in U266 cells. (A) U266 cells were cultured with 0, 25, 50, 100, or 200 ng/mL of HLJDT (•), GZFLW (♦), or HLT (▴) for 24 hours and then the cells were applied to a flow cytometer. Viable and nonviable cells were assessed by FS/SS. Values shown in this figure are the mean ± 1 SD of 3 independent experiments. *P < .05, **P < .01. (B) The mitochondrial membrane potential of U266 cells was assessed after treatment with 0, 50, 100, or 200 ng/mL of HLJDT, GZFLW, or HLT for 12 hours. Values shown in this figure are the mean ± 1SD of 3 independent experiments on flow cytometric detection of DiOC6 uptake at indicated time points. *P < .05, **P < .01. (C) Lysates from U266 cells treated with 50, 100, or 200 ng/mL of HLJDT for 12 hours were assessed for cleaved caspase by Western blotting with antibodies to caspase-9 and -3.
HLJDT induced apoptosis in U266 cells. (A) U266 cells were cultured with 0, 25, 50, 100, or 200 ng/mL of HLJDT (•), GZFLW (♦), or HLT (▴) for 24 hours and then the cells were applied to a flow cytometer. Viable and nonviable cells were assessed by FS/SS. Values shown in this figure are the mean ± 1 SD of 3 independent experiments. *P < .05, **P < .01. (B) The mitochondrial membrane potential of U266 cells was assessed after treatment with 0, 50, 100, or 200 ng/mL of HLJDT, GZFLW, or HLT for 12 hours. Values shown in this figure are the mean ± 1SD of 3 independent experiments on flow cytometric detection of DiOC6 uptake at indicated time points. *P < .05, **P < .01. (C) Lysates from U266 cells treated with 50, 100, or 200 ng/mL of HLJDT for 12 hours were assessed for cleaved caspase by Western blotting with antibodies to caspase-9 and -3.
Baicalein was identified as the active component of Scutellaria radix
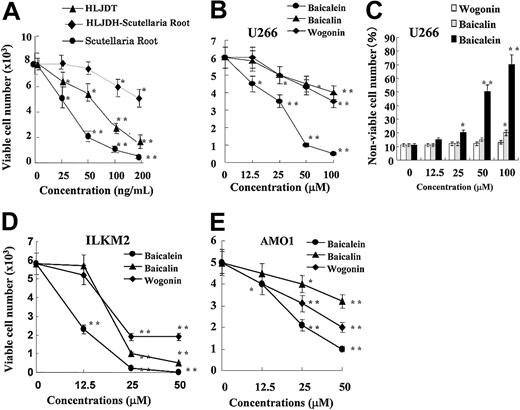
Since Scutellaria radix (root) was present in HLJDT but not in HLT or GZFLW, we tested whether it alone could suppress the proliferation of U266 cells and whether HLJDT without Scutellaria radix lost its suppressive effect. Scutellaria radix alone exhibited stronger growth inhibition (IC50 = 30 ng/mL) than HLJDT with Scutellaria radix (IC50 = 70 ng/mL), as shown in Figure 4A. We also confirmed that HLJDT without Scutellaria radix could not inhibit the proliferation of U266 cells well, in comparison with HLJDT with Scutellaria radix. Thus, Scutellaria radix was responsible for the suppressive effect of HLJDT. Next, since Scutellaria radix contains 3 major flavonoids, baicalein, baicalin, and wogonin, we examined which component was responsible for its suppressive effect. As shown in Figure 4B, baicalein had the strongest suppressive effect on the proliferation of U266 cells with an IC50 of 28 μM; the IC50s of baicalin and wogonin were greater than 200 μM. In addition, baicalein but not baicalin or wogonin induced a significant increase in the nonviable cell number over 4 days (Figure 4C). Also, baicalein suppressed the proliferation of other myeloma cell lines ILKM2 and AMO1 (Figure 4D-E). Thus, we concluded that baicalein, a component of Scutellaria radix, was responsible for the suppressive effect of HLJDT on cellular proliferation in myeloma cell lines.
