Natural killer (NK) cells hold promise for improving the therapeutic potential of allogeneic hematopoietic transplantation, but their effectiveness is limited by inhibitory HLA types. We sought to overcome this intrinsic resistance by transducing CD56+CD3- NK cells with chimeric receptors directed against CD19, a molecule widely expressed by malignant B cells. An abundance of NK cells for transduction was secured by culturing peripheral blood mononuclear cells with K562 cells expressing the NK-stimulatory molecules 4-1BB ligand and interleukin 15, which yielded a median greater than 1000-fold expansion of CD56+CD3- cells at 3 weeks of culture, without T-lymphocyte expansion. Expression of anti-CD19 receptors linked to CD3ζ overcame NK resistance and markedly enhanced NK-cell-mediated killing of leukemic cells. This result was significantly improved by adding the 4-1BB costimulatory molecule to the chimeric anti-CD19-CD3ζ receptor; the cytotoxicity produced by NK cells expressing this construct uniformly exceeded that of NK cells whose signaling receptors lacked 4-1BB, even when natural cytotoxicity was apparent. Addition of 4-1BB was also associated with increased cell activation and production of interferon γ and granulocyte-macrophage colony-stimulating factor. Our findings indicate that enforced expression of signaling receptors by NK cells might circumvent inhibitory signals, providing a novel means to enhance the effectiveness of allogeneic stem cell transplantation.
Introduction
B-cell malignancies of children and adults, such as acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), and non-Hodgkin lymphoma (NHL), are often incurable even with intensive chemotherapy. For many patients, bone marrow ablation followed by allogeneic hematopoietic stem cell transplantation is the only potentially curative option, but the disease may return after transplantation.1 The well-documented association between T-cell-mediated graft-versus-host disease (GvHD) and a delay or suppression of leukemic relapse after allogeneic stem cell transplantation2-4 has led some investigators to manipulate GvHD by infusion of donor T lymphocytes. Although this procedure can induce a measurable antineoplastic response,5-8 it carries the risk of severe GvHD, particularly in those patients (> 70%) who lack an HLA-identical donor. Moreover, in some B-cell malignancies, such as ALL, the effect of lymphocyte infusions is often inadequate.6,9,10
Besides T lymphocytes, natural killer (NK) cells also exert cytotoxicity against cancer cells.11 Recent studies have emphasized the potential of NK-cell therapy in recipients of allogeneic hematopoietic stem cell transplants. In animal models of transplantation, donor NK cells could lyse leukemic cells and host lymphohematopoietic cells without affecting nonhematopoietic tissues,12 suggesting that NK-mediated graft-versus-leukemia responses may occur in the absence of systemic disease. Because NK cells are inhibited by self-HLA molecules, which bind to killer immunoglobulin-like receptors (KIRs), these findings have led to the clinical practice of selecting hematopoietic stem cell transplantation donors with an HLA and KIR type that favors NK-cell activation and thus could be expected to promote an antileukemic effect.13-15 However, selection of the “best” donor is limited to patients who have more than one potential donor and the capacity of NK cells to lyse lymphoid cells is generally low and difficult to predict.13,15-17
Emerging evidence indicates that T lymphocytes genetically modified with chimeric receptors able to recognize a surface molecule of target cells and transduce activation signals can specifically enhance T-cell cytotoxicity against cancer cells both in vitro and in vivo.18-21 The studies presented here are based on the concept that expression of chimeric receptors on NK cells could overcome HLA-mediated inhibitory signals, thus endowing the cells with cytotoxicity against otherwise NK-resistant cells. To test this hypothesis, we first developed a novel method that allows specific and vigorous expansion of NK cells lacking T-cell receptors (CD56+CD3- cells) and their highly efficient transduction with chimeric receptors. Then, we tested the relative antileukemic effects of genetically modified NK cells bearing chimeric receptors (directed against CD19, a molecule widely expressed by malignant B cells) that deliver different primary and costimulatory signals.
Materials and methods
Cells
The CD19+ human B-lineage ALL cell lines RS4;11, OP-1, 380, 697, and KOPN57bi; the T-cell line CEM-C7; and the myeloid cell lines K562 and U-937 were available in our laboratory.21 Cells were maintained in RPMI 1640 (Gibco, Grand Island, NY) supplemented with 10% fetal calf serum (FCS; BioWhittaker, Walkersville, MD) and antibiotics.
Primary leukemia cells were obtained from 9 patients with B-lineage ALL, with appropriate informed consent and Institutional Review Board (IRB) approval; from 4 of these patients, we also studied (with Saint Jude IRB approval) cryopreserved peripheral blood samples obtained during clinical remission. An unequivocal diagnosis of B-lineage ALL was established by morphologic, cytochemical, and immunophenotypic criteria; in each case, more than 95% of the cells were positive for CD19. Peripheral blood was obtained from 8 healthy adult donors. Mononuclear cells collected from the samples by centrifugation on a Lymphoprep density step (Nycomed, Oslo, Norway) were washed twice in phosphate-buffered saline (PBS) and once in AIM-V medium (Gibco).
Plasmids and retrovirus production
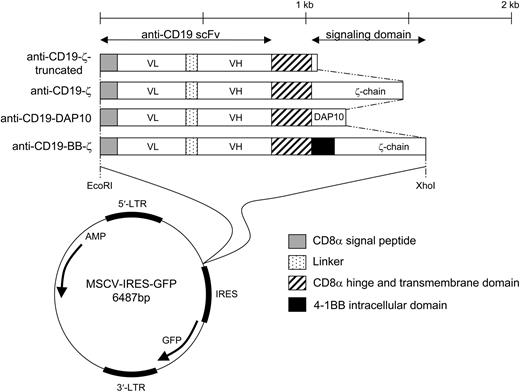
The anti-CD19-ζ, anti-CD19-BB-ζ, and anti-CD19-truncated (control) plasmids are described elsewhere.21 The pMSCV-IRES-GFP, pEQ-PAM3(-E), and pRDF constructs were obtained from the Saint Jude Vector Development and Production Shared Resource. The cDNA encoding the intracellular domains of human DAP10 and 4-1BB ligand (4-1BBL), and interleukin-15 (IL-15) with long signal peptide were subcloned by polymerase chain reaction (PCR) with a human spleen cDNA library (from Dr G. Neale, St Jude Children's Research Hospital) used as a template. An anti-CD19-DAP10 plasmid was constructed by replacing the sequence encoding CD3ζ with that encoding DAP10, using the splicing by overlapping extension by PCR (SOE-PCR) method. The cDNA encoding the signal peptide of CD8α, the mature peptide of IL-15 and the transmembrane domain of CD8α were assembled by SOE-PCR to encode a “membrane-bound” form of IL-15. The resulting expression cassettes were subcloned into EcoRI and XhoI sites of murine stem-cell virus-internal ribosome entry site-green fluorescent protein (MSCV-IRES-GFP).
