Abstract
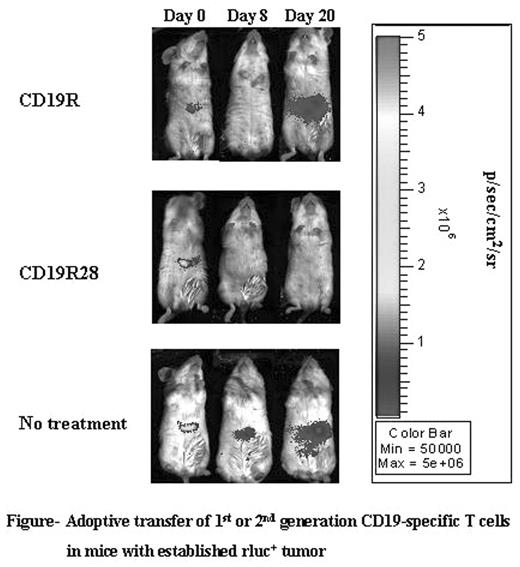
Adoptive transfer of T cells expressing chimeric immunoreceptors into cancer patients holds promise for the eradication of tumor cells. Chimeric immunoreceptors can redirect T cells to clinically important targets such as cancer cells or virally infected cells. Current adoptive immunotherapy trials with genetically modified T cells use chimeric antigen receptors (CAR’s) that connect an extracellular antigen-recognizing domain to a single intracellular signal transduction domain such as CD3-ζ. These CAR’s stimulate genetically modified T cells for lysis and cytokine production, but typically do not provide a fully-competent activation signal. Thus, when we expressed a 1st-generation CD19-specific chimeric receptor (CD19R), the genetically modified T cells demonstrated (i) antigen-specific binding, (ii) target-cell lysis in vitro and (iii) anti-tumor efficacy in vivo, but failed to produce IL-2 upon antigen recognition and exhibited a short survival (less than 10 days) in vivo. Fully-competent activation of T cells requires a second signal, typically provided by the simultaneous engagement of co-stimulatory receptors such as CD28 along with the T-cell resceptor. Since many B-lineage tumors lack or down-regulate the co-stimulatory ligands for CD28 (CD80, CD86), we designed a 2nd -generation CD19-specific immunoreceptor, designated CD19RCD28, that includes the intracellular signaling domains of CD28 and CD3-ζ. We then compared the in vitro and in vivo immunobiology of CD19-specific T cells expressing these 1st or 2nd generation CAR’s. When tested by chromium release assays both CD19R+ and CD19RCD28+ T cells were able to specifically lyse CD19+ tumor cells at a comparable efficiency. However, CD19RCD28+ T cells were able to produce IL-2 and up-regulate the anti-apoptotic protein Bcl-XL upon stimulation with CD19+ tumor cells unlike the CD19R+ T cells. Moreover, CD19RCD28+ T cells can be efficiently propagated ex vivo via the CD19-specific CAR over sequential stimulation cycles when cultured with CD19+ Epstein-Barr virus (EBV) transformed B-lymphoblastic cells. Using a biophotonic imaging system and T cells co-expressing the firefly luciferase (ffLuc) reporter gene we determined that 2nd-generation CD8+ CD19RCD28+ffLuc+ T cells persisted significantly longer in NOD/scid mice (in the absence of exogenous rhIL-2) compared with 1st-generation CD19R+ffLuc+ T cells, as measured by longitudinal bioluminescence imaging (BLI). Since these mice were engrafted with Renilla luciferase (rLuc)+ CD19+ tumor, we were able to directly correlate the presence of T cells with an anti-tumor effect, as measured by BLI of rLuc activity (Figure). Furthermore, the continued persistence of the CD19RCD28+ T cells was dependent on the presence of antigen, as adoptive transfer of CD19RCD28+ T cells could not be detected beyond 5 days by BLI in mice without tumor. Taken together, these findings demonstrate that incorporation of chimeric CD28 and CD3-ζ signaling domains in CAR’s provides a tool to increase T-cell persistence leading to improved anti-tumor efficacy.
Adoptive transfer of 1st or 2nd generation CD19-specific T cells in mice with established rluc+ tumor
Adoptive transfer of 1st or 2nd generation CD19-specific T cells in mice with established rluc+ tumor
Author notes
Corresponding author