Identification of baicalein as the active component ofScutellariaradix (root) in HLJDT. (A) U266 cells were cultured with HLJDT (▴), HLJDH without Scutellaria root (♦), or Scutellaria root alone (•) at the indicated concentration for 4 days. Viable cell number was assessed by flow cytometric detection of FS/SS. Values are the mean ± 1 SD of 3 independent experiments. *P < .05, **P < .01. U266 (B), ILKM2 (D), and AMO1 (E) cells were cultured with baicalein (•), baicalin (▴), or wogonin (♦) at the indicated concentration for 4 days. Viable cell number was assessed by flow cytometric detection of FS/SS. Values are the mean ± 1 SD of 3 independent experiments. *P < .05, **P < .01. (C) U266 cells were also cultured with baicalein (▪), baicalin (▦), or wogonin (□) at the indicated concentration for 4 days. Nonviable cells were quantitated by flow cytometric detection of FS/SS. Values are the mean ± 1 SD of 3 independent experiments. *P < .05, **P < .01.
Identification of baicalein as the active component ofScutellariaradix (root) in HLJDT. (A) U266 cells were cultured with HLJDT (▴), HLJDH without Scutellaria root (♦), or Scutellaria root alone (•) at the indicated concentration for 4 days. Viable cell number was assessed by flow cytometric detection of FS/SS. Values are the mean ± 1 SD of 3 independent experiments. *P < .05, **P < .01. U266 (B), ILKM2 (D), and AMO1 (E) cells were cultured with baicalein (•), baicalin (▴), or wogonin (♦) at the indicated concentration for 4 days. Viable cell number was assessed by flow cytometric detection of FS/SS. Values are the mean ± 1 SD of 3 independent experiments. *P < .05, **P < .01. (C) U266 cells were also cultured with baicalein (▪), baicalin (▦), or wogonin (□) at the indicated concentration for 4 days. Nonviable cells were quantitated by flow cytometric detection of FS/SS. Values are the mean ± 1 SD of 3 independent experiments. *P < .05, **P < .01.
Baicalein inhibited the survival of primary myeloma cells, especially MPC-1- immature myeloma cells in vitro
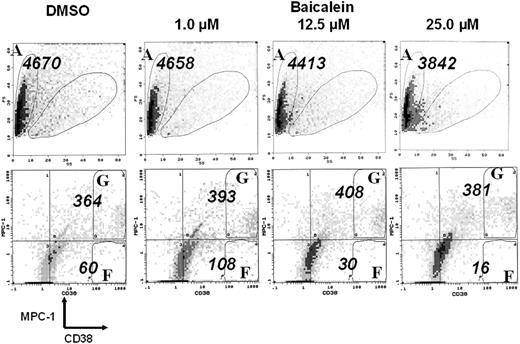
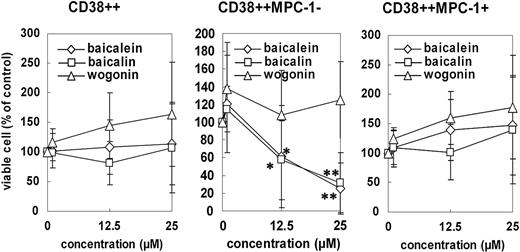
We isolated BMMNCs from 9 patients with overt myeloma and cultured them with baicalein, baicalin, or wogonin at 1.0, 12.5, or 25.0 μM, respectively, for 2 days. After 2 days' culture, the surviving cells were analyzed by multicolor staining with anti-CD38, anti-MPC-1, and anti-CD45 antibodies. As shown in Figure 5, myeloma cells were evaluated as CD38++ (regions F and G), MPC-1- immature myeloma cells as CD38++/MPC-1- (region F), and MPC-1+ intermediate/mature myeloma cells as CD38++/MPC-1+ (region G). Baicalein inhibited the survival of CD38++MPC-1- immature myeloma cells in a dose-dependent manner in vitro but did not appear to affect the survival of CD38++MPC-1+ cells. A concentration of baicalein of up to 25 μM apparently had no suppressive effect on the viability of normal myeloid cells (Figures 5, 6) or peripheral blood cells (data not shown). Data from 9 cases of overt myeloma are summarized in Figure 6. Baicalein as well as baicalin inhibited the survival of MPC-1- immature myeloma cells but not MPC-1+ myeloma cells, whereas wogonin did not affect the survival even at a concentration of 25 μM.
