Abstract
The destruction of erythrocytes and defects in erythropoiesis are among the most frequently observed causes of morbidity in severe Plasmodium falciparum malaria. The molecular mechanisms involved remain unclear, despite extensive investigation. We show here, for the first time, that tagging with the parasite rhoptry protein ring surface protein 2 (RSP2) is not restricted to the surfaces of normal erythrocytes, as previously reported, but that it extends to erythroid precursor cells in the bone marrow of anemic malaria patients. Monoclonal mouse antibodies and human sera from patients with severe anemia, reacting with RSP2-tagged erythrocytes, induced cell destruction by phagocytosis and complement activation in vitro. Our observations reveal a new parasite mechanism implicated in the destruction of normal erythrocytes and probably dyserythropoiesis in malaria patients. These data suggest that the tagging of host cells with RSP2 may trigger anemia in falciparum malaria.
Introduction
Anemia is undoubtedly the most common complication of Plasmodium falciparum infection1,2 and is particularly severe in children and pregnant women living in endemic areas. Anemia is caused partly by the loss of red blood cells, both infected erythrocytes (IEs) and uninfected erythrocytes (UEs), which are destroyed by hemolysis or phagocytosis.3 Rigidification of the membranes of IEs and UEs during infection may be an important factor in the destruction of these cells during passage through the spleen.4,5 There seem to be many causes of anemia, and the underlying mechanisms remain elusive.
Our recent studies on ring surface protein 2 (RSP2) suggest that this rhoptry molecule occasionally binds to the surface of UEs.6 RSP2 has been identified as the highly conserved Rap2 gene found in all P falciparum isolates examined to date7 (Sterkers Y., L. C., da Rocha M., Scheidig C., Lepolard C., Cowman A., G. J., and Scherf A., manuscript in preparation). Experimental evidence indicates that merozoites transfer RSP2 to the surfaces of erythrocytes, probably during aborted invasions,6 suggesting a possible link to anemia in malaria patients.
In this work, we used anti-RSP2 monoclonal antibodies and sera from P falciparum–infected anemia patients to investigate the role of RSP2 in the destruction of red blood cells. By modeling in vitro and ex vivo observations, we show that anti-RSP2 antibodies specifically react with cells of the erythroid lineage tagged with RSP2. We present experimental evidence that the antibody response against RSP2 can induce phagocytosis and complement binding to RSP2-tagged erythrocytes, leading to the destruction of otherwise normal erythrocytes. Finally, we show that merozoites can also label leukemic erythroblasts in vitro and erythroid precursors in the bone marrow of malaria patients with RSP2, possibly leading to erythropoietic dysfunction. Thus, we describe here a novel molecular mechanism of host-parasite interaction that may have severe consequences in malaria patients.
Materials and methods
Parasites and cells
P falciparum was cultured8 and synchronized9 by standard procedures. The parasites used in this study were FCR3CSA, FCR3CD36,10,11 C1-1 var1CSA knockout, C1CSA,12 placental strain no. 193 from Cameroon,13 D10,14 D10ΔRAP1 (lacking RAP1 and RAP2 on its surface),15 W2mef,16 and W2mefΔRAP3 (lacking only RAP3)17 (a gift from A. F. Cowman, The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia).
The erythroleukemic lines (erythroblasts) HEL 92.1.7 (ATCC, Rockville, MD)18 and KMOE-2 (DMSZ, Heidelberg, Germany)19 were maintained in culture, as recommended, and cocultured with parasites: 5 × 105 cells were cultured with 6 × 107 IEs at the trophozoite stage or 1.5 × 109 O+ UEs in RPMI 1640 medium (Sigma, St Quentin Fallvier, France)13 supplemented with 1 mM sodium pyruvate, 2.5 g/L glucose, 10% fetal bovine serum, at 37°C in a humidified atmosphere containing 5% CO2. Cells were isolated after reinvasion, by Ficoll 1.077 centrifugation (Eurobio, Les Ulis, France).
Peripheral blood mononuclear cells (PBMCs) from naive volunteers were isolated on Ficoll 1.077 and resuspended in monocyte medium20 at a density of 1 × 106 cells/mL. They were cultured in a glass CultureSlide Chamber (Becton Dickinson Falcon, Franklin Lakes, NJ) for 1 hour at 37°C. Nonadherent cells were eliminated by repeated washing.
Human serum samples
Serum samples were collected from Brazilian P falciparum–positive patients at Porto Velho Hospital, Rondônia, who had given informed consent. This study was approved by the local ethics committees (Centro de Pesquisa 016 [CEP016] and Comissao Nacional de Etica em Pesquisa [CONEP] 10407).
Serum sample 1 consisted of a pool of sera from nonimmune French controls (Etablissement Français du Sang [EFS]), and serum sample 2 (acute phase) was obtained from a 6-year-old anemic child with 80 000 parasites/mm3 (hemoglobin concentration [Hb], 79 g/L; hematocrit [Hct], 0.28). Serum samples 3 and 4 (acute phase) were obtained from 11-year-old children with 4000 and 2000 parasites/mm3, respectively, who developed severe anemia (Hb, 54 and 34 g/L; Hct, 0.14 and 0.13, respectively). Serum PIAG is a pool of sera from immune, asymptomatic African donors (provided by Dr P. Druilhe, Biomedical Parasitology, Pasteur Institute, France).21,22
Human bone marrow samples
With the authorization of ethics committees (CEP016 and CONEP 10407), we collected 1 mL bone marrow samples by sternum puncture23 from 5 P falciparum–infected adults who had given informed consent. Both sexes were represented among the study subjects, who were living in a hyperendemic region of Amazonia, close to Buritis, Rondônia, in Brazil. Bone marrow cells were collected in heparin-treated tubes and immediately washed 3 times with RPMI 1640, pH 7.2, supplemented with heparin. Mononuclear cells (MNCs) were isolated by centrifugation on Ficoll 1.077, washed, and immediately analyzed by liquid-phase immunofluorescence assay (L-IFA).
