Retroviruses can induce hematopoietic disease via insertional mutagenesis of cancer genes and provide valuable molecular tags for cancer gene discovery. Here we show that insertional mutagenesis can also identify genes that promote the immortalization of hematopoietic cells, which normally have only limited self-renewal. Transduction of mouse bone marrow cells with replication-incompetent murine stem cell virus (MSCV) expressing only neo, followed by serial passage in liquid culture containing stem cell factor (SCF) and interleukin-3 (IL-3), produced immortalized immature myeloid cell lines with neutrophil and macrophage differentiation potential in about 50% of the infected cultures. More than half of the lines have MSCV insertions at Evi1 or Prdm16. These loci encode transcription factor homologs and are validated human myeloid leukemia genes. Integrations are located in intron 1 or 2, where they promote expression of truncated proteins lacking the PRDI-BF1-RIZ1 homologous (PR) domain, similar to what is observed in human leukemias with EVI1 or PRDM16 mutations. Evi1 overexpression alone appears sufficient to immortalize immature myeloid cells and does not seem to require any other cooperating mutations. Genes identified by insertional mutagenesis by their nature could also be involved in immortalization of leukemic stem cells, and thus represent attractive drug targets for treating cancer.
Introduction
Emerging studies suggest that many cancers, including acute myeloid leukemia (AML),1 acute lymphoblastic leukemia (ALL),2 breast cancer,3 and central nervous system (CNS) cancer,4 arise from small populations of immortalized cancer stem cells that give rise to phenotypically diverse cancer cells that are not transplantable and have less proliferative capacity, but which represent the bulk of the cells in the tumor. Therapies that kill cancer stem cells represent potential cures for cancer, but therapies that kill the nontransplantable cancer cells can shrink the tumor but will not cure the patient because the remaining cancer stem cells will regenerate the tumor. Because several cooperating mutations are required for a cell to become cancerous,5 it has been generally assumed that these mutations accumulate in a cell that has unlimited self-renewal. Thus, one attractive possibility is that cancer stem cells are derived from normal adult stem cells. Alternatively, cancer stem cells could be derived from a more restricted progenitor with limited self-renewal if the cell acquires one or more mutations that make it immortal. If this were the case, then methods for identifying genes that make cells immortal could identify new drug targets for treating cancer. Identification of immortalization genes may also provide for a better understanding of the process of self-renewal versus differentiation in normal stem and progenitor cells.
In 1994, John Dick and colleagues showed that human AML cells could induce leukemia when transplanted into immunocompromised severe combined immunodeficient (SCID) mice.6 Limiting dilution assays showed that only 1 in 250 000 cells from the blood of a patient with AML is capable of transplanting leukemia to SCID mice. Cell fractionation experiments showed that this cell had a cell-surface phenotype similar to a hematopoietic stem cell (HSC). Subsequent experiments showed that all subtypes of AML, except M3 acute promyelocytic leukemia, share this phenotype and thus are likely derived from a HSC.
What about chronic leukemias such as chronic myeloid leukemia (CML)? The chronic phase of CML is associated with events such as overexpression of B-cell lymphoma 2 (Bcl2) and inactivation of Junb, events that increase the proliferation and survival of the HSC and myeloid progenitor populations.7 Thus, during the chronic phase of CML, the cancer-causing mutations again appear to accumulate in the HSC. Surprisingly, in the blast-crisis phase of CML, the granulocyte-macrophage progenitor (GMP) pool is greatly expanded and the cells have elevated levels of nuclear β-catenin compared with normal cells, and increased BCR-ABL expression.7 Unlike normal GMPs, CML GMPs have unlimited self-renewal in vitro. Thus, during blast crisis, the leukemic stem cell appears to be a GMP that has acquired the ability to undergo unlimited self-renewal. Consistent with this, an MSCV virus carrying the MLL-ENL fusion oncogene has been shown to immortalize purified GMPs in vitro just as well as purified HSCs.8 Both immortalized populations induce AML with similar efficiencies in mice that have received transplants, and the resulting leukemias are phenotypically the same, composed primarily of cells displaying late-stage myelomonocytic differentiation.8 These studies thus support the existence of leukemic stem cells that do not overlap with the HSC.
Another fusion oncogene, MOZ-TIF2, has also been shown to immortalize the common myeloid progenitor (CMP), the GMP and HSC and induces leukemia in hosts that have received transplants.9 This is not true, however, for the most widely studied leukemia fusion oncogene, BCR-ABL.9 BCR-ABL-transduced cells do not become immortal when serially passaged in vitro, nor do they induce leukemia in hosts that have received transplants. Thus, not all leukemia genes confer leukemic stem-cell properties to hematopoietic progenitors destined to undergo apoptotic cell death. Testing the ability of leukemia genes to immortalize primary cells in culture therefore provides a means for identifying potential leukemic stem-cell immortalization genes.
A limitation of this assay is that it only subclassifies known leukemia genes and does not identify new immortalization genes. Here we show that retroviral insertional mutagenesis can be used a tool for identifying immortalization genes, genes that by their nature might also be involved in immortalizing leukemic stem cells and thus represent potential drug targets for treating cancer.