Flow cytometric histogram of cells cultured with baicalein. Bone marrow mononuclear cells from a representative myeloma case were cultured with DMSO control or baicalein (1.0, 12.5, or 25.0 μM) in the presence of 5 ng/mL of IL-6 for 2 days. After the culture, the cells were harvested, stained with FITC-anti-CD38, PE-anti-MPC-1, and PC5-anti-CD45 antibodies and applied to a flow cytometer. Viable cell numbers of CD38++, CD38++MPC-1-, and CD38++MPC-1+ fractions were examined, and values shown in this figure are the number detected for a given period (1 minute analysis) for total cells (A), CD38++MPC-1- cells (F), and CD38++MPC-1+ cells (G), respectively.
Flow cytometric histogram of cells cultured with baicalein. Bone marrow mononuclear cells from a representative myeloma case were cultured with DMSO control or baicalein (1.0, 12.5, or 25.0 μM) in the presence of 5 ng/mL of IL-6 for 2 days. After the culture, the cells were harvested, stained with FITC-anti-CD38, PE-anti-MPC-1, and PC5-anti-CD45 antibodies and applied to a flow cytometer. Viable cell numbers of CD38++, CD38++MPC-1-, and CD38++MPC-1+ fractions were examined, and values shown in this figure are the number detected for a given period (1 minute analysis) for total cells (A), CD38++MPC-1- cells (F), and CD38++MPC-1+ cells (G), respectively.
Effect of baicalein, baicalin, or wogonin on the survival of primary myeloma cells in vitro. Bone marrow mononuclear cells from 9 myeloma patients were cultured with baicalein (⋄), baicalin (□), or wogonin (▵) in the presence of 5 ng/mL of IL-6 at the indicated concentration for 2 days. After the culture, the cells were harvested, stained with FITC-anti-CD38, PE-anti-MPC-1, and PC5-anti-CD45 antibodies and applied to a flow cytometer. Viable cell numbers of CD38++ (left), CD38++MPC-1- (middle), and CD38++MPC-1+ fractions (right) were examined, and compared with those in medium alone (DMSO control; 100%). Values shown in these figures are the mean ± 1 SD of 9 cases. *P < .05, **P < .01.
Effect of baicalein, baicalin, or wogonin on the survival of primary myeloma cells in vitro. Bone marrow mononuclear cells from 9 myeloma patients were cultured with baicalein (⋄), baicalin (□), or wogonin (▵) in the presence of 5 ng/mL of IL-6 at the indicated concentration for 2 days. After the culture, the cells were harvested, stained with FITC-anti-CD38, PE-anti-MPC-1, and PC5-anti-CD45 antibodies and applied to a flow cytometer. Viable cell numbers of CD38++ (left), CD38++MPC-1- (middle), and CD38++MPC-1+ fractions (right) were examined, and compared with those in medium alone (DMSO control; 100%). Values shown in these figures are the mean ± 1 SD of 9 cases. *P < .05, **P < .01.
Baicalein inhibited phosphorylation of IkB-α and induced activation of the mitochondria-mediated apoptotic pathway
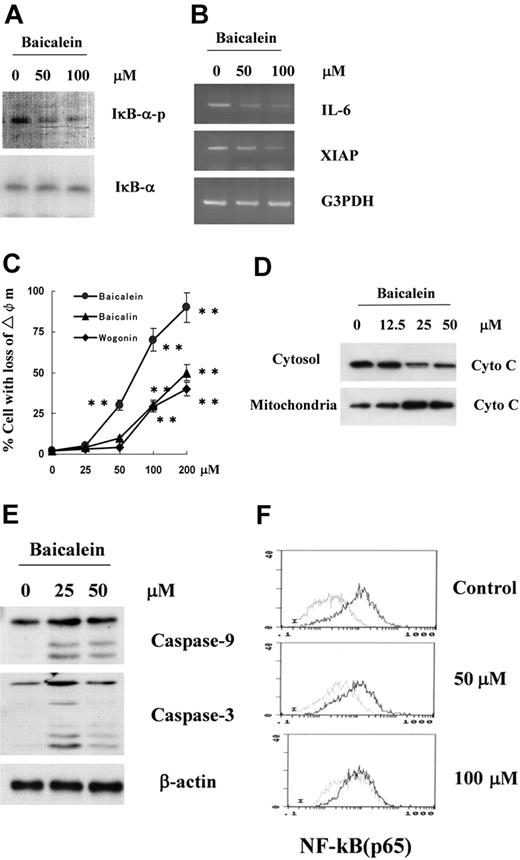
Since inhibition of NF-kB activity is considered one of the mechanisms suppressing the proliferation of myeloma cells, we examined whether baicalein inhibited the phosphorylation of IkB-α and suppressed the expression of NF-kB targets such as IL-6 and XIAP.18,19 As shown in Figure 7A, baicalein inhibited the phosphorylation of IkB-α after 1 hour in U266 cells, whereas baicalin or wogonin did not induce any changes of IkB-α phosphorylation at up to 100 μM (data not shown). Then, after 3 hours, treatment of U266 with baicalein induced suppression of the expression of NF-kB target genes such as IL-6 and XIAP (Figure 7B). Also, we confirmed that baicalein induced suppression of the nucleic translocation of NF-kB (p65) in U266 cells, as shown in Figure 7F.