Immunofluorescence of cells of the erythroid lineage
Erythrocytes were studied by L-IFA, as previously described.6 Cocultured erythroblasts (1 × 105 cells) were labeled with 40 mg/mL DAPI (4,6-diamidino-2-phenylindole, dihydrochloride; Molecular Probes, Eugene, OR) for 45 minutes at 37°C. They were then incubated with monoclonal antibody (mAb) 26G1/B4 (B4) for 30 minutes at 4°C. The cells were washed and incubated with a F(ab′)2 goat anti–mouse immunoglobulin G (IgG) Alexa-494 (Molecular Probes), diluted 1:200, for 30 minutes at 4°C. Finally, they were incubated with fluorescein isothiocyanate (FITC)–conjugated anti–glycophorin A (anti–GA) antibody (Immunotech, Marseille, France). We used a nonimmune IgG2a isotype (Immunotech) and anti–mouse IgG and anti–glycophorin A alone as negative controls.
We visualized the transfer of RSP2 to the surfaces of erythrocytes during merozoite binding on air-dried smears of the cocultured cells prepared during invasion. Slides were incubated in 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for 45 minutes before immunostaining. Merozoites were detected after 30 minutes of incubation with a mouse anti-MSP1p19 antibody (directed against merozoite surface protein 1, obtained by Dr S. Longacre [Molecular Immunology of Parasites, Pasteur Institute, France]), diluted 1:6000.24 Secondary antibody was the F(ab′)2 goat anti–mouse IgG Alexa-494 (Molecular Probes), diluted 1:200. Smears were washed, and RSP2 was detected by incubating the smears with B4 conjugated to Alexa Fluor 488 (diluted 1:100), using a kit (A-20181; Molecular Probes). Immunofluorescence signals were detected under a Nikon E800 fluorescence microscope equipped with a DXM1200 camera linked to Lucia image acquisition and treatment software (Nikon, Tokyo, Japan).
Bone marrow cells were analyzed by L-IFA with B4 and an IgG2a-negative isotype control. RSP2 and GPA were immunodetected as described in “Flow cytometry” for the erythroleukemic cell lines. We also used a biotinylated anti-CD45 mAb (Beckman-Coulter, Paris, France), detected by incubation with (7-amino-4-methylcoumarin-3 acetic acid)–streptavidin, to identify cells of the myeloid lineage (Abcys, Burlingame, CA). Immunofluorescence was analyzed in Buritis, Brazil, with an Eclipse E400 Nikon fluorescence microscope equipped with a COOLPIX 995 Nikon camera.
Fluorescence recovery after photobleaching
RSP2 is transferred to the surface of the erythrocyte at the site of contact with the merozoite and then diffuses over the surface of the cell. This redistribution process was assessed by means of fluorescence recovery after photobleaching (FRAP). IEs and UEs from the same culture were incubated for 30 minutes at 4°C with B4 and then for 30 minutes at 4°C with the secondary F(ab′)2 goat anti–mouse IgG Alexa-488 antibody (diluted 1:200). Cells were allowed to adhere for 30 minutes at 4°C in dishes (MatTeck, Ashland, MA) coated with poly-l-lysine. Nonadherent cells were removed by washing. Photobleaching experiments were carried out at 18°C with an Axiovert 200M microscope (Zeiss, Oberkochen, Germany) driven by LSM 510 software (Zeiss). Cells were photographed with a Plan Apochromat 63 ×/NA 1.4 objective. The initial 156-μm field was reduced using an 8 × digital zoom, and a digital image was captured (512 × 512 pixels). Photobleached region 1 corresponds to a circle 29 pixels (1 μm) in diameter. Once 4 images had been acquired, the zone was photobleached for 0.46 seconds using a laser set to 488 nm at full power. We then followed the fluorescence recovery on images acquired every 19 seconds. We acquired 73 such images, which were compared to 2 regions used to measure the natural decay kinetics of the fluorescence signal during the scan. The recovery curve was normalized taking this decay into account.
Flow cytometry
For the detection of RSP2, we incubated 5 × 106 parasitized cultured erythrocytes for 30 minutes at 37°C with ethidium bromide (EtBr; 5 μg/mL) and either B46 or human immune serum (diluted 1:20) adsorbed on D10ΔRAP1 erythrocytes (3 × 107 IEs per 50 μL serum). The secondary antibody was a chicken IgY (Abcam, Cambridge, United Kingdom), anti–mouse IgG, or anti–human IgG diluted 1:200, and the tertiary antibody was a goat anti–IgY conjugated to Alexa-488 (Molecular Probes), diluted 1:200. We used IgG2a and a pool of nonimmune human sera from French donors (EFS, Marseille, France) as negative controls.
We visualized RSP2 on erythroblasts by coculturing HEL 92.1.7 or KMOE-2 (1 × 105 cells) with parasites and then incubating them with B4 for 30 minutes at 4°C. The cells were washed and incubated for 30 minutes at 4°C with goat F(ab′)2 anti–mouse IgG Alexa-488 (Molecular Probes; diluted 1:200), followed by phycoerythrin (PE)–labeled anti–glycophorin A (GA) mAb (Beckman Coulter). We used the same nonimmune sera as negative controls.
