Abstract
Interaction between target cell CD47 and the inhibitory macrophage receptor signal regulatory protein α (SIRPα) counteracts macrophage phagocytosis of CD47-expressing host cells. As platelets also express CD47, we asked whether inhibitory CD47/SIRPα signaling regulates normal platelet turnover and clearance of platelets in immune thrombocytopenic purpura (ITP). CD47-/- mice had a mild spontaneous thrombocytopenia, which was not due to a decreased platelet half-life as a result of increased expression of P-selectin, CD61, or phosphatidylserine. In contrast, CD47-/- platelets were rapidly cleared when transfused into CD47+/+ recipients, whereas CD47+/- platelets had a nearly normal half-life in CD47+/+ mice under nonautoimmune conditions. CD47-/- mice were more sensitive to ITP, as compared with CD47+/+ mice. In vitro, macrophage phagocytosis of immunoglobulin G (IgG)–opsonized CD47-/- platelets was significantly higher than that for equally opsonized CD47+/+ platelets. However, when SIRPα was blocked, phagocytosis of CD47+/+ platelets increased to the level of CD47-/- platelets. Phagocytosis of opsonized CD47+/- platelets was higher than that for CD47+/+ platelets, but lower than that for CD47-/- platelets, suggesting a gene-dose effect of CD47 in this system. In conclusion, we suggest that inhibitory CD47/SIRPα signaling is involved in regulating platelet phagocytosis in ITP, and that targeting SIRPα may be a new means of reducing platelet clearance in ITP.
Introduction
Immune thrombocytopenic purpura (ITP) is an autoimmune disease characterized by low platelet counts as a result of antibody-mediated destruction of platelets.1 Platelet autoantibodies are of the immunoglobulin G (IgG) type and are most commonly directed to the glycoprotein IIb/IIIa complex. However, most patients have antibodies directed to several different platelet surface proteins.2,3 Extensive research in recent years has shown that production of antiplatelet antibodies in ITP is strongly related to the presence of autoreactive T cells and an altered cytokine environment.4 Platelets coated with IgG autoantibodies undergo accelerated clearance through Fcγ receptor–mediated phagocytosis by macrophages, usually in the spleen and liver.1 ITP can be treated with corticosteroids or intravenous gamma globulin (IVIG).5 IVIG has well-known anti-inflammatory effects, generally attributed to the ability of the IgG Fc domain to block prophagocytic Fcγ receptors on macrophages.6 However, data from the murine system have indicated that the protective effect of IVIG could also be mediated by its ability to induce expression of the inhibitory FcγRIIB receptor on splenic macrophages.7 Furthermore, the FcγRIIB-mediated protective effect of IVIG may involve signaling pathways different from that in B cells.8 Intriguingly, monoclonal antibodies to erythrocyte or leukocyte surface antigens can mimic the effect of IVIG, pointing at alternative ways to treat ITP.9 In more severe cases of ITP, and in cases of tolerance to corticosteroids, splenectomy may be required to reduce platelet destruction.1
Signal regulatory protein α (SIRPα) is an immunoglobulin (Ig) superfamily member, with 1 or 3 extracellular Ig domains (alternative splicing) and an intracellular tail with 2 immunoreceptor tyrosine-based inhibitory motifs.10,11 SIRPα is expressed preferentially by neurons and myeloid cells such as neutrophils, monocytes, and monocyte-derived cells.12,13 When expressed by macrophages and dendritic cells, SIRPα can function as an inhibitory receptor that down-regulates phagocytosis.10,14,15 The ligand for SIRPα is the cell-surface glycoprotein CD47 (integrin-associated protein, IAP), which is expressed by virtually all cells in the host.13,16,17
We have shown that CD47 can function as a marker of self on red blood cells (RBCs) and that CD47-/- RBCs were rapidly cleared when transfused into syngeneic wild-type mice, whereas they circulated normally in CD47-/- mice.15 This clearance mechanism was independent of complement and antibody, and it was based almost entirely in the spleen where the CD47-deficient cells were preferentially ingested by splenic F4/80+ red-pulp macrophages.15 By blocking SIRPα on isolated splenic macrophages, the level of phagocytosis of wild-type RBCs could be increased to that seen with CD47-/- RBCs.15 We, thus, proposed that all RBCs are recognized by splenic macrophages, which must have a receptor for RBCs. In the absence of inhibitory SIRPα signaling, this macrophage-RBC interaction is sufficient for phagocytosis to occur. CD47-/- lymphohematopoietic cells were also rapidly eliminated by splenic macrophages and dendritic cells in wild-type recipients.14 Thus, CD47 can function as an important “marker of self” to help macrophages to distinguish self from foreign.15
This concept was further extended when we found that the inhibitory signals generated by macrophage SIRPα upon ligation of CD47 also affect prophagocytic signaling mechanisms that are important for phagocytosis stimulated via Fcγ receptors and complement receptors.18 As a result, CD47-/- mice are more sensitive to spontaneous and experimental autoimmune hemolytic anemia (AIHA).19 Thus, we have hypothesized that activation of phagocytosis in macrophages in contact with target host cells is a balance between signals from activating receptors (eg, FcγR, complement receptors, or other prophagocytic receptors) and the inhibitory signal from SIRPα ligated by target cell CD47. In the macrophage, neither signal appears to be dominant, but rather the decision to phagocytose is based on an integration of the relative signaling strength from positive prophagocytic receptors and inhibitory CD47/SIRPα interactions.18
On the basis of our previous findings on the important role of inhibitory CD47/SIRPα signaling in regulating macrophage destruction of blood cells, the aim of this study was to investigate whether CD47/SIRPα interaction is also involved in regulating platelet homeostasis and platelet clearance during experimental ITP.