The RD114-pseudotyped retrovirus was generated as previously described.21 We used calcium phosphate DNA precipitation to transfect 293T cells with anti-CD19-ζ, anti-CD19-DAP10, anti-CD19-BB-ζ, or anti-CD19-truncated; pEQ-PAM3(-E); and pRDF. Conditioned medium containing retrovirus was harvested at 48 hours and 72 hours after transfection, immediately frozen in dry ice, and stored at -80°C until use.
Development of K562 derivatives, expansion of NK cells, and gene transduction
K562 cells were transduced with the construct encoding the “membrane-bound” form of IL-15. Cells were cloned by limiting dilution, and a single-cell clone with high expression of GFP and of surface IL-15 (K562-mb15) was expanded. This clone was subsequently transduced with human 4-1BBL and designated as K562-mb15-41BBL. K562 cells expressing wild-type IL-15 (K562-wt15) or 4-1BBL (K562-41BBL) were produced by a similar procedure. Peripheral blood mononuclear cells (1.5 × 106) were incubated in a 24-well tissue-culture plate with or without 106 K562-derivative stimulator cells in the presence of 10 IU/mL human IL-2 (National Cancer Institute BRB Preclinical Repository, Rockville, MD) in RPMI 1640 and 10% FCS.
Mononuclear cells stimulated with K562-mb15-41BBL were transduced with retroviruses, as previously described for T cells.21 Briefly, 14 mL polypropylene centrifuge tubes (Falcon, Lincoln Park, NJ) were coated with human fibronectin (100 μg/mL; Sigma, St Louis, MO) or RetroNectin (50 μg/mL; TaKaRa, Otsu, Japan). Prestimulated cells (2 × 105) were resuspended in the tubes in 2 to 3 mL virus-conditioned medium with Polybrene (4 μg/mL; Sigma) and centrifuged at 2400g for 2 hours (centrifugation was omitted when RetroNectin was used). The multiplicity of infection (4-6) was identical in each experiment comparing the activity of different chimeric receptors. After centrifugation, cells were left undisturbed for 24 hours in a humidified incubator at 37°C, 5% CO2. The transduction procedure was repeated on 2 successive days. After a second transduction, the cells were restimulated with K562-mb15-41BBL in the presence of 10 IU/mL IL-2. Cells were maintained in RPMI 1640, 10% FCS, and 10 IU/mL IL-2.
Detection of chimeric receptor expression and immunophenotyping
Transduced NK cells were stained with goat anti-mouse (Fab)2 polyclonal antibody conjugated with biotin (Jackson ImmunoResearch Labs, West Grove, PA) followed by streptavidin conjugated to peridinin chlorophyll protein (PerCP; Becton Dickinson, San Jose, CA). For Western blotting, cells were lysed in RIPA buffer (PBS, 1% Triton-X100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) containing 3 μg/mL pepstatin, 3 μg/mL leupeptin, 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 mM ethylenediaminetetraacetic acid (EDTA), and 5 μg/mL aprotinin. Centrifuged lysate supernatants were boiled with an equal volume of loading buffer with or without 0.1 M dithiothreitol (DTT), and then separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on a precast 10% to 20% gradient acrylamide gel (Bio-Rad, Hercules, CA). The proteins were transferred to a polyvinylidene fluoride (PVDF) membrane, which was incubated with primary mouse anti-human CD3ζ monoclonal antibody (clone 8D3; PharMingen, San Diego, CA). Membranes were then washed, incubated with a goat anti-mouse IgG horseradish peroxidase-conjugated second antibody, and developed by using the enhanced chemiluminescence system (Amersham, Little Chalfont, United Kingdom).
The following antibodies were used for immunophenotypic characterization of expanded and transduced cells: anti-CD3 conjugated to fluorescein isothiocyanate (FITC), to PerCP, or to energy-coupled dye (ECD); anti-CD10 conjugated to phycoerythrin (PE); anti-CD19 PE; anti-CD22 PE; anti-CD56 FITC, PE, or allophycocyanin (APC); anti-CD16 CyChrome (antibodies from Becton Dickinson, PharMingen, or Beckman-Coulter, Miami, FL); and anti-CD25 PE (Dako, Carpinteria, CA). Surface expression of KIR and NK activation molecules was determined with specific antibodies conjugated to FITC or PE (from Beckman-Coulter or Becton Dickinson), as previously described.15 Antibody staining was detected with a FACScan or an LSR II flow cytometer (Becton Dickinson).
Cytotoxicity assays and cytokine production
Target cells (1.5 × 105) were placed in 96-well U-bottomed tissue-culture plates (Costar, Cambridge, MA) and incubated with primary NK cells transduced with chimeric receptors at various effector-target (E/T) ratios in RPMI 1640 supplemented with 10% FCS; NK cells were cultured with 1000 U/mL IL-2 for 48 hours before the assay. Cultures were performed in the absence of exogenous IL-2. After 4 hours and 24 hours, cells were harvested, labeled with CD10 PE or CD22 PE and CD56 FITC, and assayed by flow cytometry as previously described.21-23 The numbers of target cells recovered from cultures without NK cells were used as a reference.
For cytokine production, primary NK cells (2 × 105 in 200 μL) expressing chimeric receptors were stimulated with various target cells at a 1:1 ratio for 24 hours. The levels of interferon γ (IFN-γ) and granulocyte-macrophage colony-stimulating factor (GM-CSF) in cell-free culture supernatants were determined with a Bio-Plex assay (Bio-Rad).
