Abstract
We previously reported the characterization of human osteoclast-associated receptor (hOSCAR), a novel Fc receptor γ-chain (FcRγ)–associated receptor expressed by myeloid cells. Here we show that ligation of hOSCAR by specific antibodies promotes dendritic cell (DC) survival by an extracellular signal-regulated kinase (ERK)- and phosphatidylinositol 3-kinase (PI3K)–dependent pathway, linked to expression of the Bcl-2 and Bcl-xL antiapoptotic molecules. Crosslinking of hOSCAR leads to maturation of DCs, as demonstrated by up-regulation of maturation markers, decrease in dextran uptake capacity, and secretion of immunesystem effectors such as interleukin-8 (IL-8)/CXC chemokine ligand 8 (CXCL8), IL-12 p40, monocyte chemoattractant protein-1 (MCP-1)/chemokine receptor ligand 2 (CCL2) and macrophage-derived chemokine (MDC)/CCL22. Stimulation of hOSCAR acts in conjunction with the Toll-like receptor (TLR) ligands, lipopolysaccharide (LPS), R-848, and polyinosinic-polycytidylic acid (poly(I:C)), to increase the expression of maturation markers, and to modulate cytokine release. A PI3K-dependent up-regulation of IL-10 release is observed with all the TLR ligands used, whereas regulation of IL-12 production is variable depending on the TLR stimulated. hOSCAR engagement on DCs did not significantly increase the proliferation of naive T cells; however, when co-incubated with TLR ligands, an enhanced proliferation was observed. The percentage of interferon (IFN)–γ–producing T cells is decreased when hOSCAR engagement is combined with LPS stimulation. Altogether, these data suggest that hOSCAR may modulate the responses of both innate resistance and adaptive immunity.
Introduction
Dendritic cells (DCs) are potent antigen-presenting cells that have a unique ability to prime naive T cells.1 In addition, they secrete immune-modulating factors that play a key role in the initiation and regulation of both innate resistance and adaptive immunity. DCs undergo striking changes in function and morphology depending on their maturation state and localization.1 Immature DCs, located in peripheral tissues, are considered immune sentinels and are able to capture and process antigens.2 Upon activation and maturation induced by proinflammatory signals, such as pathogen-associated molecular patterns (PAMPs),3 they migrate to the T-cell areas of secondary lymphoid organs, where they are able to present antigens to naive T cells.4,5 The outcome of the immune response (either immune priming or tolerance) is directly linked to the maturation status of DCs,6 which is generally believed to be induced by endogenous factors such as proinflammatory cytokines (tumor necrosis factor [TNF] or interleukin-1β [IL-1β])7,8 or by exogenous products such as PAMPs (eg, lipopolysaccharide [LPS], lipoteichoic acid [LTA], or viral-RNA mimics such as polyinosinic-polycytidylic acid [poly(I:C)]).9
DC activity can be regulated by both activating and inhibiting immune receptors that transduce signals through immunoreceptor tyrosine-based activating motifs (ITAM; consensus: D/Ex7D/Ex2Yx2L/Ix7Yx2L/I)10,11 and through immunoreceptor tyrosine-based inhibitory motifs (ITIM; consensus: I/V/L/SxYx2L/V),12,13 respectively. This growing family of receptors was first described in lymphocytes,14-16 but in recent years an increasing number of receptors of the lectin and the immunoglobulin superfamilies (IgSFs) linked to ITAM/ITIM signaling expressed on myeloid cells and DCs has been described.17,18 These data indicate that cells of the myeloid lineage have a large number and variety of receptors regulating their activity. These receptors signal through activating or inhibiting pathways that regulate the amplitude and the duration of the immune response triggered by pathogenic stimuli.19-21
We recently described human osteoclast-associated receptor (hOSCAR), a novel immune receptor associated with the Fc receptor γ-chain (FcRγ) and involved in endocytosis and antigen presentation through the major histocompatibility complex (MHC) class II pathway in monocyte-derived dendritic cells (mono-DCs).22 Its association with an ITAM-bearing chain confers to hOSCAR the capacity to activate myeloid cells as shown by its ability to trigger calcium flux and cytokine release.22 As hOSCAR is expressed by both immature and LPS-matured mono-DCs, we proposed that hOSCAR may have a biologic role on human DCs that is probably different to the previously described activating receptors such as the Fc receptors (FcRs) and triggering receptor expressed on myeloid cells-2 (TREM-2), the expression of which is down-regulated after activation of DCs. In contrast to the human receptor, mouse OSCAR (mOSCAR) is only expressed on osteoclasts,23 which are derived, like certain DC subsets, from the myeloid lineage.24 Data from different groups strongly suggest that in vivo ligation of mOSCAR on osteoclasts is essential for differentiation of these cells. An endogenous ligand for OSCAR on osteoblasts has been inferred from this work.23,25 Of interest, TREM-2, an activating receptor that uses the ITAM-bearing adapter DAP12, was first described in DCs and shown to promote cell survival and a partial maturation phenotype.26 TREM-2 has also been shown to be involved in osteoclast differentiation and function.27-29 These data underline the relevance of receptors associated with either FcRγ or DAP12 in the biology of cells of the myeloid lineage, including osteoclasts and DCs.
In this study we show that hOSCAR ligation induces phenotypical and functional maturation of DCs. Cytokine and chemokine secretion was induced in DCs by hOSCAR ligation, although no significant effect was noted on the ability of the treated DCs to prime naive T-cell proliferation. However, when hOSCAR ligation was associated with treatment by certain Toll-like receptor (TLR) ligands, the ability of DCs to support naive T-cell proliferation was synergistically amplified. Finally, the inflammatory effects in DCs of the TLR4-ligand LPS, but not those of the TLR7/8-ligand R-848 and of the TLR3-ligand poly(I:C), were attenuated by hOSCAR ligation which induced the production of anti-inflammatory cytokines such as IL-10 and lead to a lower level of T-helper 1 (Th1) polarization of naive T cells.
Materials and methods
Cell culture
Mono-DCs were produced by culturing purified blood monocytes for 5 days in the presence of 200 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF; Schering-Plough Research Institute, Kenilworth, NJ) and 10 ng/mL rhIL-4 (Schering-Plough Research Institute), as previously described.22 Cells were cultured in RPMI 1640 (GIBCO BRL, Gaithersburg, MD) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS; Eurobio, Les Ulis, France), 2 mM l-glutamine, 100 μg/mL gentamicin (hereafter referred to as complete medium; Schering-Plough, Levallois-Perret, France).
Peripheral blood CD11c+ myeloid DCs were purified by negative depletion of peripheral blood mononuclear cells (PBMCs) with anti-CD3 (OKT3), anti-CD8 (OKT8), anti-CD14 (MOP9.25), and anti-CD19 (4G7) monoclonal antibodies (mAbs) (all ascites from our laboratory), purified anti-CD56 (NKH-1; Beckman Coulter, Miami, FL), anti-CD16 and anti–glycophorin A mAbs (both from Immunotech, Marseille, France), and magnetic beads (Dynabeads M450; Dynal, Oslo, Norway). Enriched cells were further fluorescence-activated cell-sorted (FACS) as lineage- CD4+ (Beckman Coulter, Marseille, France) and CD11c+ (> 98% purity; Becton Dickinson, San Jose, CA). The lineage- antibody cocktail is composed of fluorescein isothiocyanate (FITC)–conjugated anti-CD3, anti-CD15 (Dako, Glostrup, Denmark), anti-CD14, anti-CD16, anti-CD19, anti-CD56 (Becton Dickinson), and anti-CD35 (BD Pharmingen, San Diego, CA).
Blood-naive CD4+ CD45RA+ T cells were prepared from PBMCs by 2 rounds of negative selection using magnetic beads (Dynal). The depletion was performed with anti-CD45RO (UCHL1), anti-CD8, anti-CD14, anti-CD19, anti-CD40 (89), anti–human leukocyte antigen-DR (HLA-DR) (L243), (all ascites from our laboratory), purified anti-CD56, anti-CD16, anti-CD35, anti–glycophorin A (all from Immunotech). Purity of the preparation was controlled by flow cytometer analysis of double labeling with anti–CD3-FITC (Dako) and anti–CD45RA-phycoerythrin (PE) (Beckman Coulter) and was equal to 96%.