Materials and methods
Mice
Generation of CD47-/- mice has been previously described.17 Male and female CD47-/- C57BL/6J or Balb/c mice, backcrossed to C57BL/6J or Balb/c (Jackson Laboratory, Bar Harbor, ME) for 16 or more generations, and their heterozygous and homozygous littermates were from our own breeding colony. Animals were kept in accordance with local guidelines and maintained in a specific pathogen-free barrier facility. All animal procedures were approved by institutional review committees.
Reagents
Mouse anti–mouse platelet monoclonal antibody (mAb 6A6, mouse IgG2a)20 (originating from Dr R. Good, University of South Florida College of Medicine, Tampa), rat anti–murine SIRPα (mAb P84; rat IgG1)16 (originating from Dr C. Lagenaur, University of Pittsburgh, PA), rat anti–murine CD47 (mAb mIAP301; rat IgG2a)17 (originating from Dr F. P. Lindberg, Washington University School of Medicine, St Louis, MO), and rat anti–murine FcγRIIB/III (mAb 2.4G2; rat IgG2b; ATCC, Manassas, VA), were purified from hybridoma supernatants by ammonium sulfate precipitation and affinity chromatography using Protein A or G High Trap columns (Amersham Bioscience, Piscataway, NJ). F(ab′)2 fragments of F(ab′)2–specific fluorescein isothiocyanate (FITC)–conjugated goat anti–mouse or goat anti–rat IgG, thrombin, annexin V–FITC, carbacyclin, and Hanks balanced salt solution were from Sigma-Aldrich, St Louis, MO. Dulbecco modified Eagle medium and fetal calf serum (FCS) were from Invitrogen, Stockholm, Sweden. Phycoerythrin-conjugated anti–mouse CD61, FITC-conjugated anti-CD62P, and isotype control antibodies were from Pharmingen, San Diego, CA. 5-chloromethylfluorescein diacetate (CMFDA) was from Molecular Probes, Eugene, OR.
Isolation and CMFDA labeling of platelets
Isolation and labeling of platelets for in vivo studies were done as previously described,21 with a few modifications. In brief, anticoagulation buffer contained the following: trisodium citrate (130 mM), disodium EDTA (ethylenediaminetetraacetic acid; 10 mM), theophylline (10 mM), and the prostaglandin I2 (PGI2) analog carbacyclin (2 μg/mL). Buffered saline glucose citrate (BSGC) contained NaCl (116 mM), trisodium citrate (13.6 mM), Na2HPO4-7H2O (8.6 mM), KH2PO2 (1.6 mM), disodium EDTA (0.9 mM), glucose (11.1 mM), and carbacyclin (1 μg/mL), pH 6.8. Blood was obtained by cardiac puncture using a syringe containing 200 μL anticoagulant buffer–BSGC ratio of 1:1. Each mouse yielded approximately 600 μL blood. The blood was further diluted to a total volume of 1.5 mL in BSGC, followed by centrifugation at 300g for 3 minutes, and platelet-rich plasma (PRP) was collected. The pellet was rediluted to a total volume of 1.5 mL in BSGC and centrifuged once more to obtain a second PRP fraction. The PRP fractions were combined, and the platelets were pelleted at 1200g for 10 minutes and resuspended in BSGC to a concentration of 5 × 108 platelets/mL. CMFDA was added to the platelets, at a final concentration of 2.5 μM, and incubated in the dark for 45 minutes at room temperature. Platelets were then centrifuged at 1200g for 10 minutes, and the pellet was suspended in sterile physiologic saline for survival experiments and BSGC for in vitro experiments.
Flow cytometric analysis of platelet CD47 and CD61 expression
Blood was collected from a tail vein and was diluted in phosphate-buffered saline (PBS) with 5 mM EDTA, washed twice in PBS, and incubated with anti–mouse CD47 (mAb miap301), followed by FITC-conjugated secondary antibody, and phycoerythrin-conjugated anti–mouse CD61 for 30 minutes on ice. After washing off unbound antibody, the samples were analyzed using FACscan flow cytometry (BD Biosciences, Mountain View, CA) and CellQuest software (BD Biosciences). Platelets were distinguished, based on size and expression of CD61.
Platelet phosphatidylserine exposure
This procedure has previously been described.22 In short, blood was collected by cardiac puncture using heparin as anticoagulant and mixed with 500 μL BSGC without carbacyclin. PRP was obtained by centrifugation at 300g for 3 minutes. The platelet concentration was adjusted to 3 × 108/mL followed by incubation at 37°C. At times indicated, 5 μL platelets were transferred to 500 μL annexin V–binding buffer. The platelets were then incubated with 1 μL annexin V–FITC for 10 minutes in the dark, followed by flow cytometric analysis.
