Abstract
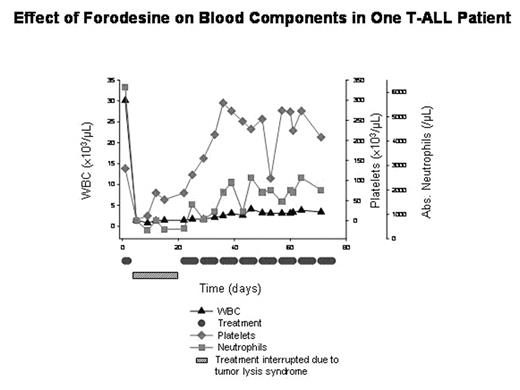
Inherited deficiency of purine nucleoside phosphorylase (PNP) is associated with a profound loss of T-cells. This rare clinical condition provides a model for developing specific inhibitors of PNP as therapy for T-cell malignancies. Forodesine is a specific PNP inhibitor that raises plasma 2′-deoxyguanosine (dGuo) and intracellular dGTP levels, leading to T-cell apoptosis. The objective of this study was to determine the efficacy and safety of forodesine in patients with relapsed or refractory T-cell leukemia. Patients received IV forodesine 40 mg/m2 5 days each week (1 cycle) for a total of 6 cycles (8 cycles if dose escalation needed after cycle 2). To date, 21 patients have been treated, 19 of which are T-ALL. Results are based on available data from 13 T-ALL patients. Median number of prior regimens was 4 (range: 1–6). Overall response rate was 38%, including 3 patients with complete response (CR) (23%) and 2 patients with partial response (PR) (15%). Three patients had stable disease and 5 patients had disease progression. Time to progression (TTP) for the 3 CR patients was 80, 119, and 160+ days. One CR patient continues to receive treatment. TTP for the 2 PR patients was 89 and 40 days. Plasma dGuo was elevated in all patients (range: 3.4 to 88.5 μM; predose levels ≤0.004 μM). Treatment was well tolerated. One serious adverse event possibly drug related was CMV pneumonitis in 1 patient. The most common adverse events possibly drug related were nausea (33%), headache (26%), anemia (20%), thrombocytopenia (20%), and weakness (20%). In 1 patient, after 2 doses of forodesine, WBC count dropped dramatically, requiring interruption of treatment for 2 weeks because of tumor lysis syndrome (Figure). After 2 cycles of treatment, the patient achieved a CR. By week 3, platelets and neutrophils recovered, indicating a specificity of forodesine for leukemic cell populations. These study results show that forodesine is active, with minimal toxicity, as a single agent in relapsed or refractory T-cell leukemia. Additional clinical efficacy and safety data will be presented.
Effect of Forodesine on Blood Components in One T-All Patient
Author notes
Corresponding author