Materials and methods
Retrovirus generation
Evi1 cDNA was excised from p58-210 by DraI and BspHI double digestion, blunt-ended, and cloned into the HpaI site of MSCVneo. Stable virus-producing cell lines were generated by transfecting either MSCVneo or MSCV-Evi1 into GP + E86 packaging cells and selecting cells that are G418-resistant using standard procedures.11 Viral titer of stable clones was determined by infecting NIH-3T3 cells.
Immortalization of bone marrow cells
Bone marrow cells were harvested from C57BL/6-Ly5.1+ mice (8 to 12 weeks old) 4 days after 5-fluorouracil (5-FU) intraperitoneal (IP) injection (150 mg/kg body weight) and cultured in Dulbecco modified Eagle medium (DMEM) plus 15% heat-inactivated fetal bovine serum (FBS), 10 ng/mL interleukin-6 (IL-6), 6 ng/mL IL-3, and 100 ng/mL stem cell factor (SCF) for 2 days. Expanded cells were subsequently plated in the same medium on top of a confluent layer of irradiated GP + E86 cells (2000 rad, 179 cGy/min) stably expressing MSCVneo or MSCV-Evi1. After infection for 24 to 48 hours, infected cells were cultured in Iscove modified Dulbecco medium (IMDM) with 20% heat-inactivated horse serum plus 100 ng/mL murine SCF and 10 ng/mL murine IL-3. Cells were passaged every 3 days and immortalized clones usually appeared after 1 month of culturing.
Splinkerette PCR
Genomic DNA was isolated from immortalized cell lines, digested with either NlaIII or MseI, and ligated to the splinkerette linker overnight.12 Nested polymerase chain reaction (PCR) was performed as described on the ligation reaction using splinkerette-specific primers and primers recognizing the long terminal repeat (LTR) of MSCV: MSCV-LTR1, 5′-TCTCGCTTCTGTTCGCGCGCTTC-3′; MSCV-LTR2, 5′-CGTCGCCCGGGTACCCGTATTC-3′. Products were separated on 2% agarose gels. Excised DNA bands were purified using MiniElute columns (Qiagen, Valencia, CA) and sequenced directly using the PRISM Big Dye Cycle Sequencing kit (Perkin Elmer, Shelton, CT) and an ABI Model 373A DNA Sequencer (Applied Biosystems, Foster City, CA).
Differentiation assays
To induce myeloid proliferation and differentiation, 20 ng/mL of murine granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), or 100 ng/mL of human macrophage colony-stimulating factor (M-CSF) was added to the culture after the 2 × 106 cells were washed with 10 mL of phosphate-buffered saline (PBS) and resuspended in IMDM with 20% heat-inactivated horse serum. PMA (phorbol 12-myristate 13-acetate) was added at 3.24 × 10-6 M final concentration.
Northern and Southern hybridization
Total RNA was isolated from cells using Trizol (Invitrogen, Carlsbad, CA) following the manufacturer's directions. 10 μg of each RNA sample was resolved, transferred, and hybridized using standard procedures.11 The full-length coding region of Evi1 was used as a hybridization probe. The Northern probe for Prdm16 was PCR amplified and confirmed by sequencing. Primer sequences are available on request. The β-actin probe was amplified using primers as described.13 Southern blotting was carried out using standard procedures.11 DNA samples of cell lines were digested with HindIII or BglII and probed with a neo-specific fragment of 760 base pair (bp) excised from MSCVneo by BamHI and EagI double digestion.
Fluorescence-activated cell-sorting (FACS) analysis
Bone marrow cells and splenocytes were harvested from animals that received transplants, and red blood cells were lysed by incubation in 3 mL of ammonium-chloride-potassium (ACK) lysis buffer on ice for 10 minutes. Cells left were then washed twice in Dulbecco (D) PBS, 1% fetal calf serum (FCS), and preincubated with rat anti-mouse CD16/CD32 antibody (2.4G2) for 10 minutes at 4°C. The cells were then washed twice again in DPBS and 1% FCS, and incubated for 30 minutes at 4°C with fluorescin isothiocyanate (FITC)-conjugated Ly5.2 (CD45.1, A20), peridinin chlorophyll protein (PerCP)-conjugated Ly5.1 (CD45.2, 104), or respective isotype control antibodies (Pharmingen, San Diego, CA). The cells were finally washed twice in DPBS, 1% FCS, fixed in 1% paraformaldehyde/PBS, and analyzed on a FACSCaliber flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
Results
Immortalization of immature myeloid cells
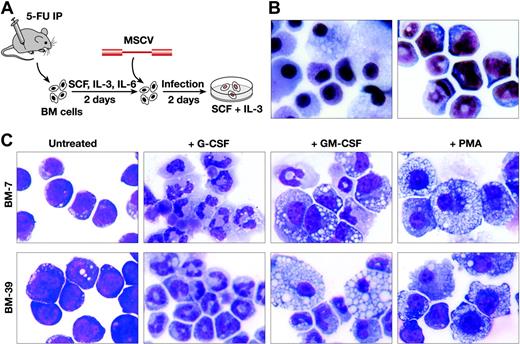
To determine whether retroviral insertional mutagenesis can be used as a tool for identifying hematopoietic cell immortalization genes, we infected bone marrow cells harvested from 5-FU-treated C57BL/6-Ly5.1+ mice that were grown in culture for 2 days in SCF, IL-3, and IL-6 in order to increase the number of cycling hematopoietic progenitor cells, with empty replication-incompetent MSCV containing only a neo gene (Figure 1A). MSCV was used because of its strong LTR activity in primitive cell types, including hematopoietic stem and immature myeloid cells, thus increasing the likelihood of activation of genes near the inserted provirus.14-16 Infected cells were then grown and serially passaged in SCF and IL-3. Under these culture conditions, normal uninfected bone marrow cells cease to proliferate after 3 to 4 weeks and only macrophages and mast cells are left in the cultures (Figure 1B, left panel). In contrast, when 1 × 106 cells were first infected for 2 days by coculturing them with packaging cells producing 3 × 106 cfu/mL MSCV, cell lines could be generated from approximately 50% of the infected cultures (Figure 1B, right panel).