Thrombin-induced CD62P expression
CD62P expression of thrombin-activated platelets was studied, using an established protocol.23 For this, blood was obtained by retro-orbital bleeding using heparinized capillary tubes. PRP was prepared by centrifugation at 300g for 3 minutes, after which platelets were sedimented at 1200g for 10 minutes and washed in wash solution (PBS/sodium azide 0.1%/HSA [human serum albumin] 1%). Platelets were then incubated with different concentrations of thrombin (0-1 U/mL) at room temperature for 10 minutes. Cells were fixated using 2% formaldehyde in PBS at room temperature for 30 minutes and washed in wash solution. Platelets were then incubated with FITC-conjugated anti–CD62P or FITC-conjugated isotype control on ice for 30 minutes, washed, and analyzed by flow cytometry.
Platelet survival experiments
Platelets were isolated and labeled with CMFDA as described in “Isolation and CMFDA labeling of platelets.” After CMFDA labeling the platelets were resuspended in sterile saline to a concentration of 3 × 108 platelets/mL. Platelet solution (350 μL) was injected intravenously in a lateral tail vein of each recipient mouse. To follow platelet survival, 5 μL blood was collected from a lateral tail vein and diluted in 95 μL PBS/5 mM EDTA and analyzed by flow cytometry, counting a total of 100 000 events. The fraction of labeled platelets in blood drawn at 10 minutes (CD47-/- to wild type) or at 2 hours (other combinations) after the injection was used as baseline values. The number of labeled platelets in samples at later time points was expressed in the percentage relative to the 10-minute or 2-hour value.
Preparation of murine bone marrow–derived macrophages
Bone marrow–derived macrophages were prepared as previously described.24 Briefly, femurs were removed from female C57BL/6 mice, and the bone marrow was flushed out with PBS/1% HSA. Following lysis of red blood cells, the bone marrow cells were resuspended in Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat-inactivated FCS, 100 U/mL penicillin and streptomycin (DMEM/10% FCS), plated on tissue-culture dishes, and incubated for 2 hours at 37°C. Thereafter, nonadherent cells were resuspended in DMEM/10% FCS supplemented with 15% L929 cell supernatant (as a source of macrophage colony-stimulating factor [M-CSF]) and plated on bacterial plastic dishes for 6 days. Mature macrophages (3 × 105/well) were then plated in 24-well tissue culture plates and allowed to adhere for 2 hours, followed by removal of nonadherent cells. The cells were then cultured in DMEM/10% FCS without M-CSF for 24 hours before use in phagocytosis experiments.
In vitro phagocytosis experiments
In vitro phagocytosis of IgG-opsonized platelets was studied essentially as previously described.22,25 For this, platelets were isolated and labeled with CMFDA, as described in “Isolation and CMFDA labeling of platelets.” resuspended in BSGC at 5 × 108/mL and incubated in the dark for 30 minutes to remove excess dye. The platelets were then pelleted at 1200g for 10 minutes and resuspended to 3 × 108/mL in Hanks balanced salt solution (HBSS) containing carbacyclin (1 μg/mL). For opsonization, mAb 6A6 was added at 0.65 μg/mL, and the platelets were incubated for 20 minutes at room temperature. The cells were then washed with HBSS and resuspended to 3 × 108/mL in DMEM/10% FCS. Before addition of platelets, macrophages were incubated with anti-SIRPα mAb P84 or isotype control mAb (75 μg/mL) for 15 minutes. Then 3 × 107 platelets were added to each well, and the plates were centrifuged at 200g for 1 minute to establish contact between macrophages and platelets. Following incubation for 30 minutes at 37°C and 5% CO2, the macrophages were washed 3 times with HBSS. To remove uningested platelets, the macrophages were treated with 0.5 mM EDTA and 0.05% trypsin in PBS for 5 minutes at 37°C, followed by detachment of the macrophages using PBS/5 mM EDTA at 4°C. Macrophages were pelleted at 200g for 5 minutes and incubated with Fc-block (20 μg/mL mAb 2.4G2) for 15 minutes on ice, before incubation with phycoerythrin-conjugated anti–mouse CD61 for 20 minutes on ice. The macrophages were washed and resuspended for flow cytometric analysis. The gates were set to separate macrophages with ingested platelets (CMFDA+/CD61-) from macrophages with platelets bound to the surface (CMFDA+/CD61+). Percentage of phagocytosis was calculated as the fraction of macrophages with ingested platelets of the total number of macrophages analyzed.
Induction of experimental thrombocytopenia
The mice were anesthetized using isoflurane before mAb 6A6 (diluted in 0.9% sterile saline) was injected intravenously in a lateral tail vein. For determination of circulating platelet counts, blood (5 μL) was obtained from a tail vein and was immediately diluted in 95 μL cold PBS/5 mM EDTA. The blood was then kept on ice until further analysis (which was within 1 hour). Blood samples were taken before administration of mAb 6A6 or saline, and at 2, 4, 6, and 24 hours after injection of mAb 6A6. Platelet counts were determined using an automatic blood analyser (Sysmex KX-21; Sysmex Europe GMBH, Norderstedt, Germany). Before analysis, the blood samples were diluted in 400 μL diluting reagent (Cellpack; Sysmex) to give a final dilution of 1/100.
