Abstract
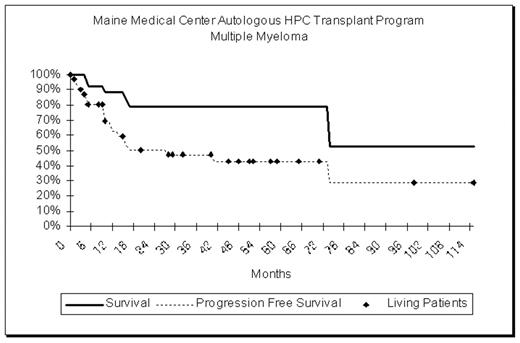
29 patients with Stage III multiple myeloma have undergone transplantation at our institution over the past 10 years. Patient selection criteria included age less than 70 years, creatinine less than 2mg/ml, no active infection, cardiac ejection fraction >40%, DLCO > 50% of predicted and no other co-morbid conditions that would jeopardize sur-vival. In addition, there was demonstrated chemo-sensitivity of the disease with at least a 50% decrease in M-component and a decrease in marrow plasmacytosis to less than 10%. After treatment to maximal effect with VAD (3–6 cycles), peripheral blood stem cells were collected. The dose of CD34 positive cells ranged from 1.8 to 17.4 x106/kg. (median 11.4 x 106/kg). 8 patients treated prior to September 2000 received high dose therapy with Melphalan 140mg/m2 and fractionated TBI, 150cGy bid for 5 doses. Patients treated after September 2000 received Melphalan 200mg/m2 without radiation. The median age at transplantation was 52 years (range 29 to 71). There were no treatment related deaths. Days to achieve AGC>500/ul ranged from 5 to 13 (average 6.8). The median length of follow up is 2.7 years. Currently 23 patients are alive, and 18 are free from progression. Overall survival at 5 years is 79%. Progression-free survival at 5 years is 43%.
Our experience suggests that carefully selected patients with chemo-responsive multiple myeloma have relatively high rates of overall and progression free survival when compared to results reported for a broader population of patients.
Author notes
Corresponding author