Abstract
Granule proteins play a major role for the bactericidal activity of neutrophils. The mechanisms for sorting of proteins to granules in neutrophils remain unknown. Serglycin proteoglycan with glycosaminoglycan side chains is the major intracellular proteoglycan of hematopoietic cells. Neutrophil elastase, a resident of primary (azurophilic) granules, binds glycosaminoglycans. Serglycin has been proposed to play a major role in sorting and packaging of granule proteins in neutrophils and other cells.
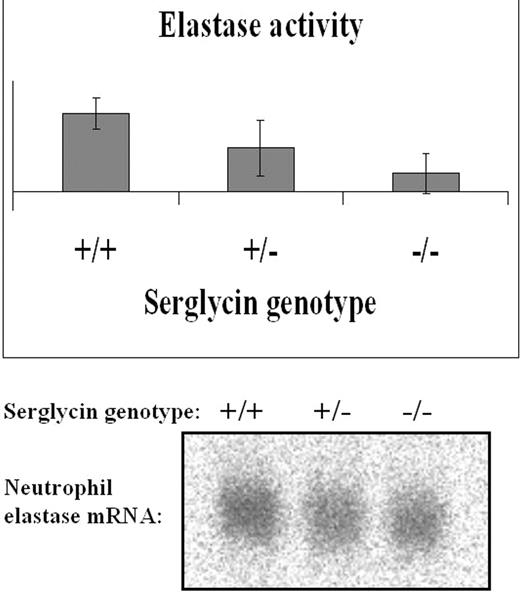
We have investigated the expression and localization of granule proteins in neutrophils from a serglycin knock out mouse. We found neutrophil elastase absent from neutrophils of serglycin knock out mice as shown by activity assay (fig. 1), Western blotting (fig. 2), and immunocytochemistry. Northern blotting (fig. 1) demonstrated the presence of neutrophil elastase mRNA both in serglycin knock out mice and in wild type mice. No other granule proteins tested (myeloperoxidase, cathepsin G, proteinase 3, bacterial permeability increasing protein (primary granules), lactoferrin, 24p3 (secondary (specific) granules), MMP-9/gelatinase B (tertiary (gelatinase) granules)) showed differences in expression or localization in neutrophils from wild type mice compared to serglycin knock out mice. Three-dimensional models of neutrophil elastase revealed a cluster of positively charged amino acids around the active site of the enzyme. This cluster may be engaged in binding the negatively charged glycosaminoglycan side chains of serglycin and thus forms the basis for serglycin mediated sorting of elastase to primary (azurophilic) granules. No counts for both bone marrow cells and peripheral blood are similar for the wild type and serglycin −/− genotypes; indicating that the lack of sorting of elastase to granules does not induce neutropenia itself. These findings disclose an important role for serglycin proteoglycan in targeting neutrophil elastase to neutrophil primary granules. This is in accordance with the recently published significance for serglycin in granule formation of mast cells. Our results also indicate that different mechanisms are responsible for sorting of different granule proteins to the same neutrophil granule subset, as none of the other tested granule proteins showed differences in localization between wild type and serglycin knock out neutrophils.
Author notes
Corresponding author