Statistical analysis
A test of equality of mean NK expansion with various stimuli was performed using analysis of variance for a randomized complete block design with each donor considered a random block. The Tukey honest significant difference procedure was used to compute simultaneous confidence intervals for each pairwise comparison of the differences of treatment means. Differences in cytotoxicities and cytokine production among NK cells bearing different chimeric receptors were analyzed by the paired Student t test.
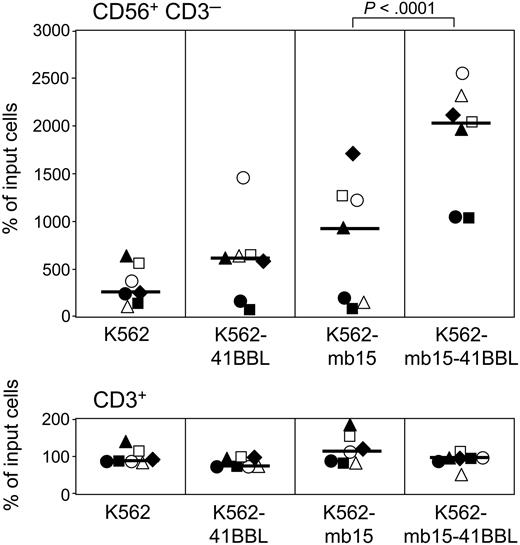
Expansion of NK cells after 1 week of culture with genetically modified K562 cells. Peripheral blood mononuclear cells from 7 healthy individuals (represented by different symbols) were cultured with various preparations of K562 at a 1:1 ratio in the presence of low-dose (10 U/mL) IL-2. Percentages of CD56+CD3- NK cells and CD3+ T lymphocytes after 7 days of culture relative to the number of input cells are shown. Each data point represents the average of 2 measurements; bars correspond to the median expansion in each group. K562 cells expressing both membrane-bound IL-15 and 4-1BBL (K562-mb15-41BBL) induced a markedly superior expansion of NK cells (P < .001 by the Tukey honest significant difference test) without inducing T-cell proliferation; there were no significant differences among other pairwise comparisons of NK expansions obtained with K562, K562-41BBL, and K562-mb15.
Expansion of NK cells after 1 week of culture with genetically modified K562 cells. Peripheral blood mononuclear cells from 7 healthy individuals (represented by different symbols) were cultured with various preparations of K562 at a 1:1 ratio in the presence of low-dose (10 U/mL) IL-2. Percentages of CD56+CD3- NK cells and CD3+ T lymphocytes after 7 days of culture relative to the number of input cells are shown. Each data point represents the average of 2 measurements; bars correspond to the median expansion in each group. K562 cells expressing both membrane-bound IL-15 and 4-1BBL (K562-mb15-41BBL) induced a markedly superior expansion of NK cells (P < .001 by the Tukey honest significant difference test) without inducing T-cell proliferation; there were no significant differences among other pairwise comparisons of NK expansions obtained with K562, K562-41BBL, and K562-mb15.
Results
Culture conditions that favor the expansion of primary NK cells
To transduce chimeric receptors into primary NK cells, we searched for stimuli that would induce specific NK-cell proliferation. In preliminary experiments, we depleted peripheral blood mononuclear cells of CD3+ T lymphocytes and stimulated the remaining cells with IL-2 (1000 U/mL) or IL-15 (10 ng/mL). Under these culture conditions there was no expansion of NK cells, which, in fact, progressively declined in numbers. With phytohe-magglutinin (PHA; 7 μg/mL) and IL-2 (1000 U/mL) as stimuli, we observed a 2- to 5-fold expansion of CD56+ CD3- NK cells after 1 week of culture. However, despite the low proportion of contaminating CD3+ cells (< 2% in 2 experiments) at the beginning of the cultures, these cells expanded more than NK cells (> 30-fold expansion), and after 1 week of culture represented approximately 35% of the cell population.
NK cells can be stimulated by contact with the human leukemia cell line K562, which lacks HLA-antigen expression,24 and genetically modified K562 cells have been used to stimulate cytotoxic T lymphocytes.25 We therefore tested whether the NK-stimulatory capacity of K562 cells could be increased through enforced expression of additional NK-stimulatory molecules, using 2 molecules that are not expressed by K562 cells and are known to stimulate NK cells. One molecule, the ligand for 4-1BB, triggers activation signals after binding to 4-1BB (CD137), a signaling molecule expressed on the surface of NK cells.26 The other molecule, IL-15, is a cytokine known to promote NK-cell development and the survival of mature NK cells.27-30 Because IL-15 has greater biologic activity when presented to NK cells bound to IL-15 receptor α (IL-15Rα) on the cell membrane of stimulatory cells, rather than in its soluble form,31-35 we made a construct containing the human IL15 gene fused to the gene encoding the human CD8α transmembrane domain and used it to transduce K562 cells. Expression of IL-15 on the surface of K562 cells was more than 5 times higher with the IL-15-CD8α construct than with wild-type IL-15 (not shown).
To test whether the modified K562 cells expressing both 4-1BBL and IL-15 (K562-mb15-41BBL cells) promote NK-cell expansion, we cultured peripheral blood mononuclear cells from 7 donors in the presence of low-dose (10 U/mL) IL-2 as well as irradiated K562 cells transduced with 4-1BBL or IL-15 (or both), or with an empty control vector. As shown in Figure 1, expression of either 4-1BBL or IL-15 by K562 cells improved the stimulation of NK-stimulatory capacity of K562 in some cases but not overall, whereas simultaneous expression of both molecules led to a consistent and striking amplification of NK cells (median recovery of CD56+CD3- cells at 1 week of culture, 2030% of input cells [range, 1020%-2520%] compared with a median recovery of 250% [range, 150%-640%] for K562 cells lacking 4-1BBL and IL-15; P < .001). In 24 experiments with cells from 8 donors, NK-cell expansion after 3 weeks of culture with K562 cells expressing both stimulatory molecules ranged from 309-fold to 12 409-fold (median, 1089-fold). Importantly, neither the modified nor unmodified K562 cells caused an expansion of T lymphocytes (Figure 1).