In vitro stimulation of mono-DCs
Anti-hOSCAR mAb and F(ab′)2 fragments mAb were produced as previously described.22 Anti-hOSCAR mAb and F(ab′)2 (clone 11.1CN5), and irrelevant mAb MOPC21 (Sigma, St Louis, MO) or anti-CD13 (Immunotech) as isotype controls were coated for 4 hours at 37°C on flat-bottom plates with a final concentration of 20 μg/mL in phosphate-buffered saline (PBS). Immature DCs were plated at a concentration of 1 × 106 cells/mL. The following activating factors were used at final concentrations of 20 ng/mL: rhTNF (Genzyme, Boston, MA), 10 ng/ml Escherichia coli LPS (Sigma), 25 μg/mL, poly(I:C) (InvivoGen, San Diego, CA), 10 μM R-848 (imidazoquinoline resiquimod synthesized in our laboratory; Schering-Plough). Supernatants and cells were collected after 24 hours and tested by enzyme-linked immunosorbent assay (ELISA) and flow cytometry, respectively. All mAbs used for tissue culture were shown to be endotoxin free, as determined by Limulus-Amebocyte Assay (BioWhittaker, Walkersville, MD). To block the effect of LPS, polymixin B (Sigma) was added to the culture at 10 μg/mL.
Flow cytometry
Cell staining was performed using PE-conjugated mouse mAb anti-CD54 (Bioscience), anti-CD40, anti-CD83, anti–DC–lysosome-associated membrane protein (LAMP) (Immunotech), anti-CD25, anti-CD80, anti-CD86, anti–HLA-DR (BD PharMingen) and FITC-conjugated mouse mAb anti-CCR7 (BD PharMingen). The staining with anti–DC-LAMP was performed using the cytofix/cytoperm kit (BD PharMingen).
Incorporation of FITC-dextran
Mono-DCs were harvested after 48 hours of stimulation as described, resuspended in complete medium supplemented with 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; Life Technologies, Paisley Park, United Kingdom) and incubated at 4°C or 37°C. FITC-dextran (Mr = 40 000 kDa; Molecular Probes, Eugene, OR) was added at a final concentration of 100 μg/mL for 30 minutes. The cells were washed 4 times with cold PBS, 5% FBS, 0.01% NaN3, and were analyzed immediately by flow cytometer. The maturation of mono-DCs was assessed by staining with PE-conjugated mouse mAb anti-CD86 (BD Pharmingen).
Detection of apoptosis
After mono-DC differentiation, the cells were harvested and washed 4 times in PBS to remove GM-CSF and IL-4. Mono-DCs stimulated with coated mAb for 3 days were harvested and the apoptotic cells were detected using the FITC–annexin V kit (BD Pharmingen).
To measure mitochondrial membrane potential, cells were incubated in complete medium containing 25 nM DiOC6(3) (3,3-dihexyloxocarbocyanine iodide; Molecular Probes) for 30 minutes at 37°C in the dark followed by flow cytometer analysis.
Intracellular labeling with FITC-conjugated mouse mAb anti–Bcl-2 (Dako) or with rabbit polyclonal Ab anti–Bcl-x (BD Biosciences), followed by PE-conjugated goat anti–rabbit (Sigma), were performed as described elsewhere.30 In blocking experiments, inhibitors (10 μM LY294002, 10 μM wortmannin, 20 μM PD98059, all from Calbiochem, San Diego, CA) were added 60 minutes before stimulation.
For biochemical analysis, mono-DCs were stimulated as described. After 3 days, cells were harvested and lysed in reducing sample buffer, and cell lysates were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and reducing Western blot, as previously described.22 The Bcl-x isoforms were detected using polyclonal Ab anti–Bcl-x and donkey anti–rabbit conjugate (Vector, Burlingame, CA).
Measurement of mono-DC secreted cytokines and chemokines
Supernatants of cells stimulated for 24 hours were collected and tested by ELISA for production of IL-1β, IL-6, IL-8/CXC chemokine ligand 8 (CXCL8), IL-10, IL-12 p40, IL-12 p70, TNF, monocyte chemoattractant protein-1 (MCP-1)/chemokine receptor ligand 2 (CCL2), interferon-γ–inducible protein-10 (IP10)/CXCL10, GM-CSF (OptEIA kits; BD Pharmingen) and macrophage-derived chemokine (MDC) and macrophage (M)–CSF (DuoSet kit; R&D Systems, Minneapolis, MN).
Semi-quantitative reverse transcriptase–polymerase chain reaction
Cells were lysed and total RNA was extracted using the RNeasy kit (Qiagen, Hilden, Germany). Genomic DNA contamination was tested by amplification (quantitative polymerase chain reaction [PCR]; Icycler IQ [Bio-Rad, Hercules, CA]) of the nontranscribed part of the human CD4 promoter (forward primer: 5′-TTCCACACTGGGCCACCTAT; reverse primer: 5′-TTGTGGGCTTACCACTGCTG; probe: CACTGGACACAATTGCCCTCAGG). Single-stranded cDNA was synthesized using a mix of random hexamer primers (Invitrogen, San Diego, CA) and oligo (dT)15 (Promega, Madison, WI) and the Superscript II RNase-H reverse transcriptase (Invitrogen). Real-time quantitative PCR was performed with a double-stranded (ds) DNA-binding dye, SYBR green I, in an Icycler IQ, in 50 μL reactions (25 μL QuantiTect SYBR Green PCR kit 2x [Qiagen], 0.4 μM each primer plus cDNA). The following primers were used: human monokine induced by IFN-γ (MIG)/CXCL9 5′-GAGATCCCACCCGAACGTCTTATC (forward) and 5′-CCTGTGAGATGAAAGGTAAGTGGGT (reverse); human IP10/CXCL10 ABI PRISM primer pairs (Applied Biosystems, Foster City, CA); human regulated on activation, normal T expressed and secreted (RANTES)/CCL5 5′-TCCCGAACCCATTTCTTCTCT (forward) and 5′-CCCAGCAGTCGTCTTTGTCA (reverse); human thymus- and activation-regulated chemokine (TARC)/CCL17 5′-CCCTTAGAAAGCTGAAGACGTG (forward) and 5′-TTGGGGTCCGAACAGATG (reverse); and human MDC/CCL22 5′-CTTGCTGTGGCGCTTCAAG (forward) and 5′-AGACGCTGTCTTCCATGTTGG (reverse). Experiments were performed in triplicate. Real-time data were acquired and analyzed using Icycler IQ Optical System software (Bio-Rad) with automatic adjustment of the baseline and threshold parameters. Gene expression levels were determined using cycle threshold values (Ct), normalized by the average expression of the housekeeping gene GAPDH (forward primer: 5′-TGCCACCACCAACTGCTTAG; reverse primer: 5′-GGATGCAGGGATAGTGTTC) and the results are expressed as gene relative expression, by applying the formula 1.8(CT GAPDH - CT gene of interest) × 10 000.
DC-T cell culture, mixed leukocyte reaction, and T-cell orientation
In mixed leukocyte reaction (MLR), 5 × 104 allogenic CD45RA+-naive T cells were cultured with serial dilutions of mono-DCs, irradiated by 30 Gy (3000 rad), and preincubated overnight with coated mAb and/or activators as described. The cells were cultured in 96–half-area-well plates (Corning, Acton, MA), in quadruplicates. The highest ratio is 2 T cells to 1 DC, and the number of mono-DCs was decreased by two thirds for each dilution. After 5 days, the cultures were pulsed with [3H]-thymidine (0.037 MBq/well [1 μCi/well]; Amersham, Buckingham, United Kingdom) for 16 hours.