Effect of baicalein on phosphorylation of IkB-α, expression of NF-kB target genes, and mitochondrial apoptotic pathway. (A) Lysates from U266 cells treated with 50 or 100 μM baicalein for 1 hour were assessed for phosphorylation of IkB-α by Western blotting with anti-phospho-IkB-α antibody. (B) Expression of IL-6 and XIAP genes, targets of NF-kB, was assessed by semiquantitative RT-PCR in U266 cells treated with 50 or 100 μM baicalein for 3 hours. (C) The mitochondrial membrane potential of U266 cells was assessed after treatment with 0, 25, 50, 100, or 200 ng/mL of baicalein (•), baicalin (▴), or wogonin (♦). Values shown in this figure are the mean ± 1 SD of 3 independent experiments on flow cytometric detection of DiOC6 uptake at indicated time points. **P < .01. (D) Cells treated with 12.5, 25, or 50 μM baicalein for 12 hours were subcellularly fractionated. Cytochrome c (Cyto C) in cytosol was assessed by Western blotting with a specific anti-cytochrome c antibody. (E) Lysates from U266 cells treated with 25 or 50 μM baicalein for 12 hours were assessed for cleaved caspase cleavage by Western blotting with antibodies to caspase-9 or -3. (F) Flow cytometric assessment of nucleic translocation of NF-kB (p65). U266 cells were treated with 50 or 100 μM of baicalein for 1 hour.
Effect of baicalein on phosphorylation of IkB-α, expression of NF-kB target genes, and mitochondrial apoptotic pathway. (A) Lysates from U266 cells treated with 50 or 100 μM baicalein for 1 hour were assessed for phosphorylation of IkB-α by Western blotting with anti-phospho-IkB-α antibody. (B) Expression of IL-6 and XIAP genes, targets of NF-kB, was assessed by semiquantitative RT-PCR in U266 cells treated with 50 or 100 μM baicalein for 3 hours. (C) The mitochondrial membrane potential of U266 cells was assessed after treatment with 0, 25, 50, 100, or 200 ng/mL of baicalein (•), baicalin (▴), or wogonin (♦). Values shown in this figure are the mean ± 1 SD of 3 independent experiments on flow cytometric detection of DiOC6 uptake at indicated time points. **P < .01. (D) Cells treated with 12.5, 25, or 50 μM baicalein for 12 hours were subcellularly fractionated. Cytochrome c (Cyto C) in cytosol was assessed by Western blotting with a specific anti-cytochrome c antibody. (E) Lysates from U266 cells treated with 25 or 50 μM baicalein for 12 hours were assessed for cleaved caspase cleavage by Western blotting with antibodies to caspase-9 or -3. (F) Flow cytometric assessment of nucleic translocation of NF-kB (p65). U266 cells were treated with 50 or 100 μM of baicalein for 1 hour.
In order to clarify whether baicalein-induced apoptosis is mediated through a mitochondrial pathway, we examined the release of cytochrome c in the cytoplasmic fraction of U266 12 hours after treatment with baicalein and attempted to detect cleaved caspase-9 and caspase-3 by Western blotting. U266 cells treated with baicalein for 12 hours exhibited an increase in cytochrome c release into the cytosol at a concentration of 12.5 μM and more release at 50 μM (Figure 7D), and cleaved bands for the activation of caspase-9 and caspase-3 were detected by the treatment with baicalein as shown in Figure 7E. These results suggest that baicalein induced inhibition of IkB-α phosphorylation followed by down-regulation of NF-kB and also induced activation of mitochondria-mediated apoptosis in myeloma cells.