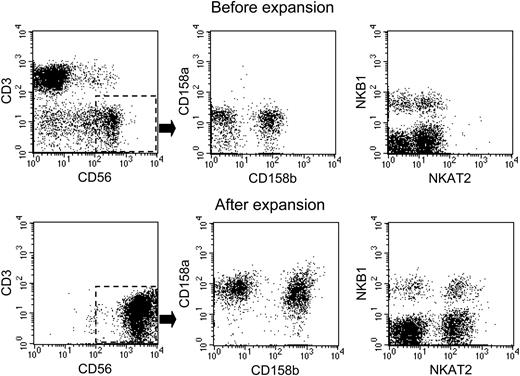
Among expanded CD56+CD3- NK cells, expression of CD56 was higher than that of unstimulated cells (Figure 2); expression of CD16 was similar to that seen on unstimulated NK cells (median CD16+ NK cells in 7 donors: 89% before expansion and 84% after expansion). We also compared the expression of KIR molecules on the expanded NK cells with that on NK cells before culture, using the monoclonal antibodies CD158a (against KIR 2DL1), CD158b (2DL2), NKB1 (3DL1), and NKAT2 (2DL3). The prevalence of NK subsets expressing these molecules after expansion resembled that of their counterparts before culture, although the level of expression of KIR molecules was higher after culture (Figure 2). Similar results were obtained for the inhibitory receptor CD94, whereas expression of the activating receptors NKp30 and NKp44 became detectable on most cells after culture (not shown). In sum, the immunophenotype of expanded NK cells reiterated that of activated NK cells, indicating that contact with K562-mb15-41BBL cells had stimulated expansion of all subsets of NK cells.
Transduction of NK cells with chimeric receptors
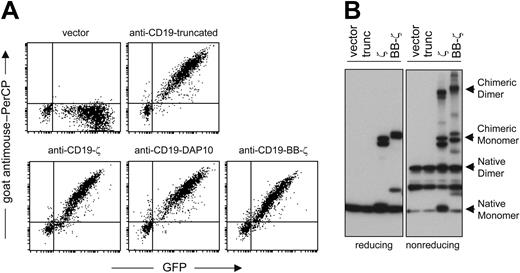
Before transducing peripheral blood mononuclear cells with retroviral vectors containing chimeric receptor constructs and GFP (Figure 3), we stimulated them with K562-mb15-41BBL cells. In 27 experiments, the median percentage of NK cells that were GFP+ at 7 to 11 days after transduction was 69% (43%-93%). Chimeric receptors were expressed at high levels on the surface of NK cells and, by Western blotting, were in both monomeric and dimeric configurations (Figure 4).
Immunophenotypic features of NK cells before and after expansion with K562-mb15-41BBL cells. Expression of CD3 and CD56, as well as expression of the KIRs CD158a (2DL1), CD158b (2DL2), NKB1 (3DL1), and NKAT2 (2DL3) on CD56+CD3- cells were examined in peripheral blood mononuclear cells from a healthy donor before (top row) and after (bottom row) 3 weeks of coculture with K562-mb15-41BBL cells and low-dose (10 U/mL) IL-2.
Immunophenotypic features of NK cells before and after expansion with K562-mb15-41BBL cells. Expression of CD3 and CD56, as well as expression of the KIRs CD158a (2DL1), CD158b (2DL2), NKB1 (3DL1), and NKAT2 (2DL3) on CD56+CD3- cells were examined in peripheral blood mononuclear cells from a healthy donor before (top row) and after (bottom row) 3 weeks of coculture with K562-mb15-41BBL cells and low-dose (10 U/mL) IL-2.
Schematic representation of the chimeric receptors used in this study. LTR indicates long terminal repeat; AMP, ampicillin resistance; and bp, base pair.
Schematic representation of the chimeric receptors used in this study. LTR indicates long terminal repeat; AMP, ampicillin resistance; and bp, base pair.
To identify the specific signals required to stimulate NK cells with chimeric receptors, and overcome inhibitory signals mediated by KIR molecules and other NK inhibitory receptors that bind to HLA class I molecules, we first compared 2 types of chimeric receptors containing different signaling domains: CD3ζ, a signal-transducing molecule containing 3 immunoreceptor tyrosine-based activation motifs (ITAMs) and linked to several activating receptors expressed on the surface of NK cells,11,36 and DAP10, a signal transducing molecule with no ITAMs linked to the activating receptor NKG2D and previously shown to trigger NK cytotoxicity.11,36,37 As a control, we used NK cells transduced with a vector containing an anti-CD19 receptor but no signaling molecules or containing GFP alone. NK cells were challenged with the CD19+ leukemic cell lines 380, 697, and RS4;11, all of which express high levels of HLA class I molecules by antibody staining. By genotyping, RS4;11 is Cw4/Cw3, Bw4 and A3; 380 is Cw4/Cw4, Bw4; and 697 is Cw3/Cw3. Hence, these cell lines were fully capable of inhibiting NK-cell cytotoxicity via binding to NK-inhibitory receptors.
Expression of chimeric receptors by NK cells expanded from peripheral blood mononuclear cells. (A) Surface receptor expression was visualized by flow cytometry after staining with a goat anti-mouse (Fab)2 polyclonal antibody conjugated with biotin followed by streptavidin PerCP (y-axes); expression of GFP is also shown (x-axes). (B) Western blot analysis of chimeric receptor expression in NK cells, under reducing or nonreducing conditions. Filter membranes were labeled with an antihuman CD3ζ antibody and a goat anti-mouse IgG horseradish peroxidase-conjugated second antibody.
Expression of chimeric receptors by NK cells expanded from peripheral blood mononuclear cells. (A) Surface receptor expression was visualized by flow cytometry after staining with a goat anti-mouse (Fab)2 polyclonal antibody conjugated with biotin followed by streptavidin PerCP (y-axes); expression of GFP is also shown (x-axes). (B) Western blot analysis of chimeric receptor expression in NK cells, under reducing or nonreducing conditions. Filter membranes were labeled with an antihuman CD3ζ antibody and a goat anti-mouse IgG horseradish peroxidase-conjugated second antibody.
