Abstract
Background: The doses and timing necessary for effective cytoreduction with monoclonal antibodies remain largely unknown. The human IgG1 CD52 MAb alemtuzumab is normally given intravenously (IV) three times per week at a standard dose of 30mg, following dose escalation. However, comparable results may be observed with fewer side effects using subcutaneously (SC) administered MAb, although the pharmacokinetics of SC administered MAb are slower than IV and therapy may have to be administered on a thrice weekly schedule for up to 18 weeks to achieve maximal efficacy. Efficacy with alemtuzumab has been shown to relate directly to serum levels of the MAb. We wished to assess whether more rapid cytoreduction with acceptable toxicities might be attained with daily alemtuzumab dosing without dose escalation.
Patients and methods: Patients with CLL (either as first-line therapy or at first or subsequent relapse) received SC alemtuzumab for 15 consecutive days at a dose of 30mg daily and thereafter three times per week for up to 8 weeks. To minimize adverse skin and possible systemic reactions, dexamethasone (12mg) was given along with acetaminophen (1g) for the first two injections only.
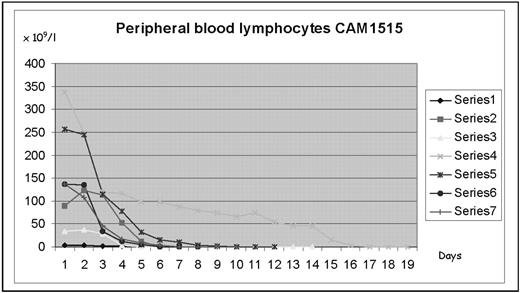
Results: 7 patients (aged 55– 73, 6 male 1 female) have been treated comprising 3 patients with Binet stage A progressive and 4 stage C. Use of steroid premedication for the first two doses virtually abolished all acute toxicities with only one patient showing any significant cutaneous reaction. CMV PCR was performed weekly. 4 patients showed low-level CMV (up to 7000 viral copies/ml) reactivation in the absence of any symptoms. All were treated successfully with oral valganciclovir 900mg bd whilst continuing with alemtuzumab without interruption. All 7 patients showed rapid reductions of peripheral blood lymphocytes as shown in the figure. Rates of drop in lymphocye count were much more rapid with daily than with conventional thrice weekly dosing schedules. Despite the more rapid reductions in lymphocytes, no patient developed tumor lysis. All patients showed significant reduction in bone marrow infiltration after the 15 days of daily therapy although a further 8 weeks of therapy was necessary to induce CR.
Conclusions; these data indicate that daily SC dosing with alemtuzumab results in rapid cytoreduction without increased adverse events. Prolonged daily dosing may result in enhanced efficacy of alemtuzumab in CLL.
Author notes
Corresponding author