To study the polarization of naive T lymphocytes, 1 × 106/mL CD45RA+ T cells were cultured with 1 × 105/mL mono-DCs stimulated as described. At 5 days, human rIL-2 (a generous gift from C. Caux-Menetrier, Centre Leon Berard, Lyon, France) was added at 10 U/mL, and the cells were expanded for 9 days. The quiescent T cells were then stimulated with 50 ng/mL phorbol 12-myristate 13-acetate (PMA) and 2 μg/mL ionomycin (Sigma) for 6 hours, with the additional presence of GolgiPlug (Pharmingen) for the last 4 hours of culture, before staining with with FITC–anti–IFN-γ (Pharmingen) and PE–anti–IL-4 (Becton Dickinson) mAb.
For the detection of secreted cytokines, quiescent T cells were restimulated with coated anti-CD3 (UCHT1; BD Pharmingen), and anti-CD28 (Sanquin, Amsterdam, the Netherlands). Supernatants were tested by ELISA for production of IFN-γ, IL-4, and IL-5 (OptEIA kits; BD Pharmingen).
Statistical analyses
Statistical analyses were performed in Microsoft Excel 5.0 (Microsoft, Redmond, WA) using 2-tailed Student t tests. A P value less than .05 was considered statistically significant. For the T-cell orientation assay, statistical analyses were performed using 2-tailed Student t tests with pair-wise comparison, to compare the percentage of IFN-γ+ cells after stimulation with TLR ligands alone or in combination with hOSCAR ligation independently on donor variability.
Results
hOSCAR crosslinking promotes DC survival
Some activating signals, such as LPS31 or anti–TREM-2 stimulation,26 have been shown to trigger prolonged survival of mono-DCs. We showed that hOSCAR crosslinking induces calcium flux and cytokine release in monocytes and mono-DCs.22 We therefore investigated whether ligation of hOSCAR increased the survival of mono-DCs cultured in the absence of GM-CSF. Mono-DC stimulation by plastic-coated anti-hOSCAR mAb that induced receptor aggregation and triggering of the ITAM-signaling pathway32 resulted in, as observed at 72 hours, morphologic changes, adherence, and conservation of cellular integrity (Figure 1A). A high percentage of anti-hOSCAR–treated mono-DCs survived in the absence of GM-CSF for more than 10 days (Figure 1B).
Stimulation of mono-DCs through hOSCAR promotes cell survival in the absence of survival factors by a PI3K-, ERK-dependent pathway. Mono-DCs were washed 4 times to remove GM-CSF and IL-4, before stimulation of the cells with plastic-coated mAb (MOPC21, anti-CD13, anti-hOSCAR), 200 ng/mL GM-CSF, or 20 ng/mL TNF. Data shown are representative of 3 independent experiments. (A) Mono-DCs were stimulated as described and photographed after 3 days. Cells were visualized using an inverted Olympus CK40 microscope (Olympus, Tokyo, Japan) with a 20×/0.50 aperture objective; final total magnification, × 200. Images were captured with a Nikon camera (Nikon, Melville, NY) and imported into Adobe Photoshop (Adobe Systems, San Jose, CA). (B) After stimulation for the indicated time, cells were harvested and counted by exclusion of dead cells with blue trypan. Cell recovery is expressed as percentage of cells put into culture at day 0. □ indicates medium only; ▪, MOPC21; ○, anti-hOSCAR; and ⬡, GM-CSF. Survival curve displays the mean and standard deviation of 3 independent cell counts from 1 representative experiment. (C) After 3 days of stimulation as described, cells were analyzed for annexin V binding, DiOC6(3) incorporation, and intracellular staining by anti–Bcl-2 and Bcl-x. Numbers in the corners correspond to the percentage of positive cells for annexin V and DiOC6(3) analysis, and indicate specific mean fluorescence intensity for Bcl-2 and Bcl-x staining (shaded histogram). The dotted line shows the binding of an isotype control mAb to the cells. (D) Lysates of mono-DCs stimulated for 3 days, as indicated, were analyzed for expression of Bcl-x isoforms by Western blot. The antiapoptotic long isoform of BcL-x corresponds to the band of 28 kDa. (E) Mono-DCs were stimulated for 3 days, as described, in the presence of PI3K inhibitor (LY294002) or ERK-pathway inhibitor (PD98059). The percentage of apoptotic cells and expression of Bcl-2 were analyzed by annexin V–FITC binding and anti–Bcl-2 labeling. The data were expressed as percentages for annexin V–FITC and ΔMFI (mean fluorescence intensity minus fluorescence detected with isotype control). □ indicates medium only; ▪, anti-hOSCAR; and ▦, GM-CSF.
Stimulation of mono-DCs through hOSCAR promotes cell survival in the absence of survival factors by a PI3K-, ERK-dependent pathway. Mono-DCs were washed 4 times to remove GM-CSF and IL-4, before stimulation of the cells with plastic-coated mAb (MOPC21, anti-CD13, anti-hOSCAR), 200 ng/mL GM-CSF, or 20 ng/mL TNF. Data shown are representative of 3 independent experiments. (A) Mono-DCs were stimulated as described and photographed after 3 days. Cells were visualized using an inverted Olympus CK40 microscope (Olympus, Tokyo, Japan) with a 20×/0.50 aperture objective; final total magnification, × 200. Images were captured with a Nikon camera (Nikon, Melville, NY) and imported into Adobe Photoshop (Adobe Systems, San Jose, CA). (B) After stimulation for the indicated time, cells were harvested and counted by exclusion of dead cells with blue trypan. Cell recovery is expressed as percentage of cells put into culture at day 0. □ indicates medium only; ▪, MOPC21; ○, anti-hOSCAR; and ⬡, GM-CSF. Survival curve displays the mean and standard deviation of 3 independent cell counts from 1 representative experiment. (C) After 3 days of stimulation as described, cells were analyzed for annexin V binding, DiOC6(3) incorporation, and intracellular staining by anti–Bcl-2 and Bcl-x. Numbers in the corners correspond to the percentage of positive cells for annexin V and DiOC6(3) analysis, and indicate specific mean fluorescence intensity for Bcl-2 and Bcl-x staining (shaded histogram). The dotted line shows the binding of an isotype control mAb to the cells. (D) Lysates of mono-DCs stimulated for 3 days, as indicated, were analyzed for expression of Bcl-x isoforms by Western blot. The antiapoptotic long isoform of BcL-x corresponds to the band of 28 kDa. (E) Mono-DCs were stimulated for 3 days, as described, in the presence of PI3K inhibitor (LY294002) or ERK-pathway inhibitor (PD98059). The percentage of apoptotic cells and expression of Bcl-2 were analyzed by annexin V–FITC binding and anti–Bcl-2 labeling. The data were expressed as percentages for annexin V–FITC and ΔMFI (mean fluorescence intensity minus fluorescence detected with isotype control). □ indicates medium only; ▪, anti-hOSCAR; and ▦, GM-CSF.
After 3 days without exogenous survival factors, a high proportion of apoptotic mono-DCs (annexin V+ cells) could be observed after culture in the presence of plastic-coated MOPC21 isotype control mAb or anti-CD13 (Figure 1C). In these culture conditions, mono-DCs were also unable to maintain their mitochondrial membrane potential, as shown by the low level of DiOC6(3) incorporation. Only a small proportion of mono-DCs cultured for 3 days in the presence of plastic-coated anti-hOSCAR underwent apoptosis and the majority of these cells incorporated DiOC6(3), indicating that their mitochondrial membrane potential was maintained (Figure 1C). A comparable level of survival was also observed in mono-DCs cultured in the presence of TNF or GM-CSF. To exclude that contaminating LPS was responsible for the rescue from apoptosis, mono-DCs were cultured in the presence of polymixin B, and no inhibition of survival induced by anti-hOSCAR was observed (data not shown).