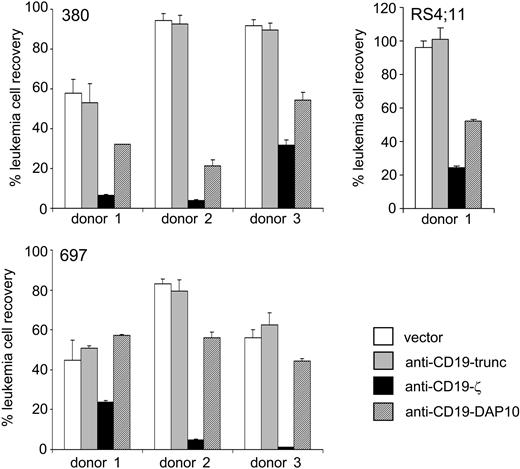
Chimeric receptors bearing CD3ζ overcome the NK resistance of leukemic cells. The results shown for 3 cell lines, 380, RS4;11, and 697, are expressed as the mean (± SD; n = 4) percentage of leukemic cell recovery after 24 hours of culture at a 1:1 E/T ratio relative to cultures without NK cells, as measured by flow cytometry. NK cells expressing anti-CD19-ζ receptors were more cytotoxic than NK cells expressing anti-CD19-DAP10 receptors, anti-CD19 receptors without signaling capacity (anti-CD19-trunc), or NK cells transduced with GFP control vector (P < .001).
Chimeric receptors bearing CD3ζ overcome the NK resistance of leukemic cells. The results shown for 3 cell lines, 380, RS4;11, and 697, are expressed as the mean (± SD; n = 4) percentage of leukemic cell recovery after 24 hours of culture at a 1:1 E/T ratio relative to cultures without NK cells, as measured by flow cytometry. NK cells expressing anti-CD19-ζ receptors were more cytotoxic than NK cells expressing anti-CD19-DAP10 receptors, anti-CD19 receptors without signaling capacity (anti-CD19-trunc), or NK cells transduced with GFP control vector (P < .001).
Expression of receptors without signaling molecules did not increase NK-mediated cytotoxicity over that exerted by NK cells transduced with the vector containing only GFP (Figure 5). By contrast, expression of anti-CD19-ζ receptors markedly enhanced NK cytotoxicity in all experiments (Figure 5), regardless of the intrinsic ability of donor NK cells to kill leukemic targets. For example, 380 cells were highly resistant to NK cells from donors 2 and 3, but were killed when these donor cells expressed anti-CD19-ζ receptors. Similar observations were made for RS4;11 cells and the NK cells of donor 1 and for 697 cells and NK cells of donor 2. Moreover, the anti-CD19-ζ receptors led to improved killing of target cells even when natural cytotoxicity was present. In all experiments, the cytotoxicity triggered by the anti-CD19-ζ receptor was enhanced over that achieved by replacing CD3ζ with DAP10 (P < .001; Figure 5).
4-1BB-mediated costimulatory signals enhance NK cytotoxicity
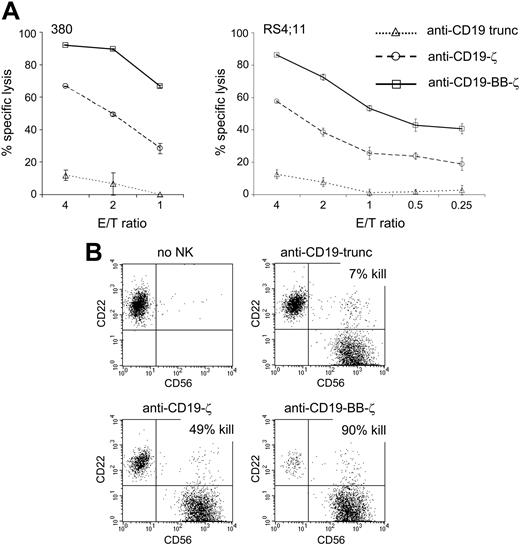
Previous studies have shown that the addition of costimulatory molecules to chimeric receptors enhances the proliferation and cytotoxicity of T lymphocytes.21,38-43 Of the 2 best known costimulatory molecules in T lymphocytes, CD28 and 4-1BB, only 4-1BB is expressed by NK cells.26,44,45 We therefore determined whether the addition of 4-1BB to the anti-CD19-ζ receptor would enhance NK cytotoxicity. In a 4-hour cytotoxicity assay, cells expressing the 4-1BB-augmented receptor showed a markedly better ability to kill CD19+ cells than did cells lacking this modification (Figure 6A-B). The superiority of NK cells bearing the anti-CD19-BB-ζ receptor was also evident in 24-hour assays with NK cells from different donors cultured at a 1:1 ratio with the leukemia cell lines 697, KOPN57bi, and OP-1 (not shown).
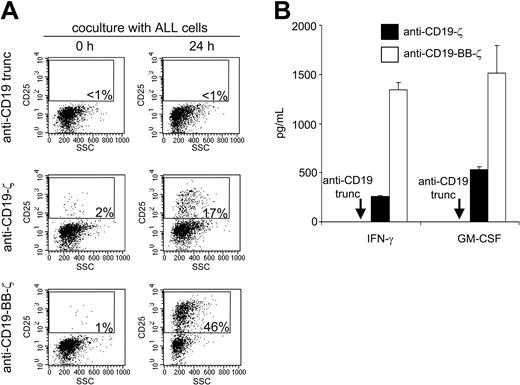
Next, we determined whether the antileukemic activity of NK cells expressing anti-CD19-BB-ζ receptors extended to primary leukemic samples. In 5 samples from children with different molecular species of ALL, NK cells expressing the 4-1BB receptors exerted strong cytotoxicity that was evident even at low E/T ratios (eg, < 1:1; Figure 7) and uniformly exceeded the activity of NK cells expressing signaling receptors that lacked 4-1BB. Even when donor NK cells had natural cytotoxicity against ALL cells and CD3ζ receptor did not improve it (patient no. 3 in Figure 7), addition of 4-1BB to the receptor significantly enhanced cytotoxicity. Consistent with their increased cytotoxicity, NK cells expressing anti-CD19-BB-ζ mediated more vigorous activation signals. As shown in Figure 8A, 46% of NK cells bearing this receptor expressed the IL-2Rα chain CD25 after 24 hours of coculture with CD19+ ALL cells, compared with only 17% of cells expressing the anti-CD19-ζ receptor and less than 1% for cells expressing receptors that lacked stimulatory capacity. Moreover, anti-CD19-BB-ζ receptors induced a much higher production of IFN-γ and GM-CSF on contact with CD19+ cells than did receptors without 4-1BB (Figure 8B).
