Abstract
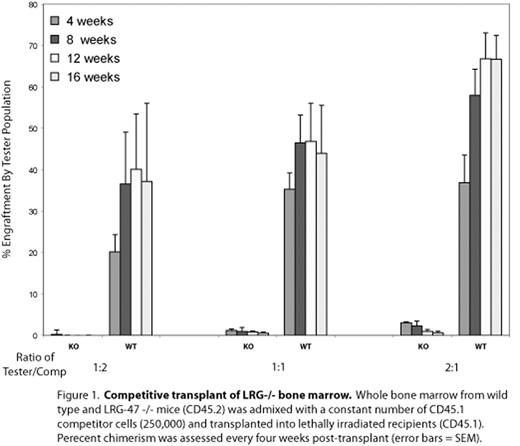
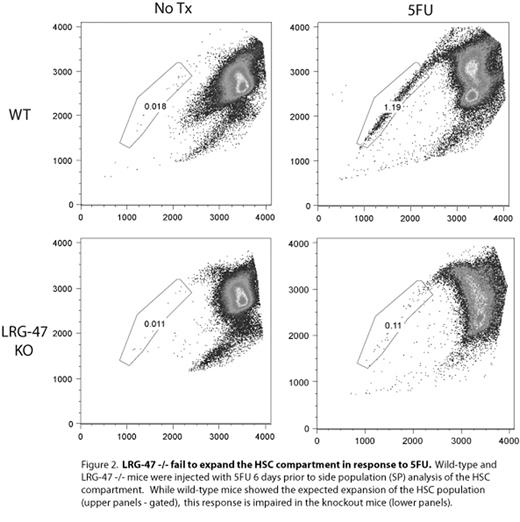
Hematopoietic stem cells (HSCs) have a remarkable capacity to respond to proliferative stimuli, as they are able to reconstitute the blood following catastrophic injuries such as chemotherapy and lethal irradiation. Most work aimed at elucidating the genetic and molecular controls on this program of activation has focused on HSCs responding to these artificial stimuli, however there is a surprising paucity of information reflecting the response of HSCs to the types of stimuli encountered in a non-laboratory setting. Here we report that LRG-47, an interferon-inducible GTPase, is required for HSCs to respond to a variety of proliferative stimuli, including mycobaterial challenge. Previously studied solely in the context of the immune response to intracellular pathogens, LRG-47 is upregulated in HSCs during 5-fluorouracil-(5FU) induced proliferation, and we now show that LRG-47 −/− HSCs exhibit profound defects. LRG-47 −/− HSCs achieve only 4–8% of wild-type engraftment activity in competitive repopulation assays (Figure 1) and, strikingly, even transplantation in 25-fold excess over wild-type competitor fails to rescue this defect. We also demonstrate that LRG-47 −/− HSCs are impaired in colony-forming ability, and that LRG-47 −/− mice exhibit both a relative and absolute failure to expand the stem cell/progenitor compartments in response to 5FU (Figure 2). Intriguingly, we also show that infectious challenge with Mycobacterium avium stimulates an expansion of the progenitor cell (LSK) compartment in wild-type mice - and that LRG-47-deficient mice are unable to mount this response. These findings implicate LRG-47 as being required for effective proliferation of HSCs in response to various stimuli. Furthermore, these results imply that expansion at the progenitor cell level is a downstream effector mechanism of the cytokine-mediated immune response to infection. Ultimately, understanding the mechanisms by which HSCs sense and respond to proliferative stimuli has far-ranging applications, and our work establishes an important connection with the immune system as a regulator of this process. Infectious processes can now arguably join ex vivo HSC manipulation, mechanisms of hematologic malignancy, and transplantation medicine as areas of importance informed by an understanding of the controls on HSC activation, proliferation and quiescence.
Competitive transplant of LRG-/- bone marrow. Whole bone marrow from wild type and LRG-47 -/- mice (CD45.2) admixed with a constant number of CD45.1 competitor cells (250,000) and transplanted into lethally irradiated recipients (CD45.1). Perecent chimerism was assessed every four weeks post-transplant (error bars = SEM).
Competitive transplant of LRG-/- bone marrow. Whole bone marrow from wild type and LRG-47 -/- mice (CD45.2) admixed with a constant number of CD45.1 competitor cells (250,000) and transplanted into lethally irradiated recipients (CD45.1). Perecent chimerism was assessed every four weeks post-transplant (error bars = SEM).
LRG-47 -/- fail to expand HSC compartment in response to SFU. Wild type and LRG-47 -/- mice were injected with SFU 6-days prior to side population (SP) analysis of HSC compartment. While wild-type mice showed the expected expansion of the HSC population (upper panels - gated), this response is impaired in the knockout mice (lower panels).
LRG-47 -/- fail to expand HSC compartment in response to SFU. Wild type and LRG-47 -/- mice were injected with SFU 6-days prior to side population (SP) analysis of HSC compartment. While wild-type mice showed the expected expansion of the HSC population (upper panels - gated), this response is impaired in the knockout mice (lower panels).
Author notes
Corresponding author