Mock-cultured erythrocytes, parasites cultured alone, and parasites cocultured with erythroleukemic cells were tested for C3 binding. Cells were incubated at 37°C for 30 minutes at 4°C with B4 and a complement-positive human serum (Sigma) diluted 1:20 and adsorbed on IEs and UEs (1 × 107 IEs or UEs per 50 μL serum). Bound complement was detected with an FITC-conjugated chicken IgY anti–human complement C3 diluted 1:400 for RSP2-positive erythrocytes and 1:200 for erythroblasts (Cedarlane, Hornby, ON, Canada). A C3-depleted serum sample (Sigma) and a human serum sample decomplemented by heating at 56°C for 30 minutes were also used as negative controls. Fluorescence was analyzed with a Coulter Epics XL flow cytometer (Coultronics, Paris, France).
In vitro phagocytosis of RSP2-positive erythrocytes
The in vitro phagocytosis test was carried out as previously described.20 A culture of IEs or UEs containing 5 × 106 cells, including 10% to 15% synchronized ring stages, was washed and incubated for 30 minutes at 37°C with B4 (IgG2a), mAb 26G1/D10 (D10) (IgG3), or human immune serum (diluted 1:20) adsorbed twice on D10ΔRAP1 or on D10 parasites (1 × 107 IEs per 50 μL serum). Erythrocytes were then dispensed into the culture wells containing adherent monocytes. Phagocytosis was allowed to occur for 60 minutes at 37°C in a humid atmosphere containing 5% CO2. Slides were washed, rapidly dried, and stained with Giemsa. Nonimmune IgG2a, IgG3, and a pool of French sera were used as negative controls. The phagocytosis index was calculated by determining the number of ring-stage infected erythrocytes (rIEs) and UEs undergoing phagocytosis per 100 cells.
Results
Transfer of rhoptry protein RSP2 to the surfaces of erythrocytes
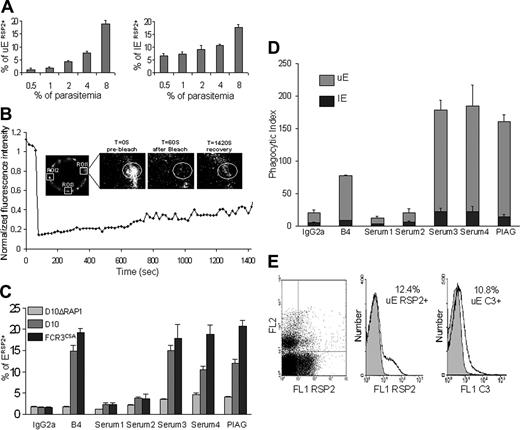
We report here that the frequency of RSP2-tagged erythrocytes increases with parasitemia. We used mouse anti-RSP2 monoclonal antibody (mAb B4)6 to analyze the RSP2-tagged UEs in populations of known parasitemia by flow cytometry. Late-stage parasites in cells with parasitemia of 0.5% to 8% were allowed to egress and then to reinvade fresh cells. Using ethidium bromide to differentiate between UEs and IEs, we found that for parasitemia levels of 0.5% to 4% (initial mature blood stage), the percentage of RSP2-tagged UEs increased in proportion with parasite load. At mature parasite levels of 8%, flow cytometry detected up to 20% RSP2-tagged erythrocytes, and liquid-phase immunofluorescence assay (L-IFA) detected up to 30% RSP2-tagged erythrocytes. Because RSP2 is transferred only if invasion is delayed or aborted,6 the number of RSP2-tagged rIEs depends on parasite load (Figure 1A) but is always considerably lower than the number of RSP2-tagged UEs. For example, at a parasitemia level of 10%, 20% of rIE are RSP2+, but this accounts for only 2% of the total erythrocyte population. L-IFA confirmed that RSP2 was transferred to a lower percentage of rIEs than UEs (data not shown).
Plasmodium merozoites contain specialized secretory organelles, rhoptries, which discharge their contents during the invasion of erythrocytes by merozoites. Discharge is usually confined to the area of merozoite contact and the parasitophorous vacuole. However, the rhoptry protein RSP2 is distributed over the entire erythrocyte surface. We analyzed the membrane mobility of RSP2 by fluorescence recovery after photobleaching (FRAP). We measured the fluorescence recovery rate of immunostained RSP2 after photobleaching a region 1 μm in diameter on RSP2-positive IEs and UEs. Two equivalent unbleached areas were used as controls to measure the natural decay kinetics of the fluorescence signal during imaging. The recovery curve was normalized taking this decay into account. FRAP analysis showed that RSP2 was transferred to the surface of the cell around the site of contact with the merozoite. It then gradually spread over the entire surface of the cell by slow, lateral movements, in IEs and UEs. Fluorescence never recovered its initial intensity (Figure 1B), consistent with the nonreplacement RSP2 model.6
Anti-RSP2 antibodies induce phagocytosis of RSP2-tagged UEs and complement activation. (A) Flow cytometry analysis showing that the percentage of RSP2-tagged erythrocytes labeled with mAb B4 depends on parasite load. Dilutions of 0.5% to 8% trophozoites were used and, after reinvasion, the percentages of RSP2-tagged UEs and RSP2-tagged IEs (±SD) were determined by staining with ethidium bromide. (B) Mobility of RSP2 on the surface of a UE. The close-up shows the region of interest (ROI 1) before, just after, and 1420 seconds after photobleaching. The graph shows the kinetics of fluorescence recovery (FRAP) (ROI 1) after correction for its natural decrease over time (ROI 2 and ROI 3). (C) Flow cytometry revealing RSP2-tagged erythrocytes in D10ΔRAP1, D10, and FCR3CSA culture, using the mAb B4, the sera from anemic patients 2, 3, and 4 and the pool of sera from immune asymptomatic African donors (PIAG). An IgG2a mAb and serum 1 were used as negative controls. (D) Comparison of the opsonization capacity of B4, the sera used in panel A and the negative controls. The phagocytosis index is the number of IEs and UEs undergoing phagocytosis per 100 monocytes counted (± SD). (E) Cultured FCR3CD36 labeled with ethidium bromide (FL2) and with mAb B4 (FL1 RSP2) or with a human anti-C3 antibody by incubation with mAb B4 and C3+ human serum (FL1 C3). IgG2a was used as an isotypic control (gray) for RSP2, and C3 immunostaining was used to assess the specific association of C3 with cytophilic antibodies such as mAb B4 (IgG2a).