Addition of the 4-1BB costimulatory molecule to the chimeric receptors augments their capacity to induce NK cytotoxicity against NK-resistant leukemic cells. (A) Expanded primary NK cells expressing chimeric receptors were incubated for 4 hours with the B-lineage ALL cell lines 380 and RS4;11 at the indicated E/T ratios. Each data point represents the mean (± SD; n = 4) percentage of ALL cell killing after culture as compared to that of parallel cultures without NK cells. At all E/T ratios, cytotoxicity of NK cells expressing chimeric receptors containing 4-1BB was significantly higher than that induced by receptors without 4-1BB (P < .001). (B) Flow cytometric dot plots show staining with anti-CD56 and anti-CD22 after a 4-hour coculture of NK cells (CD56+) and ALL cells (380; CD22+) at a 2:1 ratio. The percentage of cell killing obtained with NK cells expressing different chimeric receptors (% kill) was calculated by comparing the number of viable CD22+ ALL cells recovered after the test culture to that of parallel cultures without NK cells.
Addition of the 4-1BB costimulatory molecule to the chimeric receptors augments their capacity to induce NK cytotoxicity against NK-resistant leukemic cells. (A) Expanded primary NK cells expressing chimeric receptors were incubated for 4 hours with the B-lineage ALL cell lines 380 and RS4;11 at the indicated E/T ratios. Each data point represents the mean (± SD; n = 4) percentage of ALL cell killing after culture as compared to that of parallel cultures without NK cells. At all E/T ratios, cytotoxicity of NK cells expressing chimeric receptors containing 4-1BB was significantly higher than that induced by receptors without 4-1BB (P < .001). (B) Flow cytometric dot plots show staining with anti-CD56 and anti-CD22 after a 4-hour coculture of NK cells (CD56+) and ALL cells (380; CD22+) at a 2:1 ratio. The percentage of cell killing obtained with NK cells expressing different chimeric receptors (% kill) was calculated by comparing the number of viable CD22+ ALL cells recovered after the test culture to that of parallel cultures without NK cells.
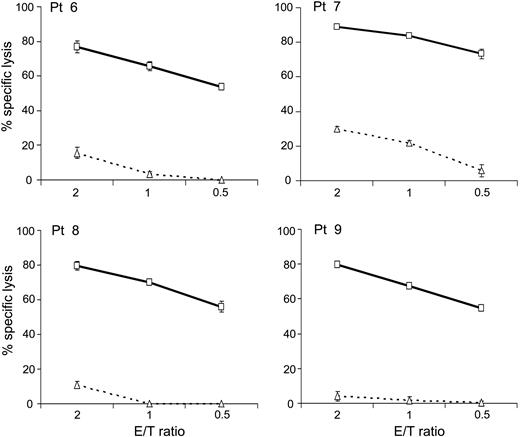
NK cells expressing 4-1BB-augmented chimeric receptors show powerful cytotoxicity against leukemic cells from patients. Expanded primary NK cells expressing chimeric receptors were incubated for 4 hours with leukemic cells from children with different subtypes of B-lineage ALL (patient [Pt] 1, hyperdiploid 47-50; Pt 2 and Pt 5, t(4;11)(q21;q23); Pt 3, t(14;?)(q32;?); Pt 4, der8, t(8;?)) at the indicated E/T ratios. Each data point represents the mean (± SD; n = 4) percentage of ALL cell killing after culture as compared to that of parallel cultures without NK cells. With the exception of the results obtained in patient 2 at a 1:1 ratio, the cytotoxicity of NK cells expressing chimeric receptors containing 4-1BB was significantly higher than that induced by receptors without 4-1BB (P < .005).
NK cells expressing 4-1BB-augmented chimeric receptors show powerful cytotoxicity against leukemic cells from patients. Expanded primary NK cells expressing chimeric receptors were incubated for 4 hours with leukemic cells from children with different subtypes of B-lineage ALL (patient [Pt] 1, hyperdiploid 47-50; Pt 2 and Pt 5, t(4;11)(q21;q23); Pt 3, t(14;?)(q32;?); Pt 4, der8, t(8;?)) at the indicated E/T ratios. Each data point represents the mean (± SD; n = 4) percentage of ALL cell killing after culture as compared to that of parallel cultures without NK cells. With the exception of the results obtained in patient 2 at a 1:1 ratio, the cytotoxicity of NK cells expressing chimeric receptors containing 4-1BB was significantly higher than that induced by receptors without 4-1BB (P < .005).
Chimeric receptors bearing 4-1BB induce a superior NK-cell activation. (A) Expanded primary NK cells expressing chimeric receptors were incubated for 24 hours with the ALL cell line RS4;11 at a 1:1 ratio. Flow cytometric dot plots illustrated CD25 expression (in the y-axes) and side scatter (SSC; in the x-axes) of GFP+ cells before and after culture. The percentages of CD25+ NK cells are indicated. (B) Production of IFN-γ and GM-CSF by NK cells expressing different chimeric receptors after 24 hours of culture with 697 cells at a 1:1 ratio (mean ± SD of 3 measurements). The 4-1BB receptors elicited a significantly higher production of both cytokines (P < .005).
Chimeric receptors bearing 4-1BB induce a superior NK-cell activation. (A) Expanded primary NK cells expressing chimeric receptors were incubated for 24 hours with the ALL cell line RS4;11 at a 1:1 ratio. Flow cytometric dot plots illustrated CD25 expression (in the y-axes) and side scatter (SSC; in the x-axes) of GFP+ cells before and after culture. The percentages of CD25+ NK cells are indicated. (B) Production of IFN-γ and GM-CSF by NK cells expressing different chimeric receptors after 24 hours of culture with 697 cells at a 1:1 ratio (mean ± SD of 3 measurements). The 4-1BB receptors elicited a significantly higher production of both cytokines (P < .005).
We asked whether the expression of signaling chimeric receptors would affect spontaneous NK activity against NK-sensitive cell lines not expressing CD19. Spontaneous cytotoxicity of NK cells from 3 donors against the CD19- leukemia cell lines K562, U937, and CEM-C7 was not diminished by expression of chimeric receptors, with or without 4-1BB (not shown).