Discussion
The constitutive activation of NF-kB is observed in primary myeloma cells as well as myeloma cell lines, and NF-kB regulates the expression of many genes (IkB-α, Bcl-xL, IL-6, and cyclin D1) important for the proliferation and survival of myeloma cells.2,20 Thus, many researchers are now exploring this transcription factor as a main target in the treatment of multiple myeloma. Several agents have been identified, including PS34121,22 as a proteasome inhibitor, thalidomide analogs23 as inhibitors of tumor necrosis factor (TNF) production, and currcumin6 as an inhibitor of IkB-α kinase (IKK). In this paper we show that Scutellaria radix and its component baicalein are also targets of NF-kB inhibitors in human myeloma cells. Baicalein inhibited the survival of primary myeloma cells, especially MPC-1- immature myeloma cells in vitro, and induced apoptosis in myeloma cell lines through the down-regulation of IkB-α phosphorylation.
We have extensively tested antiproliferative Kampo medicines having anti-inflammatory or non-anti-inflammatory activity in a human myeloma cell line, U266. In this paper, representative data concerning the anti-inflammatory agents HLJDT and HLT and the non-anti-inflammatory agent GZFLW are presented. HLJDT had the strongest inhibitory effect on cellular proliferation in U266 (ID50 = 70 ng/mL) among these Kampo medicines and also suppressed the IL-6-dependent growth of ILKM2 cells (ID50 = 55 ng/mL). Furthermore, we identified Scutellaria radix as responsible for the antiproliferative effect of HLJDH, because the removal of Scutellaria radix from HLJDT reduced its antiproliferative activity and Scutellaria radix itself had a strong antiproliferative effect on proliferation, as shown in Figure 4. In the Scutellaria radix, we found that baicalein had the strongest inhibitory effect on the proliferation of U266 cells as well as other myeloma cell lines such as ILKM2, NOP2, and AMO1 among the 3 components baicalein, baicalin, and wogonin. Baicalein has been reported to have antiproliferative effects on a human myeloid cell line, HL-60,24 a human retinal pigment epithelial cell line,25 and so on.26 Based on the reported data and our own results, the IC50 value for the antiproliferative effect of baicalein was calculated to be about 25 μM in HL-60 cells, 28 μM in U266 cells, and 16.8 μM in primary CD38++MPC-1- immature myeloma cells. These values suggest that the antiproliferative effect of baicalein is not specific to myeloma cells. Some cancer cells such as Epstein-Barr virus (EBV)-transformed B-cell lines were sensitive to baicalein at a concentration of 25 μM. However, another important finding in this paper is that baicalein had an inhibitory effect on the survival of CD38++MPC-1- immature myeloma cells but not CD38+MPC-1+ intermediate/mature myeloma cells in vitro; baicalein could not inhibit the survival of these MPC-1+ myeloma cells and normal myeloid cells or peripheral blood cells at concentrations of 25 μM to 100 μM in vitro (data not shown). Since MPC-1- immature myeloma cells as well as myeloma cell lines are proliferating or can respond to IL-6 to proliferate, it is likely that baicalein has more of an inhibitory effect on the proliferation and survival of myeloma cells than of dormant or normal plasma cells.
HLJDT, Scutellaria radix, and baicalein induced morphologic changes consistent with cellular apoptosis in myeloma cell lines U266 and ILKM2. We confirmed that the mitochondrial membrane potential was lost rapidly, and then the release of cytochrome c was potentiated followed by the activation of caspase-9 and caspase-3 after these treatments. Therefore, the apoptosis induced by HLJDT, Scutellaria radix, or baicalein may occur through a mitochondria-mediated pathway. In this pathway, down-regulation of NF-kB is considered to be critical, modulating the expression and function of B-cell lymphoma 2 (bcl-2) family proteins in the mitochondria.22,27,28 We also found that U266 cells expressed XIAP17 (X-linked inhibitor of apoptosis protein) among cellular inhibitors of apoptosis proteins such as IAP1 and 2 and its expression was down-regulated by baicalein in U266 cells. Since NF-kB upregulates the expression of XIAP and this XIAP inhibits both newly activated caspsae-9 and its effector caspase-3,25 it is also possible that down-regulation of NF-kB induced by baicalein down-regulates XIAP and the reduced XIAP expression inversely stimulates caspase-9-mediated apoptosis followed by the activation of caspase-3 and augmented induction of apoptosis.
