Abstract
The International Prognostic Scoring System (IPSS) provides relatively homogeneous prognoses for untreated patients with MDS in terms of survival and transformation to AML. In many cases, cytogenetic results are not available and prevent use of the IPSS. Our objective was to create and validate a dynamic prognostic model that would allow identification of high-risk MDS patients when cytogenetics are unavailable. We followed the methodological approach used by Greenberg et al (
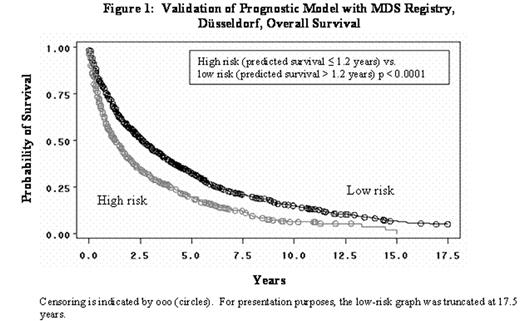
Validation of Pregnostic Model with MDS Registry, Düsselderf, Overall Survival
Validation of Pregnostic Model with MDS Registry, Düsselderf, Overall Survival
There was fair agreement between the low and high subgroups from our model with the low (low+INT-1) and high (INT-2+high) subgroups from the IPSS, Cohen’s Kappa = 0.30 (95% CI: 0.21 to 0.39) in 547 patients from the MDS Registry, Düsseldorf. A sensitivity analysis of the CALGB efficacy results was conducted by varying the ≤ 1.2 years survival criterion from our model. High-risk subgroups, defined by using predicted survival of 1.0 to 2.8 years in increments of 0.1 years, were investigated. In general, the sensitivity analyses revealed that clinically meaningful benefit was statistically demonstrated in the smaller subgroups but lost significance as the subgroups approached the full data set. The smaller subgroups were more homogeneous in predicted survival. In conclusion, our model is a valid prognostic model that bridges reasonably well to the IPSS high-risk group and identified a high-risk homogeneous subgroup of CALGB patients to further investigate the effects of azacitidine.
Author notes
Corresponding author