Anti-CD19 chimeric receptors induce NK cytotoxicity against autologous leukemic cells
To determine whether the NK-cell expansion and transduction system that we developed would be applicable to clinical samples, we studied peripheral blood samples that had been obtained (and cryopreserved) from 4 patients with childhood B-lineage ALL in clinical remission, 25 to 56 weeks from diagnosis. NK-cell expansion occurred in all 4 samples; after 1 week of culture with K562-mb15-41BBL cells, recovery of CD56+CD3- NK cells ranged from 1350% to 3680% of the input.
NK cells expressing anti-CD19 signaling receptors become highly cytotoxic against autologous leukemic cells. Peripheral blood NK cells were obtained from patients with B-lineage ALL in clinical remission. After expansion and transduction, cytotoxicity was tested against autologous leukemic lymphoblasts from diagnostic bone marrow samples. Each data point represents the mean (± SD; n = 4) percentage of ALL cell killing after culture as compared to that of parallel cultures without NK cells. The cytotoxicity of NK cells expressing anti-CD19-BB-ζ receptors (□ and solid lines) was markedly higher than that exerted by NK cells transduced with anti-CD19 truncated nonsignaling receptor (▵ and dotted line; patients 6-8) or empty vector (▵ and dotted line; patient 9).
NK cells expressing anti-CD19 signaling receptors become highly cytotoxic against autologous leukemic cells. Peripheral blood NK cells were obtained from patients with B-lineage ALL in clinical remission. After expansion and transduction, cytotoxicity was tested against autologous leukemic lymphoblasts from diagnostic bone marrow samples. Each data point represents the mean (± SD; n = 4) percentage of ALL cell killing after culture as compared to that of parallel cultures without NK cells. The cytotoxicity of NK cells expressing anti-CD19-BB-ζ receptors (□ and solid lines) was markedly higher than that exerted by NK cells transduced with anti-CD19 truncated nonsignaling receptor (▵ and dotted line; patients 6-8) or empty vector (▵ and dotted line; patient 9).
After transduction with chimeric receptors, we tested the cytotoxicity of the NK cells against autologous leukemic lymphoblasts obtained at diagnosis. As shown in Figure 9, expression of anti-CD19-BB-ζ receptors overcame NK-cell resistance of autologous cells; NK cells expressing the receptors exerted cytotoxicity, which was as powerful as that observed with allogeneic targets.
Discussion
In this study, we demonstrated that the resistance of cancer cells to NK-cell activity can be overcome by chimeric receptors expressed on primary NK cells. The stimulatory signals triggered by the receptors on contact with target cells predominated over inhibitory signals and induced powerful cytotoxicity against NK-resistant leukemic cell lines and primary leukemic cells. We found that the type of stimulatory signal delivered by the chimeric receptor was a key factor in inducing cytotoxicity. Although DAP10 signaling can elicit NK cytotoxicity,37 chimeric receptors containing this molecule in our study induced weaker NK-cell activity than that generated by CD3ζ-containing receptors, despite identical levels of surface expression. We also found that addition of the costimulatory molecule 4-1BB to the chimeric receptors markedly augmented cytotoxicity and that receptors containing both CD3ζ and 4-1BB triggered a much more robust NK-cell activation and cytokine production than did those containing only CD3ζ. The important contribution of 4-1BB signals agrees with findings that anti-4-1BB antibodies activate murine NK cells,46 and enhance their antitumor activity.26 Leukemic lymphoid cells usually do not express 4-1BBL21 ; only 2 of 284 diagnostic B-lineage ALL samples studied by gene arrays at our institution expressed 4-1BBL transcripts.47 Hence, 4-1BB signals can be delivered to NK cells only if the molecule is incorporated into the receptor.
Efficient and stable transduction of primary NK cells is notoriously difficult, prompting us to devise a new gene transduction method for the present study. Most investigators have demonstrated efficient gene transfer only in continuously growing NK-cell lines48-54 or reported methods yielding only transient gene expression.37,55,56 We achieved stable expression of chimeric receptors in primary CD56+CD3- NK cells by using an RD114-pseudotyped retroviral vector and specifically expanding primary CD56+CD3- NK cells before they were exposed to the retrovirus, a step that allowed highly efficient gene expression. Although several cytokines such as IL-2, IL-12, and IL-15 stimulate NK cells,27,57,58 their capacity to induce proliferation of resting CD56+CD3- cells has been poor, unless accessory cells are present in the cultures.24 Perussia et al59 found that contact with irradiated B-lymphoblastoid cells induced as high as a 25-fold expansion of NK cells after 2 weeks of stimulation, and Miller et al60 reported an approximate 30-fold expansion of NK cells after 18 days of culture with 1000 U/mL IL-2 and monocytes. However, these culture conditions are likely to promote the growth of CD3+ T lymphocytes as well as NK cells. Because our ultimate aim is to generate pure preparations of donor NK cells devoid of CD3+ T lymphocytes that can be infused into recipients of allogeneic hematopoietic stem cell transplants, we searched for methods that would maximize NK-cell expansion without producing T-cell mitogenicity.
Contact with K562 cells (which lack major histocompatibility complex [MHC] class I molecule expression and hence do not trigger KIR-mediated inhibitory signals in NK cells) is known to augment NK-cell proliferation in response to IL-15.24 We found that membrane-bound IL-15 and 4-1BBL, coexpressed by K562 cells, acted synergistically to augment K562-specific NK-stimulatory capacity, resulting in vigorous expansion of peripheral blood CD56+CD3- NK cells without concomitant growth of T lymphocytes. After 2 to 3 weeks of culture, we observed NK-cell expansions of up to 10 000-fold, and virtually pure populations of NK cells could be obtained, even without the need for T-cell depletion in some cases. NK cells expanded in this system retained the immunophenotypic diversity seen among peripheral blood subsets of NK cells, as well as their natural cytotoxicity against sensitive target cells, even after transduction with different chimeric receptors. Hence, this system should help studies of NK-cell biology that require specific cell expansion or gene transduction or both, but it should also be adaptable to clinical applications after generating K562-mb15-41BBL cells that comply with current good manufacturing practices for clinical trials. Recently, Harada et al reported that expansions of CD56+CD3- cells (up to 400-fold after 2 weeks) were apparently superior after contact with another HLA class I-negative cell line, the Wilms tumor cell line HFWT.61 Future studies should determine whether HFWT cells express 4-1BBL or whether enforced expression of 4-1BBL together with IL-15 results in a greater specific expansion of NK cells than seen with modified K562 cells.