More than 80 such lines have been produced and some have been maintained in culture for more than 1.5 years, suggesting they are truly immortal under these culture conditions. These cells can also be maintained in serum-free medium in the presence of SCF and IL-3 without any obvious phenotypic changes. However, all cells remain growth factor dependent, with rapid cell death ensuing in the absence of growth factor. Wright-Giemsa staining indicated that immortalized lines are composed largely of immature myeloid cells; however, some spontaneous differentiation occurs in these cultures, which typically contain anywhere from 10% to 40% mature-appearing neutrophils and 1% to 5% macrophages. The myeloid origin of these cells was also confirmed by FACS analysis, which showed that 50% to 70% of the cells are Gr-1+ (data not shown).
Immature myeloid progenitor cells with biphenotypic differentiation potential can be immortalized by infection with replication-defective MSCV. (A) Schematic representation of the immortalization procedure. Horse serum was included in the cultures along with the indicated growth factors after infection. (B) Wright-Giemsa staining of uninfected (left panel) and infected (right panel) bone marrow (BM) cells after 1 month in culture. Original magnification × 400. (C) Wright-Giemsa staining of 2 immortalized cell lines (BM-7 and BM-39) before and after treatments with G-CSF (3 days), GM-CSF (5 days), or PMA (2 days). BM-7 harbors a single MSCV Evi1 integration, while BM-39 harbors a Prdm16 integration. Both cell lines respond similarly to cytokine stimulation. Original magnification × 400. Images were obtained with the use of an Olympus Vanox AHBS3 microscope and a Nikon DXM1200F digital camera. Images were processed using Image-Pro Plus 5.1.
Immature myeloid progenitor cells with biphenotypic differentiation potential can be immortalized by infection with replication-defective MSCV. (A) Schematic representation of the immortalization procedure. Horse serum was included in the cultures along with the indicated growth factors after infection. (B) Wright-Giemsa staining of uninfected (left panel) and infected (right panel) bone marrow (BM) cells after 1 month in culture. Original magnification × 400. (C) Wright-Giemsa staining of 2 immortalized cell lines (BM-7 and BM-39) before and after treatments with G-CSF (3 days), GM-CSF (5 days), or PMA (2 days). BM-7 harbors a single MSCV Evi1 integration, while BM-39 harbors a Prdm16 integration. Both cell lines respond similarly to cytokine stimulation. Original magnification × 400. Images were obtained with the use of an Olympus Vanox AHBS3 microscope and a Nikon DXM1200F digital camera. Images were processed using Image-Pro Plus 5.1.
Immortalized lines can be further differentiated by growth factor addition
The amount of differentiation in the cultures could be increased by the addition of lineage-specific growth factors. For example, when 2 representative immortalized lines, BM-7 and BM-39, were cultured in G-CSF instead of IL-3 and SCF, the cells ceased to proliferate over 3 to 5 days and up to 95% of the cells differentiated into neutrophils (Figure 1C). In response to GM-CSF, the cells continued to expand but significantly more macrophage (up to 30%) and neutrophil (up to 70%) differentiation occurred (Figure 1C). The cells did not survive in the presence of M-CSF; however, treatment of the cells with PMA, a chemical that induces monocytic differentiation,17 induced 50% to 70% of the cells to differentiate into macrophages (Figure 1C). These cells thus demonstrate a partial differentiation block that can be overcome by growth factor addition.
Immortalized cell lines are often clonal
Since immortalized lines were not formed in the absence of MSCV infection, we hypothesized they were produced by insertional mutagenesis of an immortalization gene during the process of MSCV integration. If true, we would expect the lines to be generated from 1 or only a few infected cells since the probability of mutating an immortalization gene during integration should be low. To confirm this hypothesis, we examined the MSCV integrations in 15 immortalized lines by Southern blotting. Genomic DNA from the 15 lines was digested with HindIII, which cuts once in the provirus, and hybridized with a neomycin-specific probe. Most lines contained multiple MSCV integrations (Figure 2A). The relative intensity of the integrations within each line indicated that they were derived from 1, or at most 2, infected cells. To confirm this is the case, immortalized lines were single-cell cloned in methylcellulose and their integrations examined and compared with the parental lines. For example, when we generated 7 subclone lines from the BM-2a parental line (Figure 2B), each subclone line contained the same number and intensity of integrations as the parental line, confirming it was generated from 1 infected cell.