Proteins from the Bcl-2 family, particularly Bcl-2 and the Bcl-xL isoform, are known cell death antagonists,33,34 whereas the shorter Bcl-xS isoform has an opposite role. The survival effect of hOSCAR, as well as that of the positive controls TNF and GM-CSF, was characterized at day 3 by concomitant expression in mono-DCs of Bcl-2 and Bcl-x, as shown by intracellular staining (Figure 1C). As shown by Western blot, the staining for Bcl-x was mainly due to the presence of the antiapoptotic long isoform Bcl-xL of 28 kDa (Figure 1D).
Treatment of mono-DCs with the extracellular signal–related kinase (ERK) inhibitor PD98059 and the 2 phosphatidylinositol 3-kinase (PI3K) inhibitors, LY294002 (Figure 1E) or wortmannin (data not shown), inhibited survival (as assessed by annexin V binding) and Bcl-2 expression induced by hOSCAR ligation. In a similar fashion, Bcl-x expression was also maintained by a PI3K- and ERK-dependent pathway (data not shown). These observations indicate that hOSCAR induces survival of mono-DCs, at least in part by maintaining expression of the antiapoptotic molecules of the Bcl-2 family through activation of the PI3K and ERK signaling pathways.
hOSCAR ligation up-regulates the maturation marker expression on DCs
Immature mono-DCs were stimulated by LPS or by mAb coated onto culture plates, an efficient way of crosslinking the appropriate receptors.32 After 24 hours of stimulation, phenotypical analysis of these cells was performed by flow cytometry (Figure 2).
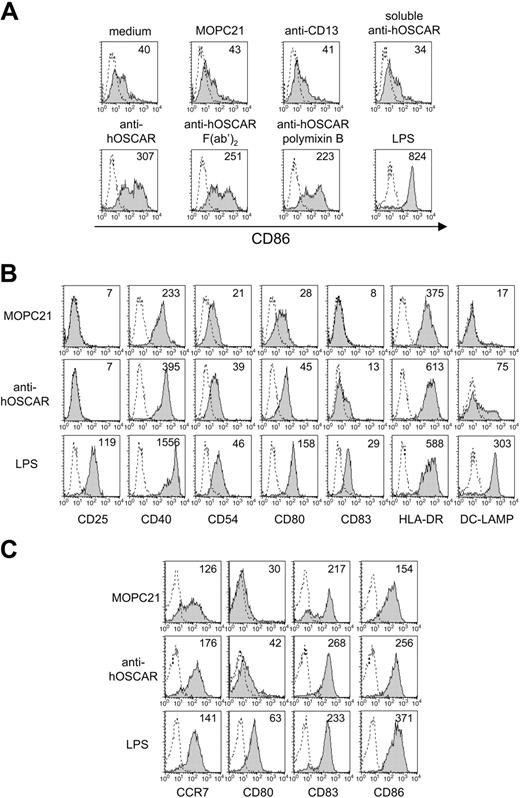
hOSCAR ligation induces expression of maturation markers on mono-DCs. Immature mono-DCs were stimulated by coated control IgG (MOPC21, anti-CD13), anti-hOSCAR whole mAb or F(ab′)2, soluble anti-hOSCAR, or 10 ng/mL LPS. Polymixin B was added to some of the cells cultured in the presence of anti-hOSCAR mAb or LPS. After 24 hours of incubation, cells were analyzed by flow cytometry for CD86 expression (A) or for the indicated markers (B). Numeric values indicate the specific mean fluorescence intensity of the staining (for shaded histograms). The dotted line shows the binding of an isotype control mAb to the cells. Data shown are representative of 3 experiments. (C) Freshly isolated blood CD11c+ DCs were cultured in the presence of coated MOPC21, anti-hOSCAR, or 10 ng/mL LPS. After 24 hours of culture, cells were analyzed by flow cytometry for the indicated markers. The dotted line shows the binding of an isotype control mAb to the cells. Data shown are representative of 6 experiments.
hOSCAR ligation induces expression of maturation markers on mono-DCs. Immature mono-DCs were stimulated by coated control IgG (MOPC21, anti-CD13), anti-hOSCAR whole mAb or F(ab′)2, soluble anti-hOSCAR, or 10 ng/mL LPS. Polymixin B was added to some of the cells cultured in the presence of anti-hOSCAR mAb or LPS. After 24 hours of incubation, cells were analyzed by flow cytometry for CD86 expression (A) or for the indicated markers (B). Numeric values indicate the specific mean fluorescence intensity of the staining (for shaded histograms). The dotted line shows the binding of an isotype control mAb to the cells. Data shown are representative of 3 experiments. (C) Freshly isolated blood CD11c+ DCs were cultured in the presence of coated MOPC21, anti-hOSCAR, or 10 ng/mL LPS. After 24 hours of culture, cells were analyzed by flow cytometry for the indicated markers. The dotted line shows the binding of an isotype control mAb to the cells. Data shown are representative of 6 experiments.
Both coated F(ab′)2 and whole mAb anti-hOSCAR were able to trigger the activation of mono-DCs as shown by up-regulation of the costimulatory molecule CD86 (Figure 2A). The up-regulation of CD86 expression induced by F(ab′)2 and whole anti-hOSCAR was consistently observed but of lower intensity compared with that observed in mono-DCs activated by LPS. Unlike anti-hOSCAR, the 2 isotype-matched controls, MOPC21 (irrelevant mAb) or anti-CD13 (a known DC surface receptor), had no significant effect on the activation state of mono-DCs. Crosslinking of hOSCAR was required for the triggering of activation, since no effect was observed with soluble mAb. All mAbs used in this study were negative for endotoxin contamination as measured by the Limulus assay. The activating effect of anti-hOSCAR was maintained in the presence of polymixin B and absent when soluble mAb was used, excluding any role of undetectable levels of endotoxins (Figure 2A).
Upon hOSCAR ligation on mono-DCs, we also observed up-regulation of CD40, CD80, and HLA-DR, and slightly increased expression of CD54 (Figure 2B). In addition, a subpopulation of mono-DCs stimulated by anti-hOSCAR up-regulated CD83 and the intracellular maturation marker DC-LAMP. With some donors (2 of 5), a slight up-regulation of CCR7 was also observed (data not shown).
The activating ability of hOSCAR was then studied on freshly isolated blood CD11c+ DCs (Figure 2C). hOSCAR ligation triggered slight up-regulation of CD80, and an increase of CD86 and CD83 expression. The increase in CCR7 expression, upon hOSCAR crosslinking, was higher and more reproducibly observed on CD11c+ DCs than on mono-DCs.
hOSCAR stimulation enhanced the ability of the TLR ligands LPS, R-848, and poly(I:C) to induce DC maturation and up-regulation of costimulatory marker expression (CD25, CD80, CD83, CD86) (Figure 3), but no alteration in the levels of HLA-DR, CD40, and CD54 (data not shown) were seen. This effect was more pronounced when suboptimal doses of TLR ligands were used and the increase of expression was particularly high with poly(I:C), a less potent mono-DC stimulator, than with LPS and R-848. Nevertheless, the up-regulation of expression is moderate, and may be due to an additive effect between ITAM and TLR signaling.
Stimulation through hOSCAR synergizes with TLR ligands to increase the expression of maturation markers expressed by mono-DCs. Mono-DCs were stimulated with coated MOPC21 (□), anti-hOSCAR (▪), and/or LPS, R-848, and poly(I:C) at the indicated dose levels. After 24 hours of culture, cells were analyzed by flow cytometry for the indicated markers. The data are expressed as ΔMFI, the mean fluorescence intensity minus the fluorescence detected with isotype control. The results presented are representative of 3 independent experiments.
Stimulation through hOSCAR synergizes with TLR ligands to increase the expression of maturation markers expressed by mono-DCs. Mono-DCs were stimulated with coated MOPC21 (□), anti-hOSCAR (▪), and/or LPS, R-848, and poly(I:C) at the indicated dose levels. After 24 hours of culture, cells were analyzed by flow cytometry for the indicated markers. The data are expressed as ΔMFI, the mean fluorescence intensity minus the fluorescence detected with isotype control. The results presented are representative of 3 independent experiments.