Statistics
Statistical analysis was performed by using the 2-tailed Student t test for paired or unpaired samples (see legends to the figures). All results are expressed as mean ± SEM.
Results
Mild spontaneous thrombocytopenia in CD47-deficient mice
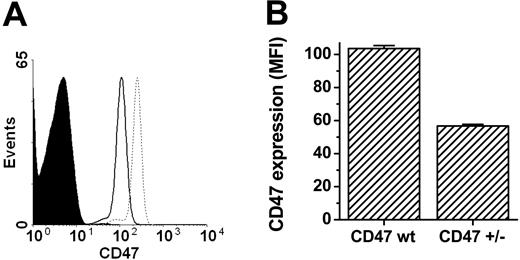
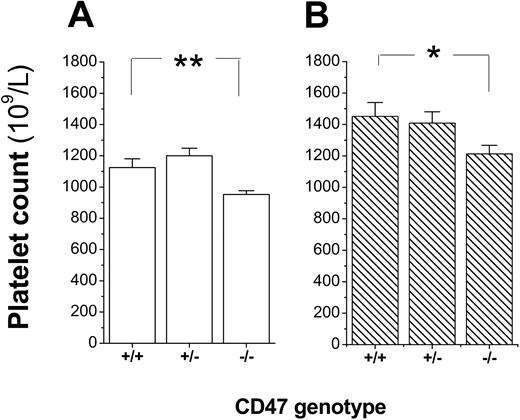
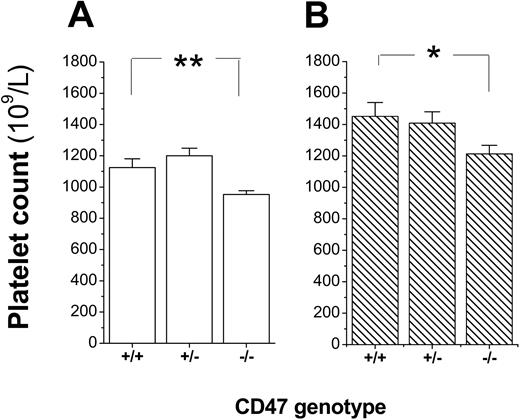
To confirm that platelets expressed CD47, platelets were incubated with the anti-CD47 mAb miap301 and analyzed by flow cytometry. As shown in Figure 1A-B, platelets from CD47+/+ and CD47+/- mice expressed CD47, whereas platelets from CD47+/- mice expressed about 55% of the CD47 level seen in CD47 wild-type mice. Further analysis of platelet counts in blood of a cohort of CD47+/+, CD47+/-, and CD47-/- C57BL/6 mice showed that both female and male CD47-/- mice had a mild spontaneous thrombocytopenia (Figure 2A-B). Virtually identical results were also found in female and male Balb/c mice (data not shown).
Platelets express CD47. (A) Flow cytometric profiles of CD47 expression on platelets from CD47-/- (filled curve), CD47+/- (solid line), and CD47+/+ mice (dotted line). (B) Comparison of CD47 expression levels on CD47+/+ (CD47 wt; n = 9) and CD47+/- (n = 6) platelets. Data are means ± SEMs. MFI indicates mean fluorescence intensity.
Platelets express CD47. (A) Flow cytometric profiles of CD47 expression on platelets from CD47-/- (filled curve), CD47+/- (solid line), and CD47+/+ mice (dotted line). (B) Comparison of CD47 expression levels on CD47+/+ (CD47 wt; n = 9) and CD47+/- (n = 6) platelets. Data are means ± SEMs. MFI indicates mean fluorescence intensity.
Mild reduction in platelet counts of CD47-/- mice. Platelet counts in 8- to 12-week-old CD47+/+, CD47+/-, and CD47-/- (A) female (n = 10, 11, and 13, respectively) or (B) male (n = 9, 2, and 12, respectively) C57BL /6 mice. Data are means ± SEMs. *P < .05 and **P < .01, using Student t test for unpaired samples.
Mild reduction in platelet counts of CD47-/- mice. Platelet counts in 8- to 12-week-old CD47+/+, CD47+/-, and CD47-/- (A) female (n = 10, 11, and 13, respectively) or (B) male (n = 9, 2, and 12, respectively) C57BL /6 mice. Data are means ± SEMs. *P < .05 and **P < .01, using Student t test for unpaired samples.