Anti-RSP2 antibodies induce phagocytosis of RSP2-tagged UEs and complement activation. (A) Flow cytometry analysis showing that the percentage of RSP2-tagged erythrocytes labeled with mAb B4 depends on parasite load. Dilutions of 0.5% to 8% trophozoites were used and, after reinvasion, the percentages of RSP2-tagged UEs and RSP2-tagged IEs (±SD) were determined by staining with ethidium bromide. (B) Mobility of RSP2 on the surface of a UE. The close-up shows the region of interest (ROI 1) before, just after, and 1420 seconds after photobleaching. The graph shows the kinetics of fluorescence recovery (FRAP) (ROI 1) after correction for its natural decrease over time (ROI 2 and ROI 3). (C) Flow cytometry revealing RSP2-tagged erythrocytes in D10ΔRAP1, D10, and FCR3CSA culture, using the mAb B4, the sera from anemic patients 2, 3, and 4 and the pool of sera from immune asymptomatic African donors (PIAG). An IgG2a mAb and serum 1 were used as negative controls. (D) Comparison of the opsonization capacity of B4, the sera used in panel A and the negative controls. The phagocytosis index is the number of IEs and UEs undergoing phagocytosis per 100 monocytes counted (± SD). (E) Cultured FCR3CD36 labeled with ethidium bromide (FL2) and with mAb B4 (FL1 RSP2) or with a human anti-C3 antibody by incubation with mAb B4 and C3+ human serum (FL1 C3). IgG2a was used as an isotypic control (gray) for RSP2, and C3 immunostaining was used to assess the specific association of C3 with cytophilic antibodies such as mAb B4 (IgG2a).
Anti-RSP2 antibodies from malaria patients react with RSP2-tagged erythrocytes
We analyzed the prevalence of antibodies against RSP2 in patients with severe anemia, using (acute phase) serum samples (serum samples 3 and 4) from two 11-year-old P falciparum–infected Brazilian children with 4000 and 2000 parasites/mm3, respectively, who developed severe anemia (Hb, 5.4 and 3.4 g/dL; Hct, 14% and 13%, respectively) and a pool of sera from nonanemic, asymptomatic, anti–falciparum immune African donors (PIAG).21 Serum 1 consisted of a pool of nonimmune French sera, and serum 2 (acute phase) was obtained from a 6-year-old anemic child with 80 000 parasites/mm3 (Hb, 7.9 g/dL; Hct, 28%). We first investigated the specificity of the serum RSP2 antibody for the detection of anti-RSP2 antibodies in the sera of infected patients incubated with cultured P falciparum IEs, by flow cytometry and L-IFA. We checked that immunolabeling was specific for RSP2 by adsorbing each human serum sample on D10ΔRAP1 IEs.
With an initial parasitemia level of 0.5% to 4%, mAb B4 labeled less than 10% of erythrocytes, and at an initial parasitemia level of 8%, mAb B4 labeled up to 20% of erythrocytes in an FCR3CSA culture.10,11 This corresponds to a 5-fold invasion rate for a 4% parasitemia level, whereas at a 8% level the invasion rate was only 3-fold (Figure 1C). Similar results were obtained with 2 other laboratory strains, D1014 and FCR3CD36 (data not shown).10,11 We used the pool of human sera from immune, asymptomatic African donors (PIAG)21 with the same population of FCR3CSA parasites and found that 20.8% of erythrocytes were RSP2-positive (2.52% IEs and 18.26% UEs). Sera 3 and 4 (Figure 1C) recognized 18.03% (2.45% IEs and 15.58% UEs) and 18.9% (2.52% IEs and 16.65% UEs) of erythrocytes, respectively, as RSP2+.
Conversely, serum 2 recognized only 3.66% of erythrocytes as RSP2+ (0.70% IEs and 2.96% UEs). Few erythrocytes (less than 3%) were labeled with the negative control IgG2a isotype (0.42% IEs and 1.19% UEs) and the pool of French sera thought to be unexposed (0.6% IEs and 1.63% UEs).
We investigated the specificity of the serum RSP2 antibody by flow cytometry and L-IFA on cultured D10ΔRAP1 cells (with a disrupted RAP1), which do not export RSP215 to the cell surface. Sera from the Brazilian patients, like the pool of sera from African donors and mAb B4, did not recognize RSP2 on the erythrocyte surface on cultured D10ΔRAP1 cells (Figure 1C). This indicates that RSP2, and possibly proteins forming complexes with RSP2, are the most prominent targets at the surface of UEs in the sera of malaria patients.