Immortalized cell lines are often clonal. (A) Southern blot analysis of the MSCV integrations present in 15 immortalized cell lines. DNA (8 μg) from each immortalized line was digested with HindIII and hybridized with a neo probe isolated from the MSCV vector. Each band represents a separate MSCV integration. (B) Southern blot analysis of 1 representative immortalized immature myeloid parental line (BM-2a) and its 7 single-cell subcloned lines (BM-2a-1-BM-2a-7).
Immortalized cell lines are often clonal. (A) Southern blot analysis of the MSCV integrations present in 15 immortalized cell lines. DNA (8 μg) from each immortalized line was digested with HindIII and hybridized with a neo probe isolated from the MSCV vector. Each band represents a separate MSCV integration. (B) Southern blot analysis of 1 representative immortalized immature myeloid parental line (BM-2a) and its 7 single-cell subcloned lines (BM-2a-1-BM-2a-7).
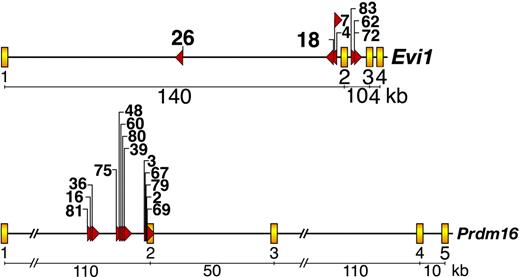
Many immortalized lines have integrations at Evi1 or Prdm16
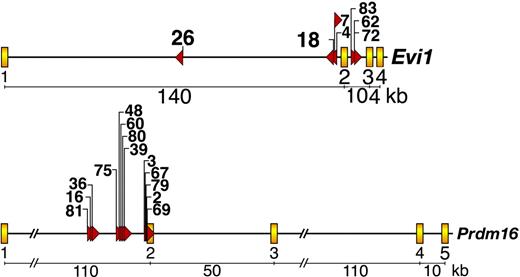
To identify the genes responsible for immortalization, we cloned over 80% of the MSCV integrations present in 37 immortalized lines12 and BLAST (basic local alignment search tool)-searched the integration site sequences against the mouse genome assembly using the University of California-Santa Cruz (UCSC) genome bioinformatics database.18 Seven lines contained MSCV integrations in the first or second intron of Evi1, while another 13 lines contained integrations in the first intron of Prdm16 (Figure 3). No lines had integrations in both genes. This was interesting because Evi1 and Prdm16 are zinc finger transcription factor homologs and validated myeloid leukemia genes. In human myeloid leukemias a number of reciprocal translocations and paracentric inversions have been identified that break at or near EVI1 or PRDM16 and result in inappropriate EVI1 or PRDM16 expression.19-22 In many cases, these rearrangements bring the ribophorin 1 gene in close proximity to EVI1 or PRDM16. This juxtaposition results in up-regulation of EVI1 or PRDM16 expression.19-22
Evi1 and Prdm16 are frequent targets of MSCV integration in immortalized immature myeloid progenitor cell lines.Evi1 MSCV integrations (top panel) and Prdm16 integrations (bottom panel). Yellow boxes depict exons, while red triangles indicate the location and orientation of integrated MSCV proviruses. Cell line numbers are listed above the red triangles.
Evi1 and Prdm16 are frequent targets of MSCV integration in immortalized immature myeloid progenitor cell lines.Evi1 MSCV integrations (top panel) and Prdm16 integrations (bottom panel). Yellow boxes depict exons, while red triangles indicate the location and orientation of integrated MSCV proviruses. Cell line numbers are listed above the red triangles.
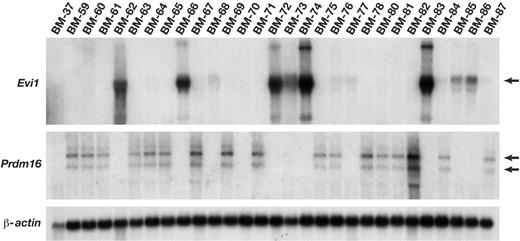
Evi1 and Prdm16 mRNA expression is activated by MSCV integrations. Northern blot analysis of Evi1 and Prdm16 expression in immortalized cell lines using probes specific for each gene. β-actin was used as a loading control. Lines BM-62, BM-72, and BM-83 have confirmed Evi1 integrations, while lines BM-60, BM-67, BM-69, BM-75, BM-80, and BM-81 have confirmed Prdm16 integrations. Lines BM-37 and BM-70 have neither integrations.
Evi1 and Prdm16 mRNA expression is activated by MSCV integrations. Northern blot analysis of Evi1 and Prdm16 expression in immortalized cell lines using probes specific for each gene. β-actin was used as a loading control. Lines BM-62, BM-72, and BM-83 have confirmed Evi1 integrations, while lines BM-60, BM-67, BM-69, BM-75, BM-80, and BM-81 have confirmed Prdm16 integrations. Lines BM-37 and BM-70 have neither integrations.