CD47-/- platelets are rapidly cleared in wild-type mice but not in CD47-/- mice
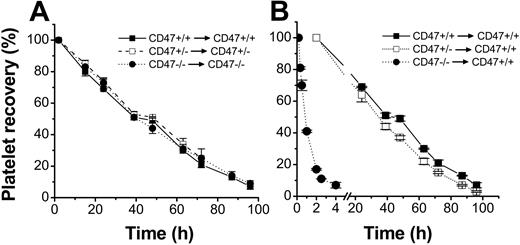
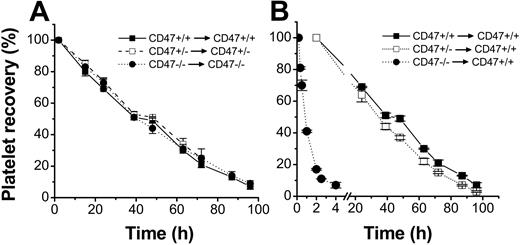
To study whether platelets in CD47-/- mice had a shorter half-life in the circulation, platelets were isolated from anticoagulated blood, taking care not to induce platelet activation, and labeled with the fluorescent cell tracker CMFDA. However, as shown in Figure 3A, the half-life of CD47+/+, CD47+/-, or CD47-/- platelets in their respective genotype recipients were about the same, with virtually complete clearance at 96 hours after injection. In contrast, when CD47-deficient platelets were injected into wild-type recipient mice, more than 90% of CD47-deficient platelets were cleared from the circulation within 4 hours after injection, with a half-life of 1.1 ± 0.1 hours (Figure 3B). Since CD47+/- platelets had 45% lower CD47 expression, as compared with wild-type platelets (Figure 1A-B), we studied whether CD47+/- platelets also had a shorter half-life when injected into wild-type mice. However, there was only a marginally shortened half-life of CD47+/- platelets, as compared with that for CD47 wild-type platelets, in wild-type recipients (30.6 ± 1.0 hours versus 33.6 ± 1.2 hours; Figure 3B). Therefore, it seemed that lack of CD47 had a dramatic effect in reducing platelet survival in CD47 wild-type mice, but that a 55% of normal CD47 expression was sufficient to prevent accelerated platelet turnover.
Role of CD47 for platelet half-life in circulation. Platelets were isolated from CD47+/+ (▪), CD47+/- (□), or CD47-/- (⬡) C57BL /6 mice, labeled with the cell tracker CMFDA, and injected into (A) recipient mice of the same genotype or (B) CD47+/+ mice. At the times indicated, 5 μL blood was sampled from a tail vein of the recipients and analyzed by flow cytometry for the fraction of fluorescent platelets of the total recipient platelets. Data were normalized to the level at 10 minutes (CD47-/- to CD47+/+) or 2 hours after injection. Data are means ± SEMs for 4 to 5 mice in each group.
Role of CD47 for platelet half-life in circulation. Platelets were isolated from CD47+/+ (▪), CD47+/- (□), or CD47-/- (⬡) C57BL /6 mice, labeled with the cell tracker CMFDA, and injected into (A) recipient mice of the same genotype or (B) CD47+/+ mice. At the times indicated, 5 μL blood was sampled from a tail vein of the recipients and analyzed by flow cytometry for the fraction of fluorescent platelets of the total recipient platelets. Data were normalized to the level at 10 minutes (CD47-/- to CD47+/+) or 2 hours after injection. Data are means ± SEMs for 4 to 5 mice in each group.
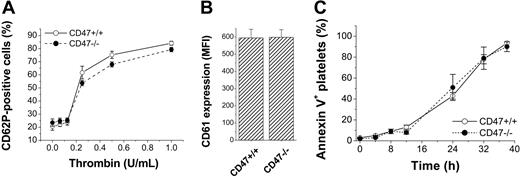
We next investigated whether the accelerated clearance of CD47-deficient platelets in wild-type mice was due to increased platelet activation or accelerated platelet senescence. There were no differences between freshly isolated CD47+/+ or CD47-/- platelets with respect to baseline expression of CD62P (P-selectin) (Figure 4A), CD61 (Figure 4B), or phosphatidylserine (Figure 4C). Following platelet activation with thrombin, both CD47+/+ and CD47-/- platelets up-regulated P-selectin to the same extent (Figure 4A). When platelets were aged in PRP in vitro, the up-regulation of cell-surface phosphatidylserine over time was the same in CD47+/+ and CD47-/- platelets (Figure 4C).
Surface phenotype of CD47-/- platelets. (A) Expression of CD62P in CD47+/+ (○) or CD47-/- (⬡) platelets incubated in the presence or absence of thrombin for 10 minutes. Data are means ± SEMs for 4 mice in each group. (B) Expression of CD61 in freshly isolated CD47+/+ or CD47-/- platelets. Data are means ± SEMs for 3 mice in each group. (C) Expression of phosphatidylserine in CD47+/+ (○) or CD47-/- (⬡) platelets aged in PRP for 0 to 40 hours at 37°C. Data are means ± SEMs for 3 mice in each group.
Surface phenotype of CD47-/- platelets. (A) Expression of CD62P in CD47+/+ (○) or CD47-/- (⬡) platelets incubated in the presence or absence of thrombin for 10 minutes. Data are means ± SEMs for 4 mice in each group. (B) Expression of CD61 in freshly isolated CD47+/+ or CD47-/- platelets. Data are means ± SEMs for 3 mice in each group. (C) Expression of phosphatidylserine in CD47+/+ (○) or CD47-/- (⬡) platelets aged in PRP for 0 to 40 hours at 37°C. Data are means ± SEMs for 3 mice in each group.