Phagocytosis and complement tagging of RSP2+ erythrocytes in the presence of anti-RSP2 antibodies
We further investigated whether cytophilic anti-RSP2 antibodies promoted the phagocytosis of RSP2-tagged erythrocytes and activated complement. We incubated RSP2-tagged erythrocytes with adherent human monocytes in the presence of anti–RSP2 mAb or human sera previously adsorbed with a culture of D10ΔRAP1 as a negative control. mAb D10, an antibody6 of the IgG3 isotype, did not induce phagocytosis (data not shown). However, mAb B4 (IgG2a) triggered the phagocytosis of RSP2-tagged erythrocytes with a phagocytosis index of 77.4% (Figure 1D). Sera 3 and 4 had phagocytosis indices of approximately 178.5% and 184.5%, respectively (Figure 1D). The pool of sera from French persons presumed to be unexposed and of serum 2 gave low background levels of phagocytosis (less than 2%). Conversely, PIAG gave a phagocytosis index of 160.4%. No such phagocytosis was observed in the presence of a non–cytophilic IgG3 (mAb D10) or if the human sera were adsorbed with the parental D10 parasite line.
Because UEs are more abundant than IEs, 90% of the erythrocytes undergoing phagocytosis were RSP2-tagged UEs (Figure 1D). Sera from patients with anti-RSP2 antibodies labeled RSP2-tagged erythrocytes, triggering the phagocytosis of these cells by adherent human monocytes.
We also investigated whether cytophilic anti-RSP2 antibodies could activate the classical complement pathway, which might account for hemolysis during anemia. We performed 2 parallel immunofluorescence experiments to correlate complement C3 binding with the number of RSP2+ cells. Cells cultured with parasites either were directly labeled with mAb B4 or were incubated with mAb B4 in the presence of human serum containing complement C3, with detection by an anti-C3 antibody. In FCR3CSA culture with 12% of RSP2-tagged UEs, 10.8% of RSP2-tagged UEs were also C3+ when labeled by mAb B4 (Figure 1E). Similar results were obtained for the binding of C3 to RSP2-tagged IEs tagged by human sera 3 and 4 but not with the French negative control serum pool (data not shown). Additional negative controls, such as nonimmune IgG2a and human sera depleted of C3 or decomplemented at 56°C, displayed no C3 binding (data not shown).
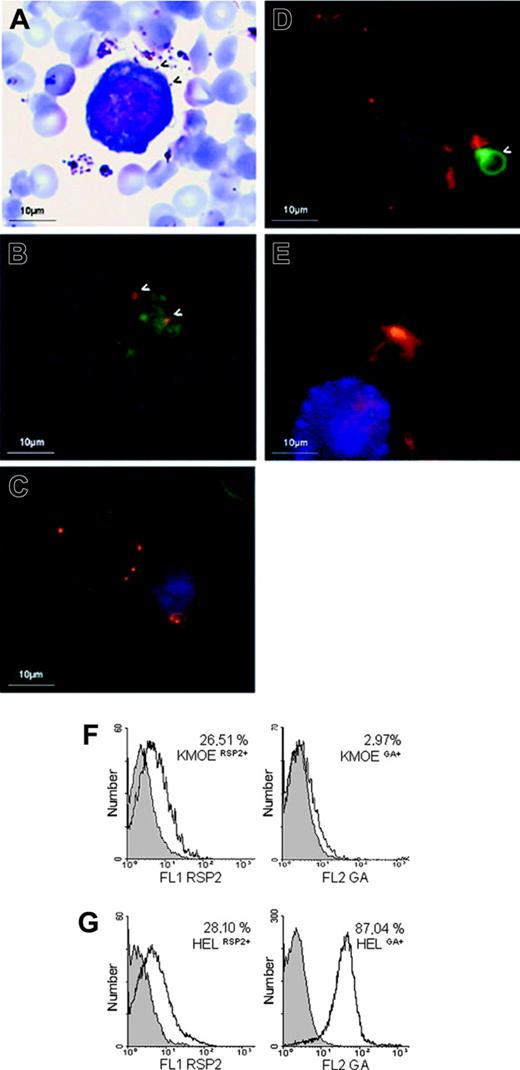
RSP2 on the surfaces of erythroid precursor cells. (A) Cocultured. erythroblasts/parasites stained with Giemsa at merozoite reinvasion, showing interaction with the surface of a HEL 92.1.7 cell. (arrows) Merozoites interacting with the erythroblast surface. (B) Double labeling (IFA) of an FCR3CSA/HEL 92.1.7 coculture at the time of reinvasion, using an anti-MSPp19 mAb (red) and B4 (green). (arrows) RSP2 transferred to the sites of interaction between merozoites and the cell. (C-D) L-IFA with the B4 (red) and anti-GA mAbs (green) of HEL 92.1.7 erythroblasts. GA+ (C) and KMOE-2 GA- (D) cells isolated from cocultures with FCR3 parasites. Arrows indicate GA+ erythrocytes. Nuclei are labeled with DAPI (blue). Independently acquired images were overlaid. (E) L-IFA with anti-RSP2 mAb B4 (red), anti–GA mAb (green), and anti-CD45 mAb (blue) of ex vivo myeloid cells. Flow cytometry quantification of RSP2 on HEL 92.1.7 and KMOE-2 erythroblasts, normalized according to GA expression (F-G). The percentage of RSP2-positive cells was determined by subtracting cells positive for the isotypic controls. We found that 26.51% of KMOE-2 GA-erythroblasts cocultured with FCR3CSA were RSP2+ (A) compared with 28.10% of HEL 92.1.7 GA+ erythroblasts (B).
