Abstract
Background: The presence of circulating myeloma cells (CMC) detected by flow cytometry at the time of diagnosis of multiple myeloma is associated with a shortened response to therapy and reduced overall survival (OS). We hypothesized that the presence of CMC at the time of stem cell collection prior to high dose therapy (HDT) and autologous stem cell transplantation (ASCT) would identifies a cohort of patients with a high risk of rapid progression.
Methods: The Mayo Clinic myeloma transplant database was queried for patients who were mobilized using cyclophosphamide and hematopoietic growth factors. CMC was determined using flow cytometry by gating on a population of CD38 bright and CD45 negative cells. The impact of CMC on OS and time to progression (TTP) and its role in the context of established prognostic parameters was evaluated.
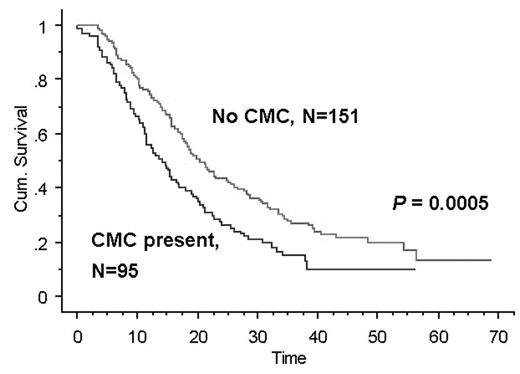
Results: Of 246 patients with MM undergoing ASCT, 95 had CMC. Patients with CMC had significantly higher plasma cell labeling index, adverse cytogenetics, B2-M and resistant disease. Complete response (CR) rates post transplant were 32% and 36% for patients with and without CMC (p=0.5034). OS was 33.2 and 58.6 months (p=0.0052) while TTP was 14.1 and 22 months respectively (p=0.0005).
On multivariate analysis, CMC remained an independent prognostic factor in a model that included cytogenetics and disease status at time of transplant (p=0.0314). A prognostic system based on the presence or absence of CMC and karyotype abnormalities was developed. Patients with neither, one or both parameters had a median, OS of 55, 48 and 21.5 months respectively (p<0.0001) while TTP was 22, 15.4 and 6.5 months for the same groups (p<0.0001).
Conclusion: The presence of CMC at the time of HDT and ASCT is an independent prognostic factor. The combination of CMC and cytogenetics provides a simple yet powerful scoring system that stratifies patients and guides their management.