The CD47/SIRPα interaction regulates phagocytosis of IgG-sensitized platelets
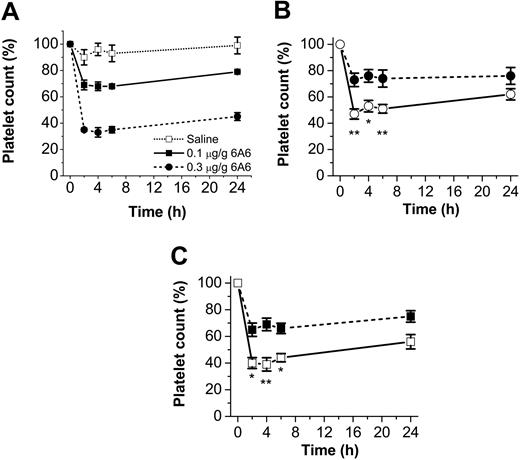
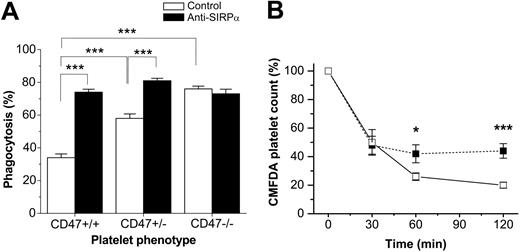
We next studied the role of the inhibitory CD47/SIRPα interaction on FcγR-mediated destruction of IgG-sensitized platelets. For this, we first induced experimental ITP in CD47-/- or CD47+/+ mice, using the pathogenic anti–mouse platelet mAb 6A6, which triggers a rapid dose-dependent consumption of platelets when injected in mice, mimicking the pathogenic effect of autoantibodies in ITP (Figure 5A).7,20 Following injection of mAb 6A6 (0.1 μg/g body weight), both CD47-/- C57BL/6 and Balb/c mice were significantly more sensitive to experimental ITP as compared with their CD47 wild-type controls (Figure 5B-C). Control experiments showed that platelets from CD47-/- and CD47+/+ mice were equally opsonized by mAb 6A6 (data not shown). On the basis of the increased sensitivity to this ITP model, the higher concentration of 0.3 μg 6A6/g was not used in CD47-/- mice. To further investigate whether the specific interaction between platelet CD47 and macrophage SIRPα was involved in regulating platelet uptake in ITP, we studied phagocytosis of platelets by wild-type macrophages in vitro. As shown in Figure 6A, 6A6-opsonized CD47+/+ platelets were phagocytosed to a significantly lower extent than equally opsonized CD47-/- platelets (34.0% ± 2.3% versus 76.0% ± 1.7% phagocytosis; P < .001) However, when macrophage SIRPα was blocked with the functional blocking mAb P84, phagocytosis of CD47+/+ platelets was increased to the same level as that seen with CD47-/- platelets (Figure 6A). The SIRPα blocking mAb had no effect on macrophage phagocytosis of CD47-/- platelets (Figure 6A).
CD47-/- mice are more sensitive to experimental ITP. Experimental ITP was induced in CD47+/+ and CD47-/- C57BL /6 or Balb/c mice, using the pathogenic IgG2a anti–mouse platelet mAb 6A6. (A) Dose-dependent effect of 6A6 in CD47+/+ mice. (B) CD47+/+ (⬡) or CD47-/- (○) C57BL /6 mice were injected with 6A6 at 0.1 μg/g body weight. Baseline platelet counts were 1179 ± 94 × 109 platelets/L for CD47+/+ mice and 980 ± 42 × 109 platelets/L for CD47-/- mice. (C) CD47+/+ (▪)or CD47-/- (□) Balb/c mice were injected with 6A6 at 0.1 μg/g body weight. Baseline platelet counts were 1332 ± 40 × 109 platelets/L for CD47+/+ mice and 1029 ± 122 × 109 platelets/L for CD47-/- mice. Data are means ± SEMs for 4 to 6 mice in each group. *P < .05 and **P < .01, as compared with CD47+/+ mice, using Student t test for unpaired samples.
CD47-/- mice are more sensitive to experimental ITP. Experimental ITP was induced in CD47+/+ and CD47-/- C57BL /6 or Balb/c mice, using the pathogenic IgG2a anti–mouse platelet mAb 6A6. (A) Dose-dependent effect of 6A6 in CD47+/+ mice. (B) CD47+/+ (⬡) or CD47-/- (○) C57BL /6 mice were injected with 6A6 at 0.1 μg/g body weight. Baseline platelet counts were 1179 ± 94 × 109 platelets/L for CD47+/+ mice and 980 ± 42 × 109 platelets/L for CD47-/- mice. (C) CD47+/+ (▪)or CD47-/- (□) Balb/c mice were injected with 6A6 at 0.1 μg/g body weight. Baseline platelet counts were 1332 ± 40 × 109 platelets/L for CD47+/+ mice and 1029 ± 122 × 109 platelets/L for CD47-/- mice. Data are means ± SEMs for 4 to 6 mice in each group. *P < .05 and **P < .01, as compared with CD47+/+ mice, using Student t test for unpaired samples.