RSP2 on the surfaces of erythroid precursor cells. (A) Cocultured. erythroblasts/parasites stained with Giemsa at merozoite reinvasion, showing interaction with the surface of a HEL 92.1.7 cell. (arrows) Merozoites interacting with the erythroblast surface. (B) Double labeling (IFA) of an FCR3CSA/HEL 92.1.7 coculture at the time of reinvasion, using an anti-MSPp19 mAb (red) and B4 (green). (arrows) RSP2 transferred to the sites of interaction between merozoites and the cell. (C-D) L-IFA with the B4 (red) and anti-GA mAbs (green) of HEL 92.1.7 erythroblasts. GA+ (C) and KMOE-2 GA- (D) cells isolated from cocultures with FCR3 parasites. Arrows indicate GA+ erythrocytes. Nuclei are labeled with DAPI (blue). Independently acquired images were overlaid. (E) L-IFA with anti-RSP2 mAb B4 (red), anti–GA mAb (green), and anti-CD45 mAb (blue) of ex vivo myeloid cells. Flow cytometry quantification of RSP2 on HEL 92.1.7 and KMOE-2 erythroblasts, normalized according to GA expression (F-G). The percentage of RSP2-positive cells was determined by subtracting cells positive for the isotypic controls. We found that 26.51% of KMOE-2 GA-erythroblasts cocultured with FCR3CSA were RSP2+ (A) compared with 28.10% of HEL 92.1.7 GA+ erythroblasts (B).
Transfer of RSP2 to the surfaces of erythroid cell precursors
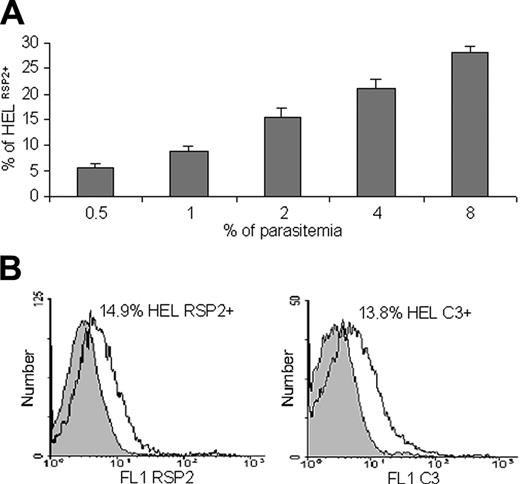
We hypothesized that RSP2 might be involved in the dysfunction of erythropoiesis observed in anemic malaria patients. We investigated RSP2 transfer in IEs with late parasite stages cocultured with 2 erythroblast lines (Figure 2A). The first erythroblast line, HEL 92.1.7, constitutively expresses glycophorin A (GA), whereas GA expression is negligible in the second, KMO-2. GA is one of the membrane proteins that acts as an erythrocyte ligand for the merozoite invasion of erythrocytes.25,26 RSP2 was transferred to the surfaces of both lines to a similar extent (Figure 2C-D), showing that GA was not associated with RSP2 transfer (Figure 2F-G) and suggesting the possible involvement of alternative pathways of merozoite invasion.26 We visualized the transfer of RSP2 to the surfaces of erythrocytes during merozoite binding (detected with a mouse anti-MSP1p19).24 RSP2 transfer was dependent on physical contact between merozoites and erythroblasts (Figure 2B), as it was for erythrocytes. RSP2 transfer to the surfaces of cells increased with the parasite load: 5.54% of cells became RSP2+ with an initial parasitemia level of 0.5% compared with 27.98% with an initial parasitemia level of 8% (Figure 3A). If merozoites and erythroblasts were prevented from coming into contact by a membrane (0.4-μm pores; Transwell-Clear, Corning Costar, Cambridge),6 RSP2 was not transferred to the erythroblast surface (data not shown).
Quantification of RSP2 erythroid precursor and binding of complement C3. (A) Flow cytometry analysis showing that the percentage of HEL 92.1.7 RSP2+ cells depends on parasite load. Results of 3 representative experiments are shown. Dilutions of 0.5% to 8% trophozoites were used, and the mean percentage (± SD) of erythroblasts cocultured with FCR3CSA that were RSP2+ was determined after reinvasion. (B) Labeling of RSP2+ HEL erythroblasts with mAb B4 (FL1 RSP2) or with a human anti-C3 antibody by incubation with mAb B4 and C3+ human serum (FL1 C3). IgG2a was used as an isotypic control (gray) for RSP2, and C3 immunostaining was used to assess the specificity of C3 binding to B4 (IgG2a).
Quantification of RSP2 erythroid precursor and binding of complement C3. (A) Flow cytometry analysis showing that the percentage of HEL 92.1.7 RSP2+ cells depends on parasite load. Results of 3 representative experiments are shown. Dilutions of 0.5% to 8% trophozoites were used, and the mean percentage (± SD) of erythroblasts cocultured with FCR3CSA that were RSP2+ was determined after reinvasion. (B) Labeling of RSP2+ HEL erythroblasts with mAb B4 (FL1 RSP2) or with a human anti-C3 antibody by incubation with mAb B4 and C3+ human serum (FL1 C3). IgG2a was used as an isotypic control (gray) for RSP2, and C3 immunostaining was used to assess the specificity of C3 binding to B4 (IgG2a).
In vivo RSP2 tagging of erythroid precursor cells in the bone marrow
We checked that RSP2 transfer was not restricted to in vitro coculture conditions by analyzing fresh bone marrow samples collected from 5 P falciparum–infected adults from a hyperendemic region of Amazonia, close to Buritis, in Brazil. In 3 samples, 1% of GA+ cells were RSP2+, whereas in the last 2 samples, 17.6% and 20% of GA+ cells were RSP2+. Parasitemia was 0.2% to 0.4% for the first 3 samples and 1% to 2% for the other 2 samples. Figure 2E shows RSP2+ erythroid precursor cells and an RSP2- myeloid cell in a P falciparum–infected human bone marrow sample. As controls, 5 bone marrow samples from uninfected French donors gave a negative result (not shown).