MSCV integration up-regulates Evi1 and Prdm16 expression
Similar to what was observed in human leukemias, Northern analysis of 29 immortalized lines showed that 8 express high levels of Evi1 (Figure 4). A few lines like BM-85 overexpress Evi1 but do not have integrations at Evi1. These lines may contain an integration at another gene that functions upstream of Evi1. Seventeen lines express high levels of Prdm16, including all lines with known integrations at Prdm16 (Figure 4). Similar to what was reported for human leukemias, 2 different-sized Prdm16 messages were expressed in the immortalized lines. Evi1 and Prdm16 expression is mutually exclusive in these lines. Since these genes are closely related, these results suggest that both genes function in the same immortalization pathway.
MSCV integration promotes the expression of proteins lacking the PR domain
Long and short isoforms of EVI1 and PRDM16 are expressed in normal cells. Long isoforms contain a PR domain that is missing in the short isoforms. The PR domain defines a subset of zinc finger genes that function as negative regulators of tumorigenesis. Other PR-domain-containing genes include BLIMP1, which maps to a tumor suppressor locus at 6q21,23 and RIZ1, which commonly undergoes deletions, rearrangements, or loss of heterozyosity in a broad spectrum of human tumors (reviewed in Huang et al24 ). In human cancer cells there is preferred expression of the short isoforms.25,26 The PR-domain is related to the SET domain, which is found in genes that regulate chromatin structure. The SET domain found in the MLL leukemia gene is a histone H3 lysine 4-specific methyltransferase that mediates heterochromatin silencing of Hox genes.27 The lack of a SET or PR domain in a gene may therefore specifically inactivate its chromatin-associated functions without affecting its other functions.
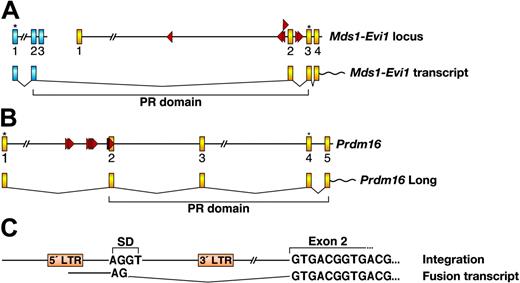
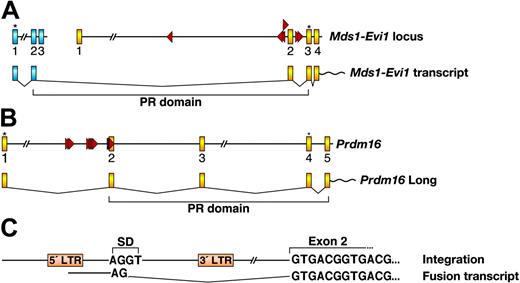
Viral integration at Evi1 and Prdm16 also promotes the expression of the short isoforms. The PR domain-containing isoform of Evi1 is generated by splicing of Evi1 to a small gene, Mds1, located more than 350 kilobase (kb) upstream (reviewed in Buonamici et al28 ). Mds1 exon 2 is fused to Evi1 exon 2, the predicted translation of which adds 188 amino acids upstream of the normal start site of Evi1, which is in exon 3 (Figure 5A). These extra amino acids, part of which contains the PR domain, are highly homologous to the PR domain of RIZ1 and BLIMP1. Interestingly, 6 out of 7 MSCV integrations at Evi1 are clustered within a 7.5-kb window around Evi1 exon 2 and the closest integration to Mds1 is over 400 kb away. Retroviruses integrate at many sites in the mouse genome and tend to behave as enhancers for close by genes. It is therefore likely that these integrations preferentially promote the transcription of the short isoform and their locations were selected during the immortalization process. These integrations could also interfere with efficiency of the splicing process used to generate the long isoform.
Integrations at Prdm16 are located in the first intron of Prdm16 and are oriented in the same transcriptional direction (Figure 5B). Fusion transcripts were readily detected in these lines by reverse transcriptase (RT)-PCR using a MSCV LTR-specific sense primer and an exon 2 specific antisense primer, indicating that MSCV integrations promote Prdm16 expression by a promoter insertion mechanism. Sequence analysis showed that the fusion transcripts started from the 5′ LTR of MSCV, which was spliced into Prdm16 exon 2 through a downstream viral internal splice donor site (Figure 5C). In humans, the long PRDM16 isoform is initiated from the first ATG located in exon 1, while the short isoform starts from an ATG in exon 4. Both translational start sites are conserved in mouse and are located in the same exons as in human (Figure 5B). In our immortalized lines, therefore, the first ATG that initiates the long isoform is excluded in the fusion transcript and only the short isoform lacking the PR domain is expressed.
MSCV integrations in lines that lack Evi1 or Prdm16 expression
Six immortalized lines do not express Evi1 or Prdm16 (ie, BM-37; Figure 4). These lines could be generated from the insertional mutagenesis of genes located downstream of Evi1 or Prdm16 or that function in other immortalization pathways. Cloning of the MSCV integrations in these lines identified a number of excellent immortalization candidates (Table 1). Three lines contain integrations in genes that are insertionally mutated by replication competent murine leukemia virus in mouse hematopoietic cancer (Cd47, Mef2d, Notch2, Supt4h) (Table 1). One of these genes, Mef2d, is mutated in human acute lymphoblastic leukemia and is a validated leukemia gene.30 BM-46 cells harbor integrations at Cbx2 and Notch2, among others (Table 1). This is interesting because Notch represents one of 5 known signaling pathways that regulate normal HSC self-renewal and cause hematopoietic disease when dysregulated by mutations.31,32 Cbx2 is a chromobox protein found in the same polycomb complex as Bmi1.33 Bmi1 also defines a signaling pathway that regulates HSC self-renewal34 and causes hematopoietic disease when mutated.35,36
MSCV integrations promote the expression of Evi1 and Prdm16 isoforms lacking the PR domain. (A) Mouse Mds1 locus and partial Evi1 locus with MSCV integrations are shown in the top panel. Mds1 and Evi1 exons are depicted as blue and yellow boxes, respectively. Red triangles indicate the location and orientation of integrated MSCV. Asterisks indicate translational start sites. The bottom panel depicts the generation of the Mds1-Evi1 fusion transcript and shows the location of the PR domain. (B) Mouse Prdm16 locus (partial) with MSCV integrations and the transcript encoding the long Prdm16 isoform containing the PR domain. Exons are depicted as yellow boxes. Translational start sites for the long and short isoforms are indicated by asterisks. (C) The nature of the fusion transcript between MSCV and Prdm16 exon 2 is shown. SD indicates splice donor in MSCV.
MSCV integrations promote the expression of Evi1 and Prdm16 isoforms lacking the PR domain. (A) Mouse Mds1 locus and partial Evi1 locus with MSCV integrations are shown in the top panel. Mds1 and Evi1 exons are depicted as blue and yellow boxes, respectively. Red triangles indicate the location and orientation of integrated MSCV. Asterisks indicate translational start sites. The bottom panel depicts the generation of the Mds1-Evi1 fusion transcript and shows the location of the PR domain. (B) Mouse Prdm16 locus (partial) with MSCV integrations and the transcript encoding the long Prdm16 isoform containing the PR domain. Exons are depicted as yellow boxes. Translational start sites for the long and short isoforms are indicated by asterisks. (C) The nature of the fusion transcript between MSCV and Prdm16 exon 2 is shown. SD indicates splice donor in MSCV.
Evi1 is an immortalization gene
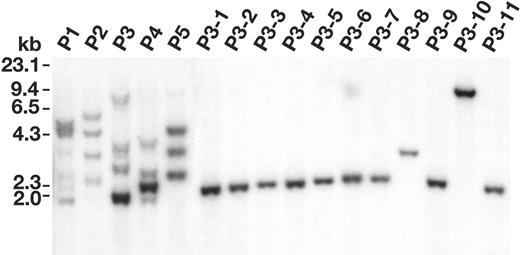
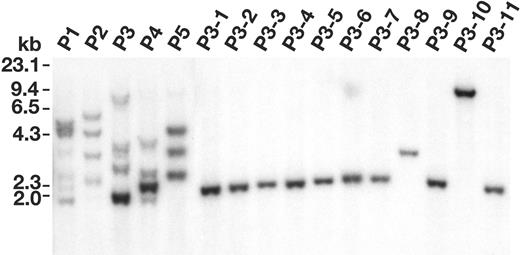
To confirm that Evi1/Prmd16 are immortalization genes we inserted 1 of them, an Evi1 cDNA lacking the PR domain, into the MSCV backbone, and then compared the ability of this virus and empty MSCV to produce immortalized lines. As indicated previously, when bone marrow cells were infected with 3 × 106 cfu/mL empty MSCV for 2 days, about 50% of the infected cultures produced immortalized lines (Table 2). Taking into account that about 30% of the immortalized cultures contain 2 clones, and the average number of insertions is 5 to 6 per immortalized cell, the frequency of immortalization is roughly 1.2 × 10-7 per starting cell per integration. However, when the viral titers were reduced to 4 × 105 cfu/mL, none of the infected cultures produced immortalized lines, presumably because too few MSCV integration events occurred in these cells to activate an immortalization gene. In contrast, when the cells were infected with 1 × 105 cfu/mL MSCV-Evi1, all of the infected cultures produced immortalized lines with properties similar to those produced by empty MSCV infection alone. MSCV-Evi1 is thus much more efficient at immortalization than empty MSCV. Southern analysis of 5 independent MSCV-Evi1 immortalized lines (P1-P5; Figure 6) showed that each contains multiple MSCV-Evi1 integrations of differing intensities, indicating that these lines are derived from several independent MSCV-Evi1 immortalized cells. Eleven single-cell subclone lines (P3-1 to P3-11) were then generated from the P3 line (Figure 6). Each subclone line harbored a single MSCV-Evi1 integration, suggesting that Evi1 overexpression alone is sufficient to immortalize immature myeloid cells. In support of this conclusion, the locations of integrations cloned from 3 subcloned lines (P3-4, P3-6, and P3-10 of Figure 6) appear to be random and do not colocalize with any previously cloned MSCV integrations. This may not be the case, however, for lines that fail to overexpress Evi1 or Prdm16. In some of these lines it is possible that 2 mutations (ie, Notch2 and Cbx2) are required for immortalization.
Evi1 can immortalize immature myeloid cells with high efficiency. Southern blot analysis of MSCV integrations in 5 immortalized cell lines (P1 to P5) generated by MSCV-Evi1 infection (10 μg/lane) and 11 cell lines subcloned from the P3 line (P3-1 to P3-11) (5 μg/lane) digested with BglII.
Evi1 can immortalize immature myeloid cells with high efficiency. Southern blot analysis of MSCV integrations in 5 immortalized cell lines (P1 to P5) generated by MSCV-Evi1 infection (10 μg/lane) and 11 cell lines subcloned from the P3 line (P3-1 to P3-11) (5 μg/lane) digested with BglII.
Evi1 and Prdm16 immortalized cells do not induce leukemia in transplanted hosts
To determine whether immortalized cells can engraft and induce leukemia in hosts that have received transplants, we transplanted 2 to 8 × 106Ly5.1+ cells from 2 lines with Evi1 integrations (BM-4, BM-7) and 1 line with a Prdm16 integration (BM-2) into lethally irradiated C57BL/6-Ly5.2+ mice along with 5 × 105 unirradiated C57BL/6-Ly5.2+ supporting bone marrow cells. Three weeks after transplantation, Ly5.1+ cells could not be detected in the spleen or bone marrow of mice that received transplants by FACS analysis, indicating that these cells did not engraft and survive in hosts that received transplants. The mice also did not develop leukemia when aged for 1 year. Evi1 and Prdm16 thus behave differently from MLL-ENL and MOZ-TIF2, which immortalize immature myeloid progenitor cells in vitro and also induce leukemia in hosts that received transplants.
Discussion
Here we show that insertional mutagenesis can be used as a tool for identifying genes that promote the immortalization of primary bone marrow progenitor cells. Several laboratories, including our own, have used retroviral insertional mutagenesis as a tool to identify leukemia genes and, in some cases, these genes have been shown to immortalize primary bone marrow progenitor cells when introduced into them. However, the genetic screen described here is much more powerful because it has the power to identify immortalization genes regardless of whether or not the gene is a known leukemia gene. This can even include genes for which there is no functional information or homology to known genes. Thus, this screen has the power to identify genes that no one would guess is an immortalization gene. The screen can also identify immortalization functions for leukemia genes that have not yet been tested for a role in immortalization. Such is the case for the immortalization genes, Evi1 and Prdm16, identified here. Both genes can block the differentiation of bone marrow progenitor cells and IL-3-dependent 32Dc13 cells to granulocytes10,26 and erythroid cells,37 and strongly favor differentiation along the megakaryocytic lineage.38 Enforced expression of Evi1 in 32Dc13 cells has can induce a differentiation block in response to GM-CSF due to the incapacity of these cells to express myeloperoxidase, resulting in their cell death.10 Likewise, Evi1 expression is known to increase the proliferation of brahma-related gene 1 (Brg1)-positive 32Dcl3 cells by binding to Brg1 and blocking its ability to repress E2f1,39 although a role for Evi1 or Prdm16 in immortalization has not been previously recognized.
In spite of their ability to immortalize hematopoietic cells, neither Evi1 nor Prdm16 immortalized cells are able to engraft and induce leukemia in susceptible hosts. Although the engraftment failure could be due to long-term culturing, primary MSCV-Evi1-infected bone marrow cells also do not induce leukemia in mice that received transplants, although they do engraft (see next paragraph), suggesting that Evi1 overexpression alone is insufficient for leukemia induction. Studies of Calmels et al40 suggest that recurrent retroviral integrations at the MSD1-EVI1 locus in nonhuman primate myeloid progenitor cells also leads to their immortalization via insertional mutagenesis. There is no evidence, however, for ongoing clonal expansion of these cells, and all animals carrying these cells are hematologically normal without evidence for leukemia. In a case where a retroviral integration at Evi1 locus did induce myeloid leukemia, a long latency was observed and stimulation by secondary transplantation and cooperation with a mutant form of neurotrophin receptor dLNGFR was required.41 Evi1 and Prdm16 thus appear to define a second group of leukemia genes that are able to immortalize bone marrow progenitor cells in vitro but are unable to induce leukemia in susceptible hosts.
What then is the role of Evi1 and Prdm16, if any, in immortalization of leukemic stem cells? The most frequent chromosomal translocations leading to EVI1 deregulation are t(3;3)(q21;q26) and inv(3)(q21q26), which are observed almost exclusively in myelodysplastic syndrome (MDS).42,43 EVI1 is also activated in MDS without apparent rearrangements of chromosome 3.44 Myelodysplasia is a hematologic disease in which genomic abnormalities accumulate in the HSC leading to serve pancytopenia, multilineage differentiation impairment, and bone marrow apoptosis. Mortality results from pancytopenia or transformation to AML. Buonamici and colleagues have shown that mice given transplants of mouse hematopoietic cells infected with an MSCV-Evi1 virus develop a fatal myeloproliferative-like disease.45 The immediate effects of Evi1 expression are hyperproliferation of bone marrow cells and down-regulation of Epor and Mpl, which are important for terminal differentiation of erythrocytes. After a relatively long latency period, the reconstituted animals developed a fatal cytopenia, accompanied by hypercellular bone marrow and severe dyserythropoiesis. The disease did not progress to AML, but instead the mice succumbed to fatal peripheral cytopenias. Since MDS is thought to be a stem cell disease, it is possible that Evi1 functions solely in the HSC in leukemia induction, and its ability to immortalize immature myeloid cells in vitro tells us nothing about its role in leukemia.