CD47/SIRPα interaction can strongly reduce macrophage phagocytosis of 6A6-opsonized platelets. (A) Platelets were isolated from CD47+/+, CD47+/-, or CD47-/- C57BL/6 mice, labeled with the cell tracker CMFDA, opsonized with 0.65 μg/mL mAb 6A6, and incubated with CD47+/+ bone marrow–derived macrophages for 30 minutes at 37°C and 5% CO2. Macrophages were pretreated with 75 μg/mL anti-SIRPα mAb P84 (▪) or isotype control mAb (□) for 15 minutes before addition of platelets. Macrophages were then treated and scored for phagocytosis as described in “Materials and methods.” Data are means ± SEMs for 3 to 4 experiments in each group. ***P < .001, using Student t test for paired or unpaired comparisons. (B) Platelets were isolated from CD47+/+ or CD47+/- C57BL /6 mice, labeled with the cell tracker CMFDA, and injected into CD47+/+ recipient mice. Experimental ITP was then induced by injection of mAb 6A6 at 0.3 μg/g body weight, and clearance of CD47+/- (□) or CD47+/+ platelets (▪) was followed as described in the legend to Figure 3. Data are means ± SEMs for 5 mice in each group. *P < .05 and ***P < .001, using Student t test for paired comparisons.
CD47/SIRPα interaction can strongly reduce macrophage phagocytosis of 6A6-opsonized platelets. (A) Platelets were isolated from CD47+/+, CD47+/-, or CD47-/- C57BL/6 mice, labeled with the cell tracker CMFDA, opsonized with 0.65 μg/mL mAb 6A6, and incubated with CD47+/+ bone marrow–derived macrophages for 30 minutes at 37°C and 5% CO2. Macrophages were pretreated with 75 μg/mL anti-SIRPα mAb P84 (▪) or isotype control mAb (□) for 15 minutes before addition of platelets. Macrophages were then treated and scored for phagocytosis as described in “Materials and methods.” Data are means ± SEMs for 3 to 4 experiments in each group. ***P < .001, using Student t test for paired or unpaired comparisons. (B) Platelets were isolated from CD47+/+ or CD47+/- C57BL /6 mice, labeled with the cell tracker CMFDA, and injected into CD47+/+ recipient mice. Experimental ITP was then induced by injection of mAb 6A6 at 0.3 μg/g body weight, and clearance of CD47+/- (□) or CD47+/+ platelets (▪) was followed as described in the legend to Figure 3. Data are means ± SEMs for 5 mice in each group. *P < .05 and ***P < .001, using Student t test for paired comparisons.
Gene dose effect of CD47 in regulation of antibody-dependent platelet clearance
In ITP experiments in which CD47+/- and CD47 wild-type mice were compared, we did not find any significant differences in the sensitivity to mAb 6A6 (not shown). However, in vitro, there was a significantly higher phagocytosis of 6A6-opsonized CD47+/- platelets by wild-type macrophages, as that seen with equally opsonized wild-type platelets (58.0% ± 2.7% versus 34.0% ± 2.3% phagocytosis; P < .001; Figure 6A). Again, when the CD47/SIRPα interaction was blocked by mAb P84, platelets of both genotypes were phagocytosed to the same extent (Figure 6A). We next compared the clearance rate of CMFDA-labeled CD47+/- or CD47 wild-type platelets in wild-type recipient mice following injection of 0.3 μg 6A6/g body weight. Using this approach, we found that there was a significantly more rapid clearance of CD47+/- platelets, as compared with that for wild-type platelets, during the initial phase of this experimental ITP model (Figure 6B).
Discussion
The present study shows that platelet CD47 expression is important in regulating normal platelet turnover and FcγR-mediated clearance of IgG-sensitized platelets. Thus, CD47-deficient platelets had a dramatically shortened half-life in the circulation of CD47 wild-type mice and were also more sensitive to Fcγ receptor–mediated clearance, both in vivo and in vitro. By blocking macrophage SIRPα, phagocytosis of CD47 wild-type platelets in vitro was increased to the level of equally opsonized CD47-/- platelets.
We have shown that normal RBCs are recognized by splenic macrophages and would be phagocytosed, were it not for the presence of CD47 on the RBC surface.15 This mechanism is based on the binding of CD47 on RBCs to the inhibitory receptor SIRPα on the macrophages, which inhibits macrophage activation and phagocytosis. The prophagocytic macrophage receptor and its ligand on the RBC, which mediate this uptake, are still unknown. This mechanism results in rapid clearance of CD47-deficient RBCs when transfused into the circulation of syngeneic wild-type recipient mice.15 Still CD47-/- mice show normal RBC levels and normal RBC half-life, and the basis for this adaptation in CD47-/- mice is still unclear. The importance of the CD47/SIRPα interaction for normal circulation of blood cells is further emphasized in this work, whereby CD47-/- platelets were rapidly cleared from the circulation of wild-type recipients. Moreover, CD47-/- platelets also circulated normally in CD47-/- mice, despite rapid clearance in CD47 wild-type mice. However, in contrast to that for RBCs, we here observed that CD47-/- mice had slightly reduced platelet counts. Despite this finding, we were unable to detect a decreased half-life of CD47-/- platelets in these mice. Interestingly, reduced platelet counts were also found in SIRPα mutant mice lacking the cytoplasmic region of the receptor, resulting in lack of inhibitory SIRPα signaling.26 This mild thrombocytopenia was explained as an increased consumption of circulating platelets and was not due to a defect in megakaryocytopoiesis or platelet production.26 This discrepancy between CD47-/- mice and SIRPα mutant mice is not yet understood but may be explained by the presence of alternative ligands for SIRPα, or a possible role for CD47 in megakaryocyte function and platelet production.