Complement tagging of erythroid precursor in the presence of anti-RSP2 antibodies
Host cells tagged with RSP2 may, therefore, be labeled in the presence of cytophilic antibodies, resulting in possible complement activation. We tested this hypothesis by coculturing erythroblasts with FCR3CSA parasites. Approximately 13.8% of cells were positive for C3 in the presence of B4; 14.9% of the erythroblasts were RSP2+ (Figure 3B). Similar results were obtained for FCR3CD36,10,11 C1-1 var1CSA knockout and C1CSA,12 placental strain no. 193,13 D10,14 W2mef,16 and W2mefΔRAP3,17 but not with parasites unable to tag cells with RSP2 (D10ΔRAP1)15 (data not shown). Thus, the C3 labeling of erythroblasts required tagging with a cytophilic anti-RSP2 antibody. Negative controls, such as nonimmune IgG2a, and human sera depleted of C3 or decomplemented at 56°C displayed no C3 binding (data not shown).
Discussion
Malarial anemia is a complex disease, and it is particularly severe in children and pregnant women living in endemic areas. Several recent studies have indicated that P falciparum–induced anemia is associated principally with the destruction of UEs1,2,27 and is exacerbated by defects in erythropoiesis.1,28,29 Extravascular hemolysis of IEs and UEs occurs in hyperparasitemic P falciparum infections and is thought to result from destruction by the “pitting” of parasites—macrophages in the spleen—decreasing the deformability of these cells. 2,5,29 The reasons for UE elimination remained enigmatic. Our results provide the first insight into the molecular basis of a specific host–parasite interaction that may be involved in the process triggering the destruction of erythrocytes in malaria patients.
We report here that the tagging of UEs with RSP2 increases with parasitemia. At 8% parasitemia, flow cytometry detected up to 20% and L-IFA up to 30% RSP2-tagged UEs. This difference probably reflects difficulties detecting weakly or unevenly labeled RSP2-tagged erythrocytes by flow cytometry. FRAP analysis showed how this parasite molecule gets to the erythrocyte surface. RSP2 is transferred to the surface of the host cell around the site of contact with the merozoite. It then gradually spreads over the entire surface of the cell by slow, lateral movements in IEs and UEs. Although the biologic relevance of these observations remains puzzling, we speculate that the presence of a parasite protein at the surfaces of uninfected host cells profoundly affects the lifespan of these cells. Indeed, our work demonstrates that RSP2-taggged host cells have features compatible with their premature destruction.
Hosts respond to P falciparum infection by increasing the proliferation and activity of macrophages responsible for the phagocytosis of IEs and apparently normal UEs.2 This phagocytosis may occur directly through membrane modifications, or it may be mediated by opsonizing antibodies. IgG and complement C3 have been detected on erythrocytes from malaria patients with severe anemia.30-33 We demonstrate here that RSP2 has features consistent with a key role in the development of anemia. Tagging RSP2-tagged erythrocytes with a cytophilic anti-RSP2 mAb and sera from immune malaria patients triggered phagocytosis by adherent human monocytes, whereas such phagocytosis was less common in the absence of noncytophilic IgG3 (with mAb D10) or nonimmune human sera.
Anti-RSP2 antibodies reacting with RSP2 molecules from various isolates are found in most persons,34 including pregnant women,11 infected with P falciparum. The maternal compartment of the placenta contains macrophages with strong phagocytic activity, which has been correlated with the frequency of severe maternal anemia.35 However, the degree of erythrocyte destruction probably depends on the quantitative and qualitative balance between cytophilic and noncytophilic antibodies. This hypothesis is supported by previous data highlighting the critical balance between IgG subclasses in the acquisition of protection against asexual blood stages of P falciparum parasites.20
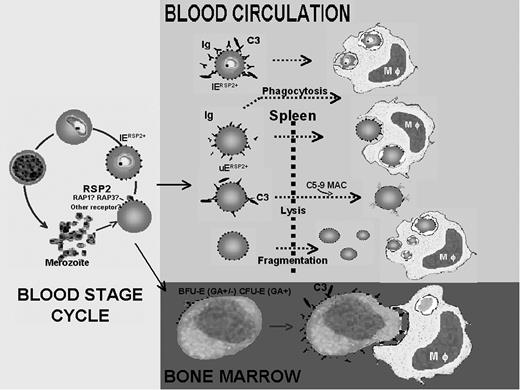
Schematic representation of the role of RSP2 in the development of severe anemia during P falciparum infection. RSP2 transferred to the surface of UEs during invasion failure or to IEs after delayed invasion is recognized by antibodies, leading to its elimination by 3 different mechanisms. RSP2+ erythrocytes tagged with opsonizing antibodies are destroyed by phagocytosis. These antibodies activate complement, which induces the lysis of RSP2+ erythrocytes. RSP2 may induce the rigidification of RSP2+ erythrocytes in the presence or absence of antibodies, resulting in their destruction in the spleen. RSP2 is involved in the dyserythropoiesis observed in severe anemia. RSP2 is transferred to the surfaces of erythroid precursors because of the presence of receptors such as GA, facilitating interaction with merozoites. From the erythroid blast-forming unit (BFU-E) or CFU-E stage after the transfer of RSP2, precursor cells may be modified by the combined action of opsonizing antibodies and complement.