Maturation of mono-DCs by anti-hOSCAR decreases their ability to uptake FITC-dextran
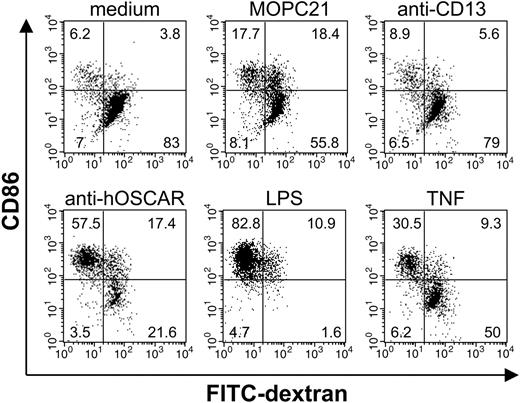
The ability to internalize and process antigens is a constitutive property of immature DCs that has been shown to decrease upon cellular maturation.7 In our experiments the majority of mono-DCs, cultured alone or in the presence of the isotype controls MOPC21 and anti-CD13, were positive for FITC-dextran uptake (80.5% ± 6.7, 74.2% ± 10.7, and 84.6 ± 6.1, respectively) (Figure 4B), and negative for CD86 expression (Figure 4A). The percentage of FITC-dextran+ cells decreased upon DC maturation (Figure 4A). Almost all the LPS-matured mono-DCs strongly expressed CD86 and were unable to uptake FITC-dextran. After treatment with anti-hOSCAR or TNF, a 39.9% ± 8.3 and a 59.3% ± 5.3 reduction, respectively, in dextran+ mono-DCs was observed (P < .001). After stimulation of mono-DCs by anti-hOSCAR and TNF, 2 populations were identifiable: CD86- cells that were still able to incorporate FITC-dextran, and fully matured CD86+ mono-DCs that had no macropinocytic/endocytic activity.
Stimulation through hOSCAR decreases the macropinocytic/endocytic activity of mono-DCs. After 48 hours of stimulation with coated antibodies (isotype controls, MOPC21 and anti-CD13 or anti-hOSCAR) or with 10 ng/mL LPS, and 20 ng/mL TNF, mono-DCs were incubated at 37°C for 30 minutes in the presence of FITC-dextran. The cells were then harvested, stained with anti-CD86–PE, and immediately analyzed by flow cytometry. Numbers in the corners correspond to the percentage of cells in each panel. Data shown are representative of 4 independent experiments.
Stimulation through hOSCAR decreases the macropinocytic/endocytic activity of mono-DCs. After 48 hours of stimulation with coated antibodies (isotype controls, MOPC21 and anti-CD13 or anti-hOSCAR) or with 10 ng/mL LPS, and 20 ng/mL TNF, mono-DCs were incubated at 37°C for 30 minutes in the presence of FITC-dextran. The cells were then harvested, stained with anti-CD86–PE, and immediately analyzed by flow cytometry. Numbers in the corners correspond to the percentage of cells in each panel. Data shown are representative of 4 independent experiments.
hOSCAR activation induces the secretion of cytokines and chemokines
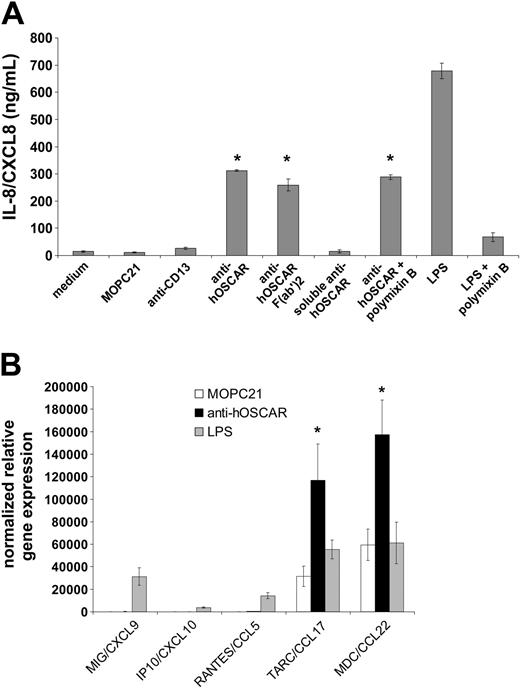
We investigated the ability of hOSCAR ligation on mono-DCs to trigger cytokine and chemokine secretion (Table 1). Stimulation of this receptor triggered production of high amounts of IL-8/CXCL8 (Figure 5), which were not produced in response to isotype controls (MOPC21 anti-CD13) or soluble anti-hOSCAR. Coated F(ab′)2 and whole anti-hOSCAR mAb in the presence of polymixin B were able to trigger the activation of DCs as potently as the whole mAb, demonstrating that the activation observed was not mediated by FcR engagement or by contaminating endotoxin (Figure 5A). hOSCAR ligation also induced secretion of M-CSF and small amounts of IL-12 p40 (Table 1). However, unlike LPS, stimulation through hOSCAR did not induce secretion of significant amounts of IL-1β, IL-6, IL-10, IL-12 p70, TNF, and GM-CSF (Table 1). Significant amounts of MCP-1/CCL2 and MDC/CCL22, but not IP10/CXCL10 were detected in the supernatants of hOSCAR-stimulated mono-DCs.
Cytokine and chemokine secretion by mono-DCs upon stimulation through hOSCAR. (A) Immature mono-DCs were stimulated with plastic-coated isotype controls (MOPC21, anti-CD13), anti-hOSCAR F(ab′)2 and whole mAb, soluble anti-hOSCAR, and LPS. As stated, polymixin B was added to the culture to block LPS activity. After 24 hours, supernatants were tested by ELISA for IL-8/CXCL8 secretion. Data are mean ± SD of triplicate samples from 1 representative experiment out of 3 performed with similar results. Statistical significances of *P < .001 are given by comparison to values obtained with the negative control anti-CD13. (B) Mono-DCs were stimulated with plastic-coated isotype control MOPC21, anti-hOSCAR, and LPS. After 2 hours of stimulation, cells were harvested and lysed. The transcripts coding for chemokines were analyzed by real-time PCR using SYBR-Green. Data are given after normalization of Ct (cycle threshold) value of 1 gene with the expression of GAPDH transcripts. Data are mean ± SD of triplicate samples from 1 representative experiment out of 3 performed with similar results. *P < .01 are given by comparison to values obtained with the negative control MOPC21.
Cytokine and chemokine secretion by mono-DCs upon stimulation through hOSCAR. (A) Immature mono-DCs were stimulated with plastic-coated isotype controls (MOPC21, anti-CD13), anti-hOSCAR F(ab′)2 and whole mAb, soluble anti-hOSCAR, and LPS. As stated, polymixin B was added to the culture to block LPS activity. After 24 hours, supernatants were tested by ELISA for IL-8/CXCL8 secretion. Data are mean ± SD of triplicate samples from 1 representative experiment out of 3 performed with similar results. Statistical significances of *P < .001 are given by comparison to values obtained with the negative control anti-CD13. (B) Mono-DCs were stimulated with plastic-coated isotype control MOPC21, anti-hOSCAR, and LPS. After 2 hours of stimulation, cells were harvested and lysed. The transcripts coding for chemokines were analyzed by real-time PCR using SYBR-Green. Data are given after normalization of Ct (cycle threshold) value of 1 gene with the expression of GAPDH transcripts. Data are mean ± SD of triplicate samples from 1 representative experiment out of 3 performed with similar results. *P < .01 are given by comparison to values obtained with the negative control MOPC21.
