Abstract
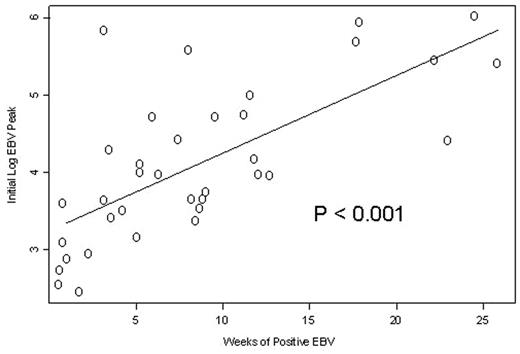
Although reactivation of Cytomegalovirus (CMV) and Epstein-Barr virus (EBV) during periods of immune suppression following solid organ and allogeneic hematopoietic stem cell transplantation (HSCT) can cause fatal complications, the natural history of viral reactivation and the potential for serious complications following antibody-based immunosuppressive (I/S) treatments for bone marrow failure syndromes is not defined. We monitored blood for EBV and CMV reactivation by polymerase chain reaction (PCR) weekly in 39 consecutive patients (total of 44 I/S courses) with aplastic anemia (AA). During the observation period of 3–6 months per patient, none developed clinical features of EBV or CMV reactivation, nor received antiviral therapy. Four regimens were studied: (1) HC: horse ATG + cyclosporine (CsA) (18 pts); (2) HCS: horse ATG + CsA + sirolimus (15 pts); (3) RC: rabbit ATG (Thymoglobulin®) + CsA (5 pts); and (4) CP: alemtuzumab (Campath®) (6 pts). Overall EBV reactivation occurred in 32/39 (82%) seropositive patients at a median of 14 days in the HC arm, 11 days in the HCS arm, 15 days in the RC arm and 6 days in the CP arm from starting I/S. The median peak EBV copies for HC recipients was 4,340 (0–54,250) copies/106 mononuclear cells (MNCs); for HCS 1,420 (0–466,000) copies/106 MNCs; for RC 370,000 (97,000–1,025,000) copies/106 MNCs, and for CP 965 copies/106 MNCs (0–655,000) (P= 0.026). The median duration of PCR positivity for EBV was higher in the RC group (18 weeks) compared to HC (6 weeks), HCS (4 weeks) and CP (1 week) (P=0.002). Higher peak values were associated with a longer duration of viremia following initial I/S (P<0.001)(Figure). CMV reactivation occurred only in 12/29 (41%) seropositive patients, 10 CMV seronegative patients showed no reactivation by PCR. The EBV reactivations in the alemtuzumab arm were less frequent and short lived, presumably because the antibody eliminates the B cell population which is the source for reactivating virus. A more prolonged lymphocyte depletion was seen with RC and CP treated patients when compared to HC and HCS. Our results indicate that subclinical reactivation of both EBV and CMV is common in patients with AA receiving I/S and that different regimens are associated with different intensity of I/S as measured by viral load and lymphocyte count. Our data suggests that higher and longer EBV reactivations are seen following rabbit ATG compared to horse ATG or alemtuzumab, and for the whole cohort, the duration of positivity for EBV PCR correlated to the initial degree of EBV reactivation. While all reactivations were subclinical, our findings suggest that viral reactivation should be monitored when developing new and more potent I/S regimens for treatment of hematologic and non-hematologic disorders as the threshold to the development of CMV or EBV disease is not well understood.
Author notes
Corresponding author