Phosphatidylserine and P-selectin are potential platelet surface structures involved in regulating platelet half-life in vivo. However, so far, phosphatidylserine is the only platelet surface structure which has been shown to decrease platelet survival in circulation.27 In this study, we did not find an increased phosphatidylserine exposure on freshly isolated CD47-/- platelets, or an accelerated phosphatidylserine exposure during in vitro aging, as compared with that in platelets from CD47+/+ mice. It is likely that there are more unknown platelet surface structures, which are involved in regulating platelet half-life in circulation. One problem with identifying the nature of platelets at the stage of being more prone to rapid elimination in CD47-deficient mice is that such platelets are unlikely to be recovered in blood samples taken from these mice, simply because they would already have been recognized and eliminated. Furthermore, to isolate and label platelets in vitro for transfusion experiments, without causing platelet activation, inhibitors such as PGI2 or prostaglandin E1, are needed.21,25 The drawback of this treatment could be that changes to platelets that normally occur in the circulation may not take place when these cells are transfused back into the recipient. Such a possibility could explain why we were unable to find a shortened half-life of platelets in CD47-/- mice, despite the presence of mild thrombocytopenia in these mice.
Lack of CD47 expression promotes a markedly accelerated development of spontaneous AIHA in the autoimmune-prone nonobese diabetic mice and results in increased sensitivity to experimental AIHA induced by both IgG1 and IgG2a anti-RBC monoclonal autoantibodies in nonautoimmune C57BL/6 mice.19 We have previously suggested that, since CD47 is expressed on virtually all cells in the host, but not on foreign microorganisms, CD47/SIRPα signaling is critically involved not only in prevention against autoantibody-mediated autoimmune manifestations but also in host defense against foreign antigens, by modulating the prophagocytic signals mediated through the activation of macrophage Fcγ and complement receptors.18 Therefore, we hypothesized here that, if the interaction between platelet CD47 and macrophage SIRPα plays a similar role in regulating platelet phagocytosis in ITP, as seen for RBC phagocytosis in AIHA, CD47-/- mice would be more sensitive to experimental ITP. As predicted, CD47-/- mice were twice as sensitive to ITP as compared with the wild-type mice, suggesting that the inhibitory CD47/SIRPα signaling pathway is involved in reducing clearance of platelets in antibody-induced ITP. The important role of the CD47/SIRPα interaction was further pinpointed in vitro, whereby we found that 6A6-opsonized CD47+/+ platelets were phagocytosed to a significantly lower extent than equally opsonized CD47-/- platelets. However, pretreatment of the macrophages with a functional blocking SIRPα antibody increased the phagocytosis of CD47+/+ platelets to that seen with CD47-/- platelets. Importantly, the SIRPα antibody did not affect phagocytosis of CD47-/- platelets. Thus, inhibitory CD47/SIRPα signaling can strongly reduce macrophage phagocytosis of IgG-sensitized platelets.
The pathogenic effect of the IgG2a mAb 6A6 depends on macrophage-mediated platelet phagocytosis through the low-affinity activating IgG Fc receptor FcγRIII,7 indicating that SIRPα signaling has a profound effect on the pathogenic effects mediated through this Fcγ receptor. These data are in good agreement with our previous findings of a strong CD47/SIRPα-mediated inhibitory effect on IgG2a-mediated RBC phagocytosis.19 Of particular interest, CD47 heterozygous mice did not show a significantly increased sensitivity to experimental ITP, as compared with wild-type mice. However, by following the clearance of labeled CD47+/- platelets or CD47 wild-type platelets in wild-type recipients during our experimental model of ITP, we found that the 45% reduction in platelet CD47 expression on CD47+/- platelets is enough to significantly increase the platelet clearance rate in this model. Furthermore, using an in vitro phagocytosis assay, we showed that the uptake of 6A6-opsonized CD47+/- platelets was significantly stronger than that for CD47+/+ platelets, but weaker than that for CD47-/- platelets. Again, the difference in phagocytosis between either platelet genotype was abolished when SIRPα was blocked. Thus, not only does absence of platelet CD47 expression increase platelet clearance in ITP, but less than 50% reduction of platelet CD47 is sufficient to significantly increase the platelet phagocytosis in this system. These data further strengthen our hypothesis that the net phagocytic activity in the macrophage is determined by the integrated signals from prophagocytic receptors, such as Fcγ receptors, and inhibitory CD47/SIRPα interactions.18,28
In conclusion, the present study extends our previous findings and supports our hypothesis by showing that the interaction between platelet CD47 and macrophage SIRPα also plays an important role in regulating normal platelet homeostasis and autoimmune elimination of opsonized platelets in ITP. Furthermore, the present finding of a gene dose effect of inhibitory CD47/SIRPα signaling opens the possibility that CD47 mimetics might provide a novel alternative to therapeutically manipulate this system in ITP.
Prepublished online as Blood First Edition Paper, January 21, 2005; DOI 10.1182/blood-2004-08-2980.
Supported by grants from the Swedish Research Council (06P-14098, 31X-14286), the National Institutes of Health (GM57573-06), the Swedish Society of Medicine, the Åke Wiberg Foundation, and the Faculty of Medicine, Umeå University.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Thomas H. Steinberg for critical evaluation of the manuscript and Dr Göran Roos for providing flow cytometry resources.