In order to determine the type of chemokines induced upon hOSCAR crosslinking, we assayed cDNA prepared from mono-DCs incubated for 2 hours with MOPC21, anti-hOSCAR, and LPS using realtime semi-quantitative reverse transcription–PCR (Figure 5B). Crosslinking of hOSCAR triggered high expression levels of TARC/CCL17 and MDC/CCL22. These chemokine attract Th2 effectors and regulatory T cells.35,36 In contrast to LPS, hOSCAR ligation did not induce significant transcription of MIG/CXCL9, IP10/CXCL10, and RANTES/CCL5, chemokines able to recruit Th1 T cells.35,37
hOSCAR ligation modulates cytokine production by mono-DCs exposed to TLR ligands
The diversity and quantity of cytokines and chemokines produced by hOSCAR-stimulated mono-DCs was more restricted than that of LPS-stimulated DCs (Table 1). As some ITAM-signaling receptors, such as TREM-1,19,20,38 synergize with other activators, we investigated the modulation of TLR-mediated cytokine secretion upon hOSCAR engagement.
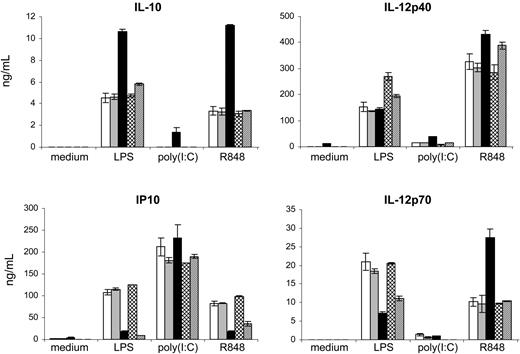
Costimulation of the cells with anti-hOSCAR and any of the TLR ligands used strongly augmented the secretion of IL-10 (Figure 6). This effect was dependent on PI3K signaling, as shown by its inhibition when cells were pretreated with LY294002. IL-12 p40 production were slightly up-regulated with poly(I:C) and R-848, while no modification was observed in combination with LPS. hOSCAR costimulation decreased the secretion of IL-12 p70 induced by LPS and up-regulated that induced by R-848. The increase of IL-12 p70 production was dependent on the PI3K pathway, while the down-regulation observed with LPS was not altered by PI3K inhibitors. In addition, the secretion of IP10/CXCL10 upon LPS and R-848 stimulation was strongly suppressed by the ligation of hOSCAR in a PI3K-independent mechanism. Overall, only the increase of IL-10 and IL-12 p70 production induced by hOSCAR engagement was clearly PI3K dependent, suggesting that another pathway is involved in the inhibitory activity of hOSCAR on cytokine release.
hOSCAR ligation modulates the pattern of cytokines/chemokines secreted by mono-DCs upon TLR stimulation. Mono-DCs were stimulated for 24 hours with plastic-coated MOPC21, anti-hOSCAR in combination with 10 ng/mL LPS, 25 μg/mL poly(I:C), or 10 μM R-848. Supernatant fluids were tested by ELISA for IL-10, IL-12 p40, IL-12 p70, and IP10/CXCL10 secretion. When indicated, cells were pretreated with the PI3K inhibitor LY294002. □ indicates medium only; ▦, MOPC21; ▪, anti-hOSCAR; ▩, LY94002; and ▨, anti-hOSCAR + LY294002. Data are mean ± SD of triplicate samples from 1 representative experiment out of 4 performed with similar results. Values are shown in ng/mL/106 cells.
hOSCAR ligation modulates the pattern of cytokines/chemokines secreted by mono-DCs upon TLR stimulation. Mono-DCs were stimulated for 24 hours with plastic-coated MOPC21, anti-hOSCAR in combination with 10 ng/mL LPS, 25 μg/mL poly(I:C), or 10 μM R-848. Supernatant fluids were tested by ELISA for IL-10, IL-12 p40, IL-12 p70, and IP10/CXCL10 secretion. When indicated, cells were pretreated with the PI3K inhibitor LY294002. □ indicates medium only; ▦, MOPC21; ▪, anti-hOSCAR; ▩, LY94002; and ▨, anti-hOSCAR + LY294002. Data are mean ± SD of triplicate samples from 1 representative experiment out of 4 performed with similar results. Values are shown in ng/mL/106 cells.
Costimulation by hOSCAR ligation and TLR ligands enhances the ability of mono-DCs to induce allogeneic CD45RA+ T-cell proliferation
We tested whether anti-hOSCAR stimulation, alone or in combination with other activators, triggered or enhanced the ability of mono-DCs to induce allogeneic CD45RA+ T-cell proliferation. Anti-hOSCAR–treated mono-DCs did not promote a significant CD45RA+-naive T-cell proliferation (Figure 7A). When the mono-DCs were stimulated by both anti-hOSCAR and TLR ligands (LPS, poly(I:C), and R-848), T-cell proliferation was enhanced compared with that induced by mono-DCs treated with the TLR ligands only (Figure 7B). This effect was particularly strong with LPS and R-848. No synergy was observed with TNF stimulation.
hOSCAR ligation, in combination with TLR ligands, increases the ability of mono-DCs to stimulate MLR and modulates Th1 polarization. (A) Proliferative response of CD45RA+-naive T cells cultured for 5 days with DCs treated with coated MOPC21, anti-CD13, anti-hOSCAR, and TNF was estimated by incorporation of [3H]-thymidine. Data are mean ± SD of quadruplicate samples from 1 representative experiment out of 4. (B) Mono-DCs were stimulated with coated anti-hOSCAR and/or activators (20 ng/mL TNF, 10 ng/mL LPS, 25 μg/mL poly(I:C), 10 μM R-848). After 5 days of culture with CD45RA+ T cells, the lymphocyte proliferation was measured. Data are mean ± SD of quadruplicate samples from 1 representative experiment out of 4 performed with similar results. (C) Polarization of CD45RA+-naive T cells cultured with mono-DCs stimulated with anti-hOSCAR and/or TLR ligands. After 6 hours of PMA/ionomycin stimulation and 4 hours of culture with GolgiPlug, T cells were intracellularly stained with FITC–anti–IFN-γ and PE–anti–IL-4, and analyzed by flow cytometer. Data shown are representative of 6 experiments. Numbers correspond to the percentage of cells in the lower right quadrant. (D) Secretion of IFN-γ by T cells cultured with mono-DCs stimulated with anti-hOSCAR and/or TLR ligands. Supernatant of T cells restimulated by anti-CD3 and anti-CD28 were analyzed by ELISA for the presence of IFN-γ, IL-4, and IL-5. No IL-4 and IL-5 were detected. Data are mean ± SD of triplicate samples from 1 representative experiment out of 3 performed with similar results. *P < .01 are given by comparison to values obtained with T cells cultured in the presence of MOPC21-stimulated DCs.
hOSCAR ligation, in combination with TLR ligands, increases the ability of mono-DCs to stimulate MLR and modulates Th1 polarization. (A) Proliferative response of CD45RA+-naive T cells cultured for 5 days with DCs treated with coated MOPC21, anti-CD13, anti-hOSCAR, and TNF was estimated by incorporation of [3H]-thymidine. Data are mean ± SD of quadruplicate samples from 1 representative experiment out of 4. (B) Mono-DCs were stimulated with coated anti-hOSCAR and/or activators (20 ng/mL TNF, 10 ng/mL LPS, 25 μg/mL poly(I:C), 10 μM R-848). After 5 days of culture with CD45RA+ T cells, the lymphocyte proliferation was measured. Data are mean ± SD of quadruplicate samples from 1 representative experiment out of 4 performed with similar results. (C) Polarization of CD45RA+-naive T cells cultured with mono-DCs stimulated with anti-hOSCAR and/or TLR ligands. After 6 hours of PMA/ionomycin stimulation and 4 hours of culture with GolgiPlug, T cells were intracellularly stained with FITC–anti–IFN-γ and PE–anti–IL-4, and analyzed by flow cytometer. Data shown are representative of 6 experiments. Numbers correspond to the percentage of cells in the lower right quadrant. (D) Secretion of IFN-γ by T cells cultured with mono-DCs stimulated with anti-hOSCAR and/or TLR ligands. Supernatant of T cells restimulated by anti-CD3 and anti-CD28 were analyzed by ELISA for the presence of IFN-γ, IL-4, and IL-5. No IL-4 and IL-5 were detected. Data are mean ± SD of triplicate samples from 1 representative experiment out of 3 performed with similar results. *P < .01 are given by comparison to values obtained with T cells cultured in the presence of MOPC21-stimulated DCs.