Schematic representation of the role of RSP2 in the development of severe anemia during P falciparum infection. RSP2 transferred to the surface of UEs during invasion failure or to IEs after delayed invasion is recognized by antibodies, leading to its elimination by 3 different mechanisms. RSP2+ erythrocytes tagged with opsonizing antibodies are destroyed by phagocytosis. These antibodies activate complement, which induces the lysis of RSP2+ erythrocytes. RSP2 may induce the rigidification of RSP2+ erythrocytes in the presence or absence of antibodies, resulting in their destruction in the spleen. RSP2 is involved in the dyserythropoiesis observed in severe anemia. RSP2 is transferred to the surfaces of erythroid precursors because of the presence of receptors such as GA, facilitating interaction with merozoites. From the erythroid blast-forming unit (BFU-E) or CFU-E stage after the transfer of RSP2, precursor cells may be modified by the combined action of opsonizing antibodies and complement.
We found that complement C3 was present only on erythrocytes labeled with a cytophilic antibody, such as B4, suggesting a possible activation of the C5-C9 membrane attack complex and, therefore, the loss of RSP2-tagged erythrocytes by hemolysis. This possibility has been raised before, when the deposition of surface IgG and changes in the surface expression of complement regulatory protein are correlated with severe malarial anemia.31,32 However, no specific target antigen was identified that could trigger this reaction. Rigidification of the membrane of RSP2-tagged erythrocytes may lead to cell destruction, even in the absence of antibodies. This possibility is being investigated (Sterkers et al, manuscript in preparation). Another study36 recently demonstrated in vitro that the interaction of hematin with erythrocytes results in changes to the erythrocyte membrane that may contribute to the decrease in cell deformability associated with severe malaria.
Several studies1,2,29 have clearly demonstrated a slow rate of compensation of the massive erythrocyte loss in anemic subjects because of defective erythropoiesis. Acutely infected children have normal or small numbers of erythroid precursors in their bone marrow.1 Conversely, children with chronic anemia present major changes in erythroid cells, including erythroblast multinuclearity, karyorrhexis, incomplete or unequal mitotic divisions, intercytoplasmic bridges, and cytoplasmic membrane budding.1,37 The functional abnormality has not yet been defined, but the proportion of erythroid progenitors in the G2 phase is higher than that in healthy controls.1,2,29
We observed the transfer of RSP2 to erythroleukemic lines in culture conditions, but only if the cells were in direct contact with merozoites. Importantly, the investigation of bone marrow samples from P falciparum–infected patients showed that erythroid precursor cells are also tagged with RSP2 in vivo. We conclude that merozoites can cross the endothelial barrier from the sinus, where mature blood stages sequester in the parenchyma during inflammation. This is consistent with the findings of other studies reporting the presence of ingested merozoites in banded neutrophils and occasionally of mature blood stages in the parenchyma of P falciparum–infected bone marrow.29 In any case, RSP2+ cells would be labeled in the presence of cytophilic antibodies, resulting in possible complement activation. This may account for the decrease in the number of erythroid colony-forming units (CFU-Es) and other stages of the erythroid cell lineage. We hypothesize that RSP2–antibody complement complexes on the surfaces of erythroblasts are involved in the decline in precursor cells numbers because of phagocytosis or in the morphologic alterations observed in erythroid cells in bone marrow. It has been suggested that, in some conditions, activated bone marrow macrophages may damage precursor cells and destroy them by phagocytosis.29 Under our experimental conditions, we observed the appearance of multinuclearity in RSP2+ erythroblasts (HEL 92.1.7) incubated with adherent human monocytes, but we observed no phagocytosis, even after 16 hours of coculture.
Based on our in vitro and ex vivo observations, we propose a model illustrating a possible role of RSP2 in anemia (Figure 4). This model does not take into account the possibility that the balance in numbers of cytophilic and nonopsonizing antibodies may determine the development of severe anemia, as previously described in the development of a protective anti–P falciparum immune response.20 Other rhoptry antigens that form a complex with RSP2/RAP2 may also be involved in the development of severe anemia.
Previous studies have shown that anti-RSP2 antibodies effectively inhibit invasion6,7 in vitro and confer partial protection against P falciparum infection in Saimiri monkeys.38 However, our results suggest that caution is required concerning the inclusion of RSP2/RAP2 in vaccines because the resultant immune response might worsen anemia.
In conclusion, we have identified a novel molecular mechanism of host–parasite interaction that may set the conditions for the destruction of normal erythrocytes in malaria patients. This provides new insight into a possible mechanism of malarial anemia. Further epidemiologic studies are now required to determine the prevalence of RSP2-tagged cells of the erythroid lineage in anemic and nonanemic patients and to identify the critical regions of RSP2/RAP2 involved in the insertion into the membrane of erythroid cells. The knowledge obtained in these studies suggests possible new intervention strategies for severe anemia.
Prepublished online as Blood First Edition Paper, July 26, 2005; DOI 10.1182/blood-2005-04-1574.
Supported by grants from program Biology and Pathology of the Malaria Parasite network (BIOMALPAR), a Sixth Framework Programme (FP6)–funded network of excellence; Direction Générale pour l'Armement/Projet d'Etude en Amont (DGA/PEA) no. 980814 from Paludisme (PAL) +2000 of the Ministere de l'Education National de la Recherche et de la Technologie (MENRT); Action Concertee Iniciative (ACI) program, Institut National de la Santé et de la Recherche Médicale (INSERM) MIC no. 0318; Malaria Antigen Discovery Program Malaria in Pregnancy Initiative; and no. 29202 from the Melinda and Bill Gates Foundation. Layez Corinne is a PhD fellow supported by MENRT grant 10731-2003.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr R. Stuart and Dr A. Scherf for critical reading of the manuscript; Prof A.F. Cowman for D10ΔRAP1, W2mef, and W2mefΔRAP3 parasites; Dr S. Longacre-Andre for the anti-MSP1 mAb p19; Dr P. Druilhe for the serum PIAG; and P. Roux for technical help with FRAP done in Plate-Forme Imagerie Dynamique (PFID) Institut Pasteur.