Hereditary pyropoikilocytosis (HPP) is a severe hemolytic anemia due to abnormalities of the red blood cell (RBC) membrane skeleton. In the original HPP kindred, there is compound heterozygosity for an allele encoding a structural variant of α-spectrin (L207P) and an α-spectrin allele associated with a defect in α-spectrin production. To identify the molecular defect in the production-defective allele, reticulocyte α-spectrin cDNA from one of the original HPP patients was analyzed. Transcripts from the production-defective, non-L207P allele demonstrated a pattern of abnormal splicing between exons 22 and 23, resulting in insertion of intronic fragments with an in-frame premature termination codon. A G to A substitution at position +5 of the donor consensus splice site of IVS 22 was identified in the inserts. Following gene transfer into tissue culture cells, there was complete absence of normally spliced α-spectrin gene transcripts derived from a minigene containing the IVS 22 +5 mutation.
Introduction
Hereditary pyropoikilocytosis (HPP)1 is a severe hemolytic anemia first described by Zarkowsky et al.2 Erythrocytes from HPP patients have morphology similar to that seen in patients suffering severe burns and frequently exhibit increased thermal sensitivity. There is a significant relationship between HPP and hereditary elliptocytosis (HE).1 Approximately one third of parents or siblings of patients with HPP has typical HE. Many HPP patients experience severe hemolytic anemia that evolves into typical HE later in life. In many cases of HE and HPP, a defect of the erythrocyte membrane protein α spectrin has been identified. Spectrin, composed of heterodimers of 2 subunits, α and β spectrin, is the major protein of the membrane skeleton. αβ spectrin heterodimers self-associate into tetramers and oligomers that provide the strength and flexibility to the erythrocyte membrane.1
α-spectrin mutations associated with HE and HPP are structural variants located in the heterodimer self-association region, the αI domain. In some cases, HPP is associated with homozygosity or compound heterozygosity for structural defects of the αI domain of spectrin.1 In other cases, including the original kindred reported by Zarkowsky et al,2 there is compound heterozygosity for an αI domain structural variant and a production-defective α-spectrin allele in trans associated with markedly decreased or absent normal α spectrin on the membrane.3,4 The parent who transmits the production-defective spectrin allele is clinically normal with unremarkable erythrocyte morphology because α spectrin is normally synthesized in a 2- to 3-fold excess and output from a single normal α-spectrin allele is sufficient to maintain membrane integrity.2,5,6
The precise molecular basis for the production-defective α-spectrin allele in HPP has not been identified. In some cases, defective mRNA accumulation has been demonstrated, suggesting a defect in mRNA processing.4 This report describes the identification and characterization of the molecular defect associated with the production-defective α-spectrin allele in the original HPP kindred described by Zarkowsky et al.2 The defect was found to be a splicing abnormality in trans to a previously described structural defect of the αI domain. We have named this variant α spectrinSt Louis II.
Study design
Patients
Clinical, hematologic, biochemical, and partial genetic characterization of the original HPP family have been previously described.2,4,5,7,8 Partial tryptic digestion and 2-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of spectrin revealed absence of the normal 80-kDa αI peptide and the presence of a new 50-kDa peptide.5 The molecular basis of the abnormal 50-kDa peptide was identified as a missense mutation, Leu207Pro (spectrinSt Louis).7,8 In other studies, reverse transcriptase-polymerase chain reaction (RT-PCR) demonstrated a decrease in α-spectrin mRNA accumulation associated with the non-L207P allele.4
Genomic DNA PCR, sequencing, mRNA isolation, and RT-PCR
Minigene construct
Genomic DNA fragments containing exons 21 to 23 of the α-spectrin gene, with either the wild-type or mutant intron 22 sequence, were inserted into a cytomegalovirus (CMV) promoter-driven expression plasmid (pcDNA3; Invitrogen, Carlsbad, CA). Cos-7 cells were transiently transfected with the expression plasmid. RNA was extracted after 24 hours, reverse transcribed, and amplified.
Results and discussion
Structural studies of α spectrin genes in affected patients
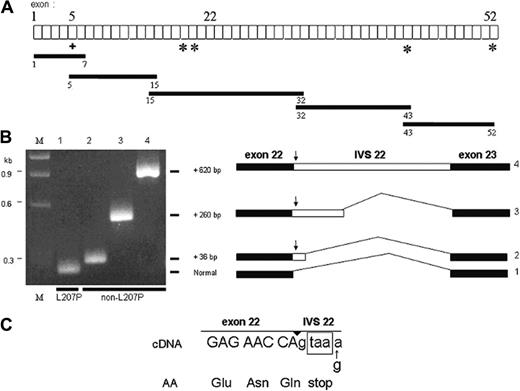
Sequencing of the promoter region of both α-spectrin alleles from -794 to +1 using PCR-amplified genomic DNA from one of the HPP probands detected no abnormalities.11 The alleles were distinguished by the presence of an XbaI polymorphism in intron 2. Using an RT-PCR-based strategy, with reticulocyte RNA as template, the entire coding region of the α-spectrin mRNA was amplified in overlapping cDNA fragments 1.0- to 2.6-kb in length (Figure 1A): exons 1 to 7 (1.0 kb), exons 5 to 15 (1.5 kb), exons 15 to 32 (2.6 kb), exons 32 to 43 (1.5 kb), and exons 43 to 52 (1.6 kb). Amplification products were subcloned and sequenced. Each fragment contained at least one single nucleotide polymorphism (SNP) or the Leu207Pro mutation that allowed discrimination of the 2 different alleles.
In addition to the fragment of expected size, 3 abnormally long cDNA fragments were identified in the exon 15 to 32 amplification product. Sequence analysis revealed that all of the fragments were derived from the non-L207P allele. One fragment contained the entire intervening sequence (IVS) 22 of the gene (Figure 1B). Sequence analysis of the other fragments revealed the presence of 2 other types of intronic insertions between exon 22 and exon 23: (1) a 36-base pair (bp) sequence and (2) a 260-bp insertion, both derived from the 5′ end of intron 22 (Figure 1B). The 3′ end of these 2 shorter inserts was immediately followed by a potential GT donor splice site dinucleotide located in the normal sequence of IVS 22, indicating an appropriate basis for alternative spicing. All 3 of the inserts introduce into the aberrantly spliced mRNA an in-frame TAA termination codon derived from nucleotides +2 to +4 of IVS 22. This suggests that nonsense-mediated decay12,13 is probably responsible for the previously observed quantitative deficiency of the non-L207P α-spectrin mRNA in one of the HPP probands.4 The cDNA fragments derived from the L207P allele had a normal sequence at the exon 22/23 junction (Figure 1B).
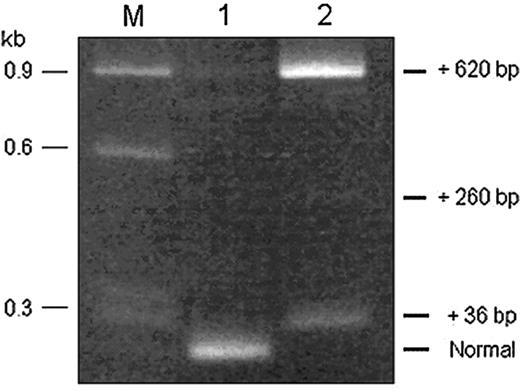
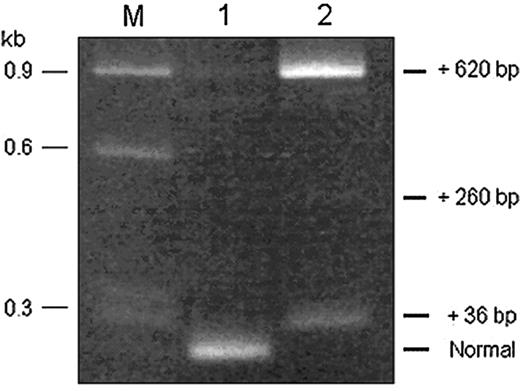
Diagram of α-spectrin gene exons (not to scale) and strategy used to obtain overlapping cDNA fragments, indicated by the black bars. (A) The + indicates the L207P mutation and the asterisks indicate single nucleotide polymorphisms (SNPs) used as linkage markers to distinguish between the 2 α-spectrin alleles in the subcloned amplified cDNA fragments. (B) Reamplification of PCR products from different subclones of the exon 15 to 32 cDNA fragment, using primers to encompass exons 22 and 23. All subclones of the L207P allele had a normal-sized band (no. 1). However, subclones of the non-L207P allele demonstrated 3 different higher molecular weight bands corresponding to fragments with incremental sizes of 36, 260, or 620 bp (nos. 2, 3, and 4, respectively). M indicates size markers. To the right of the gel are diagrams of the different splicing isoforms, with insertions derived from the 5′ end of IVS 22 (open bar). The arrow indicates the in-frame stop codon located at the very 5′ end of the retained intronic sequence. (C) Nucleotide sequence at the junction of exon 22 and IVS 22 in the cDNAs corresponding to the non-L207P allele of the HPP proband. The encoded amino acid sequence is shown below the sequence, and the exon/intron junction is indicated by the arrowhead above the sequence. The in-frame termination codon is shown in the box.
Diagram of α-spectrin gene exons (not to scale) and strategy used to obtain overlapping cDNA fragments, indicated by the black bars. (A) The + indicates the L207P mutation and the asterisks indicate single nucleotide polymorphisms (SNPs) used as linkage markers to distinguish between the 2 α-spectrin alleles in the subcloned amplified cDNA fragments. (B) Reamplification of PCR products from different subclones of the exon 15 to 32 cDNA fragment, using primers to encompass exons 22 and 23. All subclones of the L207P allele had a normal-sized band (no. 1). However, subclones of the non-L207P allele demonstrated 3 different higher molecular weight bands corresponding to fragments with incremental sizes of 36, 260, or 620 bp (nos. 2, 3, and 4, respectively). M indicates size markers. To the right of the gel are diagrams of the different splicing isoforms, with insertions derived from the 5′ end of IVS 22 (open bar). The arrow indicates the in-frame stop codon located at the very 5′ end of the retained intronic sequence. (C) Nucleotide sequence at the junction of exon 22 and IVS 22 in the cDNAs corresponding to the non-L207P allele of the HPP proband. The encoded amino acid sequence is shown below the sequence, and the exon/intron junction is indicated by the arrowhead above the sequence. The in-frame termination codon is shown in the box.
A G to A base substitution was observed at position +5 of the consensus donor splice site at the 5′ end of all of the IVS 22 inserts (Figure 1C). Heterozygosity for G and A at position +5 of IVS 22 was confirmed by sequence analysis of PCR products of genomic DNA from the 2 HPP probands and their asymptomatic mother (data not shown). The nucleotide G is highly conserved at position +5 of consensus donor splice sites of introns. Mutations of G to A (or another nucleotide) at this position have been associated with abnormal splicing and severe gene expression defects in a number of genes, including β-globin and many other genes.14 No other differences were found in the entire IVS 22 sequence in genomic DNA from the HPP probands or their mother.
This G to A base change, which is detectable by denaturing high-performance liquid chromatography (DHPLC)15 analysis of the PCR product of α-spectrin gene exon 22 and its flanking intronic DNA, was not found in the analysis of 50 unrelated individuals with various types of hemolytic anemia including 4 cases of HPP with a similar inheritance pattern to that of these index cases. Of the HPP patients tested, 2 were known to have a quantitative deficiency of the α-spectrin mRNA associated with the non-HE structural variant allele,4 in a pattern similar to that seen in the one of the affected siblings in the α-spectrinSt Louis family.
Minigene model for splicing assays
To evaluate the functional significance of the G to A substitution in IVS 22, a minigene model was developed (“Study design”) to study splicing in transfected tissue culture cells. The RT-PCR products derived from RNA of cells transfected with the wild-type minigene contained primarily the expected normally sized product and a faint band at the position corresponding to that of a product containing unspliced IVS 22 (Figure 2). RT-PCR of RNA from cells transfected with the mutant (IVS 22, +5 G to A) minigene revealed absence of the normal fragment and the presence of prominent abnormally sized fragments corresponding to IVS 22 insertions of 36 bp and 620 bp, the complete intron; in addition, a faint band was observed at the position corresponding to the IVS 22 insert of 260 bp (Figure 2).
RT-PCR products, obtained using primers encompassing exons 22 and 23, of RNA extracted from Cos-7 cells transfected with minigene expression constructs containing either the normal or mutant IVS 22 donor splice site. The RT-PCR product derived from RNA of cells expressing the wild-type construct (lane 1) had a predominant band of normal size. The RT-PCR product derived from RNA of cells expressing the IVS 22 mutant construct (lane 2) yielded no visible normally sized band but, instead, displayed 2 prominent higher molecular weight bands, corresponding to fragments with incremental sizes of 36 and 620 bp and a faint band of incremental size of 260 bp. The sequence composition of the different products was verified by sequencing and shown to be concordant with the splicing events illustrated in Figure 1B. M indicates size markers.
RT-PCR products, obtained using primers encompassing exons 22 and 23, of RNA extracted from Cos-7 cells transfected with minigene expression constructs containing either the normal or mutant IVS 22 donor splice site. The RT-PCR product derived from RNA of cells expressing the wild-type construct (lane 1) had a predominant band of normal size. The RT-PCR product derived from RNA of cells expressing the IVS 22 mutant construct (lane 2) yielded no visible normally sized band but, instead, displayed 2 prominent higher molecular weight bands, corresponding to fragments with incremental sizes of 36 and 620 bp and a faint band of incremental size of 260 bp. The sequence composition of the different products was verified by sequencing and shown to be concordant with the splicing events illustrated in Figure 1B. M indicates size markers.
Mutations associated with quantitative defects of α-spectrin gene expression
Only a few mutations of the α-spectrin gene have been reported to cause a quantitative deficiency of α-spectrin synthesis. The low-expression Lyon allele (αLELY) is associated with an alternative splicing defect causing in-frame skipping of exon 46 in approximately 50% of the transcripts from the affected gene, presumably due to a base change at position -12 in IVS 45.16 When inherited in trans to an HE allele, the resulting hematologic phenotype of the disorder is usually more severe than that of simple heterozygosity for HE.1,16 Of interest, the Leu207Pro HE mutation occurred on the chromosomal background of the αLELY allele.8 Other HE alleles associated with abnormal splicing of α-spectrin mRNA expected to be associated with nonsense-mediated mRNA decay have been described, but quantitative analyses have not been performed.1
The other characterized α-spectrin gene mutations causing decreased α-spectrin chain production have been associated with hereditary spherocytosis (HS) rather than HE/HPP. These include the following: the low-expression Prague allele (αLEPRA) associated with a base change at position -99 of IVS 30 and an enhanced aberrant alternative splicing defect causing, in approximately 85% of the transcripts from the affected gene, retention of the distal 70 bp of intron 30 that results in the insertion of an in-frame premature termination codon.17 The αLEPRA base change is also linked to an A970D polymorphism in exon 21 called αIIa or αBug Hill, which is frequently found in patients with severe recessive HS.17,18 A more severe (“virtually null”) production deficit has been reported in the αLELY-Bicetre allele associated with a G to A substitution of the last base of exon 51,19 causing no change in amino acid sequence, but associated with abnormal alternative splicing resulting in either complete or partial loss of exon 51 in the mature mRNA.
In summary, this study completes the characterization of the molecular defects in the first cases of HPP initially reported 30 years ago and has identified a rare null allele of the α-spectrin gene inherited in trans to the more common L207P α-spectrin structural variant.
Prepublished online as Blood First Edition Paper, September 8, 2005; DOI 10.1182/blood-2005-05-1813.
Supported in part by the following grants of the National Institutes of Health (NIH): RO1-DK19482 (B.G.F.) and R01-HL65448 (P.G.G.).
Presented as an oral presentation at the 46th annual meeting of the American Society of Hematology, San Diego, CA, December 6, 2004.20
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We sincerely thank Timothy Graubert, MD, and his colleagues at Washington University School of Medicine, as well as Harold S. Zarkowsky, MD, for their invaluable assistance in obtaining blood samples from the probands and their family members.