We next investigated the intracellular cytokine profile of T cells stimulated for 6 hours with PMA/ionomycin after having been cultured for 14 days with mono-DCs stimulated by anti-hOSCAR and/or TLR ligands. Anti-hOSCAR stimulation of mono-DCs did not enhance their ability to prime T cells for IFN-γ production, compared with no stimulation or the use of isotype controls (Figure 7C). LPS-, poly(I:C)-, and R-848–stimulated mono-DCs polarized a proportion of CD45RA+-naive T cells into IFN-γ–producing cells. As shown in Figure 5C, simultaneous stimulation by hOSCAR significantly neutralized the ability of LPS to endow mono-DCs with the ability to prime T cells for IFN-γ production. In a series of 6 experiments with different donors, an average decrease of 71% in the percentage of IFN-γ–producing T cells primed by mono-DCs stimulated with both anti-hOSCAR and LPS compared with LPS alone was observed (P = .002, 2-tailed Student t tests with pair-wise comparison). Modulation of IFN-γ production when hOSCAR stimulation was combined with poly(I:C) or R-848 was not consistent (P = .49 and P = .46, respectively). The absence of IL-4 and IL-5 production and the effect on IFN-γ production, in addition to intracellular cytokine staining, were confirmed by measuring cytokine secretion by ELISA (Figure 7D).
Discussion
Recently, we and others22,23,25 have shown that OSCAR is an FcRγ-associated molecule, implicated in the functional development and maturation of cells of the myeloid lineage. In mice the expression and function of OSCAR is restricted to osteoclasts,23 cells of the macrophage lineage. In humans, OSCAR is more widely expressed and it is present on peripheral blood monocytes and myeloid DCs, as well as on in vitro–derived macrophages, granulocytes, and mono-DCs.22 The expression of hOSCAR at all stages of DC development and maturation provides a unique tool to examine FcRγ-induced activation on these cells, as other receptors associated with this or other ITAM-bearing signaling chains are present uniquely on immature DCs or on particular subpopulations. The outcome of signaling through FcRγ activated by engagement of the associated Fc receptors on human DCs in terms of cell maturation is controversial,39-41 and a possible effect on DC survival has not previously been investigated.
In the absence of growth factors or upon serum starvation, most cells enter into a state of programmed cell death. hOSCAR ligation prevented the entry of mono-DCs into apoptosis in the absence of survival factors. The survival of hOSCAR-stimulated mono-DCs was accompanied by maintenance of the mitochondrial membrane potential and expression of the antiapoptotic proteins Bcl-2 and Bcl-xL, 2 molecules that prevent mitochondrial pore formation.33 These observations demonstrate that hOSCAR ligation triggers an intracellular antiapoptotic pathway. Among the receptors signaling through FcRγ, FcRϵRI was shown to block apoptosis in human monocytes after survival factor withdrawal, by directly inducing Bcl-2 and Bcl-xL expression.42 We have shown that the antiapoptotic effects of hOSCAR stimulation in mono-DCs are dependent on both PI3K and ERK pathways. In other cell types, growth factor–induced cell survival has been shown to be due to PI3K that through protein kinase B (Akt/PKB) activation allows the expression of the antiapoptotic protein Bcl-243 and Bcl-x.44 PI3K31 and ERK45 have been shown to be involved in LPS-induced mono-DC survival. Bouchon et al26 recently demonstrated that crosslinking of TREM-2, a receptor signaling through the ITAM-bearing chain DAP12, promotes survival of mono-DCs by an ERK-dependent, but PI3K-independent pathway. The difference in the involvement of ERK and PI3K in the survival effect, as well as the capacity of hOSCAR to induce cytokine secretion, may indicate clear differences in signaling pathways of the 2 ITAM-bearing chains, FcRγ for hOSCAR, and DAP12 for TREM-2.
We had previously shown that mono-DCs stimulated by anti-hOSCAR secrete IL-8/CXCL8 and IL-12 p40.22 However, engagement of hOSCAR alone on DCs does not induce secretion of large amounts of proinflammatory cytokines such as TNF and IL-12 p70, and induces less IL-12 p40 than TLR ligands. This suggests that the principal role of hOSCAR in DCs is not proinflammatory. Chemokines secreted by DCs are also important in the amplification and orientation of the adaptive immune response.46 hOSCAR ligation on DCs resulted in the secretion of significant amounts of IL-8/CXCL8, MCP-1/CCL2, and MDC/CCL22 chemokines that recruit immune effector cells, including neutrophils, monocytes, DCs, and natural killer cells.47 Of interest, hOSCAR stimulated mono-DCs produced the CCR4 ligands MDC/CCL22 and TARC/CCL17, chemokines known to be involved in the recruitment of Th2 effector cells and regulatory T cells,35,36 whereas IP10/CXCL10, RANTES/CCL5, and MIG/CXCL9, chemokines targeting CXCR3 and CCR5 expressed on Th1 cells, are not produced.35,37 These data suggest that hOSCAR engagement alone preferentially triggers the production of chemokines recruiting Th2 and regulatory cells.
hOSCAR ligation, coupled to the ITAM signaling pathway, clearly induces phenotypic and functional maturation of human mono-DCs and ex vivo blood CD11c+ DCs, as shown by the up-regulation of specific maturation markers and loss of antigen uptake capacity. Compared with the classical, strong DC activation triggered by TLR4 targeting by LPS, the phenotypic maturation induced by anti-hOSCAR is moderate but consistently observed using several markers, demonstrating that hOSCAR is an activating receptor on DCs. Although hOSCAR stimulation leads to up-regulation of molecules involved in costimulation, anti-hOSCAR-stimulated mono-DCs are unable to trigger naive T-cell proliferation.
Since OSCAR, by up-regulating CCR7 expression, also mediates a cellular maturation program that potentially leads to the migration of DCs to the lymph nodes, and interactions with T and B cells, the induction of cellular survival may be essential to maintain viability during this critical stage in the initiation of the adaptive response. Interestingly, other receptors that promote immune cell maturation and migration, such as FcR and members of the TREM family, also promote cellular survival,26,42 indicating that the 2 pathways may be essential in the immune response. The positive effect of hOSCAR ligation on DC survival allows the continuation of long-term DC activity, especially during and after migration. Migration of DCs is observed under normal steady-state conditions, particularly “veiled” cells, which represent DCs migrating to the lymph node. Some degree of maturation has been shown for steady-state migrating DCs: modulation of adhesion molecules and chemokine receptors, up-regulation of MHC, and costimulatory molecules. In the absence of microbial or inflammatory stimulation these DCs are not presumed to produce proinflammatory cytokines. This semimature stage of DCs are thus proposed to induce tolerance,6 and may correspond to this one induced upon hOSCAR engagement. The inability of hOSCAR engagement to endow DCs with the ability to stimulate proliferation of allogeneic naive T cells suggests that in absence of other maturation stimuli, hOSCAR-stimulated DCs, after migration into lymph nodes, may preferentially induce tolerance, and this effect may be augmented by the secretion of chemokines able to recruit Th2 and regulatory T cells that negatively regulate Th1 polarized responses.
The finding that transmembrane receptor activator of nuclear factor–κB ligand (RANKL)48 and secreted IFN-γ49 expressed by T cells are able to activate and to inhibit the development and functions of bone-resorbing cells, respectively, underscores the dynamic relationship between bone and the immune system. Increasingly, cell surface receptors are demonstrated to be expressed by both bone-remodeling cells and immune cells: the inhibiting natural killer (NK) receptors NKRP1 recognizing Clr/OCIL molecules50,51 expressed by DCs, macrophages, and osteoblasts, and which are involved in the inhibition of osteoclast differentiation;52 and TREM-2 expressed by both osteoclasts28,29 and DCs26 and having potential ligands on NK cells53 and in the bone environment.25 We can now add hOSCAR to the known receptors playing a role in both bone homeostasis and the activation of the immune response. The presence of a hOSCAR potential ligand in the immune system environment can be postulated, allowing regulation of the precarious balance between immune activation and suppression, to favor elimination of pathogens without deleterious effects against the host.
Large differences in maturation status of DCs were previously shown for different activators, such as TNF and LPS,54 and were postulated to determine the tolerogenic or immunogenic ability of DCs.6 Here we show that hOSCAR engagement induces a semimature status with expression of costimulatory molecules but absence of proinflammatory cytokines. Partial maturation, without up-regulation of CD83 and CD54 or cytokine release, has been previously described in mono-DCs stimulated with anti–TREM-2.26 In this study, Bouchon et al26 showed that ITAM signaling induced up-regulation of DC maturation markers by an ERK- and protein tyrosine kinase (PTK)–dependent and NF-kB and stress-activated protein kinase (SAPK)–independent pathways, whereas LPS maturation involved NF-kB and SAPK, and moderately involved ERK. hOSCAR engagement triggers signals through the FcRγ-ITAM and recruits ERK and PI3K, as demonstrated in survival assay. Thus, the partial maturation status might be due to the lack of NF-kB and SAPK activation, in contrast to the stimulation with a strong activator, such as TLR ligands. This underlines the fine-tuning of DC activation depending on the stimulator used and the signaling pathway triggered. This partial maturation can be reverted to a fully mature state by simultaneous PAMP-mediated activation of DCs through TLR and the NF-κB pathway.6 This is consistent with our observation that the activation of DCs with both TLR ligands and anti-OSCAR increased the expression of markers such as CD25 and CD86. In addition, when DCs were costimulated by hOSCAR and the TLR ligands LPS, poly(I:C), and R-848, they became more potent accessory cells able to sustain an enhanced CD45RA+ T-cell proliferation. However, DCs stimulated by both hOSCAR and LPS were less efficient in the polarization of Th1 effector cells and the induction of IFN-γ secretion than DCs incubated with LPS alone. The LPS-induced ability of DCs to sustain Th1 polarization is decreased by the engagement of hOSCAR, probably due to the concomitant increase in secretion of IL-10 and decrease of IL-12 p70 production, an effect seen only with LPS and not with other TLR ligands. The hOSCAR-mediated increase in IL-10 production induced by all of the tested TLR ligands was mediated by PI3K. The involvement of PI3K in IL-10 induction is in agreement with previous description of its role on IL-10 production upon stimulation of CD40, TLR2, or CD28.55-57 Recent studies indicate that PI3K is also an endogenous suppressor of IL-12 and TNF production triggered by LPS. This may act to limit excessive Th1 polarization causing undesirable immune responses;58-60 however, we did not observe a role of PI3K on hOSCAR-mediated IL-12 inhibition in response to LPS. In contrast, the increase of secretion of IL-12 observed with anti-hOSCAR and R-848 was PI3K dependent. Differences in the Toll-interleukin 1 receptor (TIR) domain adapters used by each TLR, linked to alternative downstream activation programs, probably account for the observed differential DC stimulation capacities,9,61 as well as for the different modulation of cytokine release when hOSCAR is engaged. In combination with LPS, signals induced by hOSCAR engagement might contribute to control inflammation and to down-regulate Th1-polarized responses.
Many receptors are known to be involved in the first line of defense, and the initiation of the innate immune response.62-64 Extensive studies on TLR using mice deficient for a specific TLR or for TLR-signaling mediators have demonstrated an almost complete requirement for these molecules in a broad range of innate immune responses.63 Emerging data from the literature19-21,65,66 suggest that one important role of ITAM-linked receptors expressed by monocytes, macrophages, and neutrophils is to enhance TLR-mediated signaling and subsequently, the proinflammatory responses. The data presented here address the question of ITAM-linked receptors and TLR cooperation in DCs and indicate that FcRγ signaling through hOSCAR ligation generates intracellular signals, which can modulate the acquired immune responses induced by DCs upon TLR-mediated activation. This effect is specific to the targeted TLR, and probably also to the signaling pathway induced, as shown by the differences in cytokine and T-cell functional modulation observed with LPS, poly(I:C), and R-848. Interestingly, this effect is present at suboptimal doses of TLR ligands, as would most probably be encountered in physiologic conditions. The fact that the response differs between TLRs shows that hOSCAR may help to determine a need to limit inflammation (in the case of LPS triggering) once events leading to adaptive immunity are initiated, or a need to continue an antiviral response, recruiting NK cells and DCs when only TLR3 or TLR8 are engaged. In the absence of a pathogen-derived signal, hOSCAR engagement promotes a semimature state, without the ability to induce T-cell proliferation. Thus, we have shown that both innate and adaptive immune responses by DCs can be characterized by a complex series of events, dependent on receptor engagement and the initial signals received by the cells.
Prepublished online as Blood First Edition Paper, January 13, 2005; DOI 10.1182/blood-2004-07-2809.
Supported by a grant from the Fondation Marcel Mérieux, Lyon, France (E.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank I. Durand for cell sorting, C. Massacrier and I. Perrot for technical help on T-cell experiments, B. Salaun for invaluable advice on survival experiments, M. Vatan and D. Lepot for editorial assistance, and colleagues from EFS-Lyon who provided us with blood samples.



![Figure 7. hOSCAR ligation, in combination with TLR ligands, increases the ability of mono-DCs to stimulate MLR and modulates Th1 polarization. (A) Proliferative response of CD45RA+-naive T cells cultured for 5 days with DCs treated with coated MOPC21, anti-CD13, anti-hOSCAR, and TNF was estimated by incorporation of [3H]-thymidine. Data are mean ± SD of quadruplicate samples from 1 representative experiment out of 4. (B) Mono-DCs were stimulated with coated anti-hOSCAR and/or activators (20 ng/mL TNF, 10 ng/mL LPS, 25 μg/mL poly(I:C), 10 μM R-848). After 5 days of culture with CD45RA+ T cells, the lymphocyte proliferation was measured. Data are mean ± SD of quadruplicate samples from 1 representative experiment out of 4 performed with similar results. (C) Polarization of CD45RA+-naive T cells cultured with mono-DCs stimulated with anti-hOSCAR and/or TLR ligands. After 6 hours of PMA/ionomycin stimulation and 4 hours of culture with GolgiPlug, T cells were intracellularly stained with FITC–anti–IFN-γ and PE–anti–IL-4, and analyzed by flow cytometer. Data shown are representative of 6 experiments. Numbers correspond to the percentage of cells in the lower right quadrant. (D) Secretion of IFN-γ by T cells cultured with mono-DCs stimulated with anti-hOSCAR and/or TLR ligands. Supernatant of T cells restimulated by anti-CD3 and anti-CD28 were analyzed by ELISA for the presence of IFN-γ, IL-4, and IL-5. No IL-4 and IL-5 were detected. Data are mean ± SD of triplicate samples from 1 representative experiment out of 3 performed with similar results. *P < .01 are given by comparison to values obtained with T cells cultured in the presence of MOPC21-stimulated DCs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/9/10.1182_blood-2004-07-2809/6/m_zh80090577760007.jpeg?Expires=1767704523&Signature=pcbFEpSNfF480EUj01FAjw2FppMVPHOZRmvy6WKcYG8Rho4dLBT3n1PUGjIuWEsxWUy3M~Q0rmT1ppJXBNQg-flExP-U6LshzqJSh4WAncT4QPX86jOMYn-GTvMIUwL9XQbd0fXa9xTUrBgBNW5vNuedxJ~SHOFLdbrhycD~oTP6sDQ-gq2pnTo2yNvJ~sjUr8qHFtLUyjMV4FxYNV0dGC9t-T2NMCRRY6qtOxXHNiwelQ80397NQgOVQkTjWafhTknCLTPX4u83KEK~Uu4yKfoewTtN8pgdTIW~azVUCcjCbUCdZjIoeSgdj6ezrfSkM7j9YLomC0C~gJgBSN12tQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