In the context of allogeneic hematopoietic stem cell transplantation, infusions of activated donor T cells would carry an unacceptably high risk of severe GvHD, particularly in recipients of haploidentical or mismatched transplants. By contrast, infusions of pure CD56+CD3- NK cells should not impose that risk.12 Most clinical studies of the therapeutic effects of NK cells have been performed in an autologous setting and have yielded only moderately promising results.11,62 This is not surprising because NK-cell activity is inhibited by surface receptors that recognize autologous HLA molecules expressed by both normal and neoplastic cells. Allogeneic NK cells may be more effective, but even in an allogeneic setting the capacity of NK cells to kill malignant lymphoid cells is generally modest and often negligible.17 Leung et al15 detected NK cytotoxicity against an ALL cell line expressing particularly low levels of inhibitory HLA molecules, but cytotoxicity was much lower than that observed against the NK-cell target K562; only about 50% of the ALL cells were killed at an effector-target ratio of 40:1. In that study, RS4;11 cells, which express HLA-C alleles that bind the most commonly expressed KIRs, were NK resistant, whereas these cells, as well as autologous leukemic cells, were highly sensitive to NK cells expressing anti-CD19 signaling receptors in our study. Thus, NK cells expressing signaling chimeric receptors have much more powerful antileukemic activity than unmodified NK cells and can kill target cells irrespective of their HLA profile.
An increased understanding of the signals leading to immune cell activation, together with progress in gene cloning and transfer, have made the treatment of cancer with “adoptively acquired immunity”63 a realistic goal. Clinical precedents, such as administration of T-cell clones that target cytomegalovirus epitopes64 or Epstein-Barr virus-specific antigens,65 attest to the clinical feasibility of adoptive immune cell therapy. Nonetheless, potential limitations may affect the effectiveness of cell therapy guided by chimeric receptors. One is that the murine scFv portion of the chimeric receptor or the fusion sites of the human regions that compose it may trigger a host immune response leading to elimination of the modified cells.18 Although the impact of such an event in a clinical setting remains to be determined, we anticipate that immune responses against modified NK cells will be limited in immunosuppressed patients after hematopoietic stem cell transplantation. Another potential limitation is that adoptively transferred cells may have inadequate persistence in vivo, although a recent study showed that NK cells obtained from haploidentical donors and activated ex vivo could expand in patients when infused after administration of high-dose cyclophosphamide and fludarabine, which caused an increase in endogenous IL-15.66 We speculate that such expansions would also occur with genetically modified NK cells and suggest that further studies to identify signaling molecules that promote NK-cell proliferation when incorporated into chimeric receptors are warranted. In patients at a high risk of leukemia or lymphoma relapse, the expected benefits of genetically modified NK cells will outweigh the risk of insertional oncogenesis posed by the use of retroviruses for chimeric receptor transduction.67 We also predict that the coexpression of suicide genes will become a useful safety measure in clinical studies68 ; this strategy would also ensure that the elimination of normal CD19+ B-lineage cells is only temporary.
Novel therapies that bypass cellular mechanisms of drug resistance are urgently needed for patients with refractory leukemia and lymphoma. NK-cell alloreactivity is a powerful new tool for improving the therapeutic potential of allogeneic hematopoietic stem cell transplantation. The results of this study indicate that signaling receptors can enhance the efficacy of NK-cell alloreactivity and widen its applicability. We envisage initial clinical trials in which donor NK cells, collected by apheresis, are expanded ex vivo as described here, transduced with chimeric receptors, and then infused after transplantation in patients with B-lineage ALL. The target molecule for the chimeric receptors, CD19, was selected because it is one of the most widely expressed surface antigens among B-cell malignancies, including ALL, CLL, and NHL. In these malignancies, CD19 is highly expressed on the surface of virtually all cells but has limited or no expression in normal tissues.69 However, the NK-cell strategy of immunotherapy we describe would not have to be directed to the CD19 antigen but could be applied to any of the numerous molecules identified as potential targets for chimeric receptor-based cell therapy in patients with cancer.18
Prepublished online as Blood First Edition Paper, March 8, 2005; DOI 10.1182/blood-2004-12-4797.
Supported by grants CA58297 and CA21765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Martha Holladay and Jim Houston for KIR expression analysis, Victoria Turner for cell line genotyping, Stanley Pounds for assistance with statistical analysis, the St Jude Vector Development and Production Shared Resource for retroviral vectors and the St. Jude Tissue Resource laboratory for providing patient samples.

![Figure 7. NK cells expressing 4-1BB-augmented chimeric receptors show powerful cytotoxicity against leukemic cells from patients. Expanded primary NK cells expressing chimeric receptors were incubated for 4 hours with leukemic cells from children with different subtypes of B-lineage ALL (patient [Pt] 1, hyperdiploid 47-50; Pt 2 and Pt 5, t(4;11)(q21;q23); Pt 3, t(14;?)(q32;?); Pt 4, der8, t(8;?)) at the indicated E/T ratios. Each data point represents the mean (± SD; n = 4) percentage of ALL cell killing after culture as compared to that of parallel cultures without NK cells. With the exception of the results obtained in patient 2 at a 1:1 ratio, the cytotoxicity of NK cells expressing chimeric receptors containing 4-1BB was significantly higher than that induced by receptors without 4-1BB (P < .005).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-12-4797/4/m_zh80130580460007.jpeg?Expires=1766338225&Signature=C80Tsi2sf6R4Run7lQx7EcDIoQRxjaOmwhtF5Ti4EJ0ePFs7ucCEHksZbe3sKEp~WmZ403-O6GM2dTjPINrtyumT079Mje3nN0yDSg49X6WtZs3HXxwIwAOpH4Wt2yUIUB6osDjfBCxQopOreRwreHt3kTgukOiW76k1V1-JqgfoBm7J3VkLdn4TZW4cizYDtG9ZB9v~C5d4RABd6ZlaaisThMQLhGn8zW3b9FFOkSDdeXusOPh80gTs3yZ4NARDw2HPe9OXOM7-N6V--3o1-MgLGNDUNhTsB06reIafJ-kFVH~wS9ASrbAtm10z~EJdX415vOp8CCZFRLguaq8jBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)