Abstract
Background: TT2 introduced T into the frontline therapy for MM in a randomized phase III trial design (ASCO 2005). In comparison to TT1, TT2 applied: (1) more intensive chemotherapy for induction prior to and introduced CCT after melphalan 200mg/sqm-based tandem autotransplants, designed to improve survival in high-risk patients with CA; (2) DEX pulsing was added during the 1st year of interferon (IFN) maintenance therapy. We now report on the outcome of patients treated on the “no thalidomide” arm of TT2 (TT2-) in comparison to TT1, in order to evaluate the effect of dose-intensification during induction and post-transplant therapy.
Patients and Methods: 231 patients were enrolled in TT1 (median follow-up, 11yr) and 345 in TT2- (median follow-up, 3.5yr). Completion rates of 1st/2nd transplants were 195/165 (84%/71%) with TT1 and 292/235 (85%/68%) with TT2-. In TT2-, 64% started CCT and 36% received DEX consolidation (when platelets failed to recover or no benefit was documented from induction DCEP). TT2- and TT1 were compared in terms of pre-transplant-1 and final CR rates (intent-to treat), EFS/OS from treatment start, 1st transplant and from last (2nd or 1st) transplant. EFS and OS were examined in the context of baseline prognostic variables including CA.
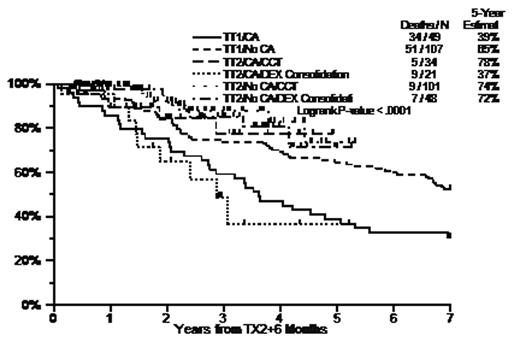
Results: Compared to TT1, TT2- induced similar pre-Tx1 CR rates (11% vs 12%, p=0.8) and final CR rates (41.3% vs 40.7%, p=0.9). The median onset of CR was 8.9mo for TT2 vs 8.4mo for TT1 (p=0.4). 5-year EFS/OS were 45%/63% with TT2- vs 28%/57% with TT1 (p<0.001/p=0.06). 4-yr post-transplant-1 EFS/OS were 48%/65% with TT2- vs 28%/56% with TT1 (p<0.001/p=0.02); 4-yr post-transplant-2 EFS/OS were 46%/64% with TT2- and 31%/50% with TT1 (both p=0.01). Pre-study CA, high LDH (>=190U/L), and low Hb (<10g/dL) were independently, significantly associated with poorer post-transplant-2 EFS and OS (p<0.05); independent of these factors, TT2- improved post-transplant-2 EFS and OS compared to TT1 (p<0.001/p=0.033). In the presence of CA, TT2- with CCT vs TT2- with DEX improved 4-yr OS (measured from a 6-mo landmark post-transplant-2) from 37% (similar to 39% with TT1 [no DEX or CCT]) to 78%, which is comparable to the 65% for TT1 without CA.
Conclusion: In this historical comparison of TT1 with TT2-, the more intensive induction chemotherapy with TT2- did not improve CR. However, post-transplant CCT benefited the 1/3 of patients with CA, doubling 4-yr post-transplant OS in comparison with TT1 in this high-risk subgroup. A phase 3 randomized trial addressing the CCT concept is warranted.
Author notes
Corresponding author