Down-regulation of NF-kB activity is considered to be involved in the suppression of cellular proliferation and induction of apoptosis in myeloma cells2 induced by HLJDT, Scutellaria radix, or baicalein. In this paper, we showed that baicalein suppressed the phosphorylation of IkB-α, but not expression of IkB-α itself, in a dose-dependent manner shortly after its application. Further, we confirmed that NF-kB activity after baicalein treatment was markedly reduced through the flow cytometric detection of phosphorylated NF-kB p65(Ser536) in U266 cells (Figure 7F). Our findings are consistent with previous reports that showed an inhibition of binding to the NF-kB site by baicalein in lipopolysaccharide (LPS)-activated murine macrophages,26 human hepatoma cells,27 and murine microglia cells.28 However, it remains to be clarified how the phosphorylation of IkB-α is down-regulated by baicalein. The phosphorylation is catalyzed by the activation of a complex consisting of 2 kinases, IKK-α and IKK-β, together with a modifying subunit of IKK-γ (NF-kB essential modulator [NEMO]).29,30 At present, it is not clear whether baicalein suppresses the kinase activity of IKK(s) as a kinase inhibitor directly or indirectly, or whether baicalein inhibits the expression of IKK(s) itself or not. With respect to its activity as a kinase inhibitor, baicalein was reported to inhibit Raf-1-mediated phosphorylation; baicalein functions as an inhibitor for mitogen-induced extracellular kinase 1 (MEK-1) kinase.31 Also, a recent report showed that baicalein was an effective inhibitor of protein kinase CK2.32
As for the actions of baicalein, it should be noted that baicalein has a wide variety of effects in many types of cells including myeloma cells. For example, baicalein suppresses the activity of cyclooxygenase 2 (COX-2) followed by the inhibition of prostaglandin E2 and inhibition of cell proliferation in cancer cells33 ; this suppression of COX-2 activity may be due to a suppression of COX-2 gene expression induced by the down-regulation of NF-kB. Baicalein is also a specific inhibitor for 12-lipoxygenase (12-LOX), which is a key regulator of tumor cell proliferation and motility, the regulation of apoptosis, and tumor angiogenesis.34-36 Furthermore, baicalein is a potent inhibitor for α-glucosidase, which catalyzes the final step in the digestion of carbohydrates.37 Based on these observations, we cannot exclude the possibility that the baicalein-induced inhibition of myeloma cell proliferation and induction of apoptosis result from not only the down-regulation of NF-kB activity but also various other actions of baicalein.
Finally, baicalein or Scutellaria radix (root) may be useful oral medicines for therapy to treat multiple myeloma. Given our finding that both baicalein and Scutellaria radix also inhibit the production of IL-6 in bone marrow mononuclear cells or myeloma cell lines (U266; data not shown), the oral administration of baicalein or Scutellaria radix (or HLJDH) could result in both a direct inhibition of myeloma cell proliferation and a reduction of IL-6, a growth and survival factor for myeloma cells.10,38,39 Notably, baicalein selectively affects the survival of MPC-1- immature myeloma cells, which contain IL-6-responsive CD45+ myeloma cells, and this effect would contribute to the inhibition of relapse during maintenance therapy in multiple myeloma. The results presented here clearly show that baicalein (or Scutellaria radix) can directly inhibit the proliferation of myeloma cells and present enough evidence for clinical trials to treat multiple myeloma.
Prepublished online as Blood First Edition Paper, December 30, 2004; DOI 10.1182/blood-2004-10-3915.
Supported in part by grants from the Ministry of Education, Science, Sports and Culture of Japan and the Ministry of Health, Labour and Welfare of Japan. S.A. is the recipient of a Postdoctoral Fellowship Award for Foreign Researchers (P04500) from the Japan Society for the Promotion of Science (JSPS).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Tsumura Central Research Laboratories; Tsumura and Co for providing traditional Chinese medicines; and the staff of the Science Research Center, Yamaguchi University for support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal