Abstract
We previously showed that costimulated Th2/Tc2 cells abrogate graft rejection. In recent experiments, we found that ex vivo rapamycin generates Th2 cells (Th2R cells) that more potently prevent GVHD relative to control Th2 cells. We thus hypothesized that rapamycin may improve the ability of Th2/Tc2 cells to abrogate graft rejection. To test this hypothesis, we utilized a recently defined model of rejection involving lethal host irradiation and subsequent quantitative host T cell addback [B6(H-2b) into BALB/c(H-2d), TBI:10.5 Gy; 0.1x10^6 host T cell addback]. To generate stem-cell enriched allografts, donor mice were treated with G-CSF (5 microgram/d for 5 d) and resultant spleen cells were expanded for 6 d in rhTPO, rmSCF and rhFLT3L: relative to input cells, the final product was T cell depleted (%CD3 reduced from 9.7±0.6% to 0.4±0.03%) and stem-cell enriched by KLS analysis (%c-kit+Lin-Sca-1+ increased from 5.7±1.8% to 49.08±2.8%) and by functional analysis (%side population increased from 0.24±0.04% to 1.7±0.2). In an initial experiment that did not involve host T cell addback, we determined that 10x10^6 of the HSC product was radioprotective in 100% of recipients (10/10 subjects, 90 d follow-up) and yielded 100% donor chimerism without clinical GVHD. To generate donor Th2/Tc2 and Th2/Tc2R cells, donor T cells were costimulated with anti-CD3/CD28 coated beads and expanded in media supplemented with rmIL-4, rhIL-2, rhIL-7 either without or with high dose rapamycin (10 micromolar). Host-vs-graft (HVG) responses were quantified at d 5 post transplant by the following method: (a) spleen cell harvest and enumeration; (b) 24 h host (syngeneic) or donor (allogeneic) dendritic cell stimulation; (c) cell-surface flow cytometry with anti-CD4, anti-CD8 and anti-host (H-2d) antibodies; (d) Miltenyi IFN-gamma cytokine capture flow cytometry; and (e) calculation of absolute number of host anti-donor alloreactive CD4+ and CD8+ T cells per spleen. HSC transfer without Th2/Tc2 cells induced robust CD4- and CD8-mediated HVG responses (Fig.1, cohort 2>cohort 1; p<0.001). HSC transfer augmented with donor Th2/Tc2R cells fully abrogated HVG responses; in marked contrast, HSC transfer augmented with control Th2/Tc2 cells only partially reduced HVG responses. Rapamycin generated Th2/Tc2 cells were more potent than control Th2/Tc2 cells with respect to abrogation of both CD4- and CD8-mediated HVG responses (p<0.05). The mechanism of Th2/Tc2R cells abrogation of HVG responses involved both CD4+Th2R and CD8+Tc2R cell components, and did not require IL-4, perforin, or fas ligand. At day 90 post transplant, Th2/Tc2R cell recipients had nominal GVHD (<5% weight loss) and consistent alloengraftment (>99% chimerism, 9 of 10 recipients; rejection, 1 of 10 recipients); in marked contrast, control Th2/Tc2 cell recipients had either graft rejection (9 of 10 recipients) or mixed chimerism (1 of 10 recipients). In conclusion, ex vivo rapamycin generates donor Th2/Tc2 cells that potently abrogate HVG responses and HSC graft rejection through a mechanism that involves CD4+Th2 and CD8+Tc2 cells and non-classical molecular effectors.
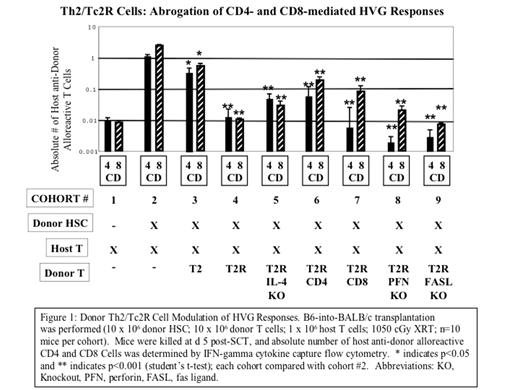
Donor Th2/Tc2R Cell Modulation of HVG Responses. B6-into-BALB/c transplantation was performed (10 x 106 donor HSC; 10 x 106 donor T cells; 1 x 106 host T cells; 1050 cGy XRT; n=10 mice per cohort). Mice were killed at d 5 post-SCT, and absolute number of host anti-donor alloreactive CD4 and CD8 Cells was determined by IFN-gamma cytokine capture flow cytometry. * indicates p<0.05 and ** indicates p<0.001 (student’s t-test); each cohort compared with cohort #2. Abbreviations: KO, Knockout, PFN, perfocin, FASL, fas ligand.
Donor Th2/Tc2R Cell Modulation of HVG Responses. B6-into-BALB/c transplantation was performed (10 x 106 donor HSC; 10 x 106 donor T cells; 1 x 106 host T cells; 1050 cGy XRT; n=10 mice per cohort). Mice were killed at d 5 post-SCT, and absolute number of host anti-donor alloreactive CD4 and CD8 Cells was determined by IFN-gamma cytokine capture flow cytometry. * indicates p<0.05 and ** indicates p<0.001 (student’s t-test); each cohort compared with cohort #2. Abbreviations: KO, Knockout, PFN, perfocin, FASL, fas ligand.
Author notes
Corresponding author