Alternatively, EVI1 chromosomal rearrangements have also been observed in all French-American-British (FAB) subtypes of AML (but in subtype M3 only as a second event)46,47 and in the blast crisis phase of CML.48 EVI1 expression also increases in CML during blast crisis, raising the possibility that EVI1 plays a role in CML progression,49,50 a hypothesis supported by the increased EVI1 expression observed in CML patients undergoing blast crisis that lack EVI1 chromosome rearrangements and the correlation between EVI1 activation and progression of CML observed by several groups.49,50 It is therefore possible that Evi1 has duel functions in leukemia induction. In MDS or CML during the chronic stage of the disease, EVI1 expression may increase the proliferation of the HSC and/or inhibit its differentiation, allowing for other cooperating mutations to accumulate. During the blastcrisis phase of CML, EVI1 might also participate in the immortalization of the GMP, which accumulating evidence suggests is the leukemia stem cell during the blast-crisis stage.7 While Evi1 expression alone appears sufficient to immortalize immature mouse myeloid cells in vitro, the cells do not engraft or induce leukemia in hosts that receive transplants. This could be due to the effect of the culture conditions or represent a species difference between human and mouse. Alternatively, Evi1 could require 1 or more other cooperating mutations that accumulate during the chronic phase of the disease in order for Evi1 immortalized GMP cells to survive and expand during blast crisis. Fusion oncogenes like MLL-ENL and MOZ-TIF2 might not be so dependent on these cooperating mutations, explaining why they induce leukemia in hosts that receive transplants.
Evi1 immortalized myeloid lines produced by MSCV infection are growth factor dependent and die in the absence of growth factors. Prevention of cell death is one of the crucial events in myeloid leukemogenesis. In human leukemia, cell-death antagonists such as Bcl2 play an important role in the transformation of myeloid cells. The fusion oncogene AML1-ETO directly up-regulates Bcl2 expression51 and activating mutations due to retroviral integration have been identified in the murine Bclxl gene in both myeloid and T-cell leukemias.51 Loss-of-function mutations in Fas signaling are also associated with lymphoid hyperplasia. Intercrosses between hMRP8Bcl2 and Fas-deficient Faslpr/lpr mice leads to the development of AML in 15% of the hMRP8Bcl2, Faslpr/lpr mice, with an expansion of myeloblasts in all hematopoietic tissues and substantially lower numbers of granulocytes in the bone marrow and blood.52 Enforced Bcl2 expression also greatly increases the incidence of CML-like disease in hMPR8BCR-ABL mice53 as well as the incidence of acute promyelocytic leukemia in hMPR8PML/Rara mice.53 Mutations in one or both of these pathways may thus be critical for allowing Evi1 immortalized immature myeloid cells to induce leukemia in susceptible hosts in the absence of high concentration of SCF and IL-3.
Increased self-renewal in the CML GMP is associated with activation of β-catenin during the blast crisis phase of CML and increased expression of BCR-ABL.7 The cause of β-catenin activation and increased BCR-ABL expression is unknown. One intriguing possibility is that 1 or both of these effects is mediated by increased EVI1 signaling, either alone or in combination with other genes that are also expressed during blast crisis. EVI1 is a zinc finger transcription factor and is likely to have many downstream targets. However, despite considerable effort, little is known about the genes regulated by EVI1.
Many other cytokines beside those used in these studies have been identified that promote the proliferation of hematopoietic cells. By serially passaging MSCV-infected cells in other cytokines it may thus be possible to select immortalized lines that are blocked at other stages of hematopoiesis and thereby identify immortalization genes for other types of hematopoietic cancers. In support of this notion, in preliminary studies we have found that passaging MSCV-infected cells in SCF and Fms-like tyrosine kinase 3 ligand (FLT3L) can select for immortalized cells that are blocked at a very early stage of hematopoiesis (Y.D., unpublished results, October 2004). Cloning and sequence analysis of the MSCV integration sites in these cells suggest that immortalization is independent of the Evi1/Prdm16 pathway. Insertional mutagenesis is thus not limited to finding immortalization genes for immature myeloid cells. Since our screening method employs a replication-defective virus, it is also not limited to murine cells. The MSCV LTR is active in human cells.55 By using amphotropic packaging cells it may therefore also be possible to perform these screens directly in human cells.
Prepublished online as Blood First Edition Paper, August 18, 2005; DOI 10.1182/blood-2005-03-1113.
Supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Y.D. designed and performed research, analyzed data, and wrote the manuscript; N.A.J. designed research and wrote the manuscript; and N.G.C. designed research, analyzed data, and wrote the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Jonathan Keller for helpful discussion and Dan Logsdon for assistance with the transplantation studies.