The 911 amino acid band 3 (SLC4A1) is the major intrinsic membrane protein of red cells and is the principal
Introduction
The 911 amino acid human erythroid AE1 (eAE1)
Band 3 is characterized by 3 distinct structural and functional domains: (1) the membrane spanning domain, which traverses the bilayer and functions as a chloride/bicarbonate exchanger; (2) the short C-terminal cytoplasmic domain that binds carbonic anhydrase II, and (3) the N-terminal cytoplasmic domain that interacts with a number of proteins, including ankyrin, protein 4.2, protein 4.1, glycolytic enzymes (eg, aldolase and glyceraldeyde-3-phosphate dehydrogenase [GAPDH]), hemoglobin, hemichromes, and different protein tyrosine kinases.1,2,6-13 Furthermore, band 3 has recently been shown to assemble a macromolecular protein complex of integral proteins including the Rh complex (Rh-associated glycoprotein, Rh polypeptides, CD47, and glycophorin B) and glycophorin A.14
The majority of the human SLC4A1 mutations reported to date2,7 result in autosomal-dominant forms of hereditary spherocytosis (HS) as a consequence of moderate decrease in membrane content of eAE1 protein. Importantly, in all these cases of HS mutant polypeptide is not assembled into the membrane.2,7 As such, the consequence of the assembly of mutant band 3 into the membrane on its function in red cells has not been defined.
The availability of knock-out animal models enabled the identification of the effect of complete deficiency of eAE1 on red-cell physiology. Band 3 null red cells have been described in a natural strain of cattle and in 2 strains of knock-out mice.15-17 Marked membrane loss leading to spherocytic morphology is a feature of red cells in these animals. In addition to the absence of band 3, the mouse red cells also exhibit complete deficiency of glycophorin A.15,18,19 In mice, the absence of band 3 leads to a severe life-threatening hemolytic anemia. So far, only one case of complete deficiency of band 3 in humans has been described. In this case, both the erythrocytic and kidney band 3 were absent, resulting in life-threatening neonatal anemia and distal renal tubular acidosis.20
Here we report a novel variant of eAE1, called band 3 Neapolis, in which N-terminal 11 amino acids are truncated. In the homozygous state, red-cell membranes contain only the mutant protein albeit at a much lower level. Detailed biochemical and functional characterization of these red cells has enabled us to obtain novel insights into the function of the N-terminal domain of band 3.
Materials and methods
Studies on SLC4A1 gene and band 3 cDNA
Blood samples were drawn from healthy volunteers, the proband, and his parents. The study was approved by the Institutional Review Board of the Second University of Naples, and was performed in accordance with the World Medical Association's Declaration of Helsinki of 1975, as revised in 2000. Written informed consent to molecular genetic analysis, data analysis, and publication was obtained from all of the participants. DNA and total reticulocyte RNA were isolated as previously described.21 Sequencing of band 3 genomic DNA and cDNA was performed as previously reported.21,22
Antibodies and immunoblotting
Monoclonal antibodies to band 3, alpha and beta spectrin, glycophorin A (GPA), and glycophorin C (GPC) were obtained from Sigma (Sigma Chemical, St Louis, MO). Polyclonal antibodies to aldolase, GAPDH and CD47, were provided by Santa Cruz Biotechnologies (Santa Cruz, CA). A monoclonal antibody (CDB3) directed against the N-terminus of band 3 was previously described.23 Immunoblotting was performed according to the protocols reported previously.24,25
Polymerase chain reaction (PCR) analysis
Reticulocyte RNA was reverse transcribed to obtain random-primed cDNA using Moloney murine leukemia virus-reverse transcriptase (MMLV-RT) (Gibco-Invitrogen, Carlsbad, CA). The PCR conditions and the primers employed for amplification of cDNA were as follows: 5′-aacgagtgggaacgtagctg-3′ (forward); 5′-cttcatattcctcctgctccag-3′ (reverse); 18 to 30 cycles with each cycle, consisting of 95°C for 30 seconds, 55°C for 1 minute, and 68°C for 3 minutes. In order to use the same amount of cDNA in each reaction, GAPDH amplification was performed first for normalization purposes as previously reported.26 All reaction products were run on 2% Tris borate EDTA (ethylenediaminetetraacetic acid) (TBE)-agarose gel stained with ethidium bromide. Normally spliced band 3 PCR product (exon1-exon2-exon3) was 216 bp long, while mutant band 3 products were 132 bp (exon1-exon3) and 341 bp (exon1-exon2-intron2-exon3).
To perform real-time RT-PCR on reticulocyte cDNA, we synthesized the following primers: SLC4A1/e1AF, 5′-agggaccctgaggctcgtgagcag-3′; SLC4A1/e1BF, 5′-ttcaccaagggaccctgaggctcg-3′; SLC4A1/e2-3R, 5′-tgtcttcataatcatcctgcagct-3′; SLC4A1/e1-3F, 5′-ctgaggctcgtgagcaggatgatt-3′; SLC4A1/e4R, 5′-tctgtggctgttgcctcggtgtcg-3′; SLC4A1/i2F, 5′-cacagcccagcctcagcggccact-3′; and SLC4A1/i2R, 5′-actgctgatgccagggaacacccg-3′.
Combinations of these primers were used to set up 5 different reaction mixtures: (1) SLC4A1/e1AF and SLC4A1/e2-3R that will generate a product of 113 bp derived by the correct joining of exons 1-2-3, since the reverse primer can only anneal to the junction 2-3; (2) SLC4A1/e1BF and SLC4A1/e2-3R that will generate a product of 120 bp with a different forward primer but correct joining of exons 1-2-3; (3) SLC4A1/e1-3F and SLC4A1/e4R that will generate a product of 132 bp derived by the skipping of exon 2 and the aberrant joining of exons 1-3-4, since the forward primer can only anneal to the junction 1-3 and the reverse primer is located in exon 4; (4) SLC4A1/i2F and SLC4A1/e4R that will generate a product of 147 bp derived by the retention of intron 2 and the aberrant joining of exon2-intron2-exon3-exon4, since the forward primer can only anneal to intron 2 and the reverse primer is located in exon 4; and (5) SLC4A1/e1BF and SLC4A1/i2R that will generate a product of 129 bp derived by the retention of intron 2 and the aberrant joining of exon1-exon2-intron2, since the forward primer is placed on exon 1 and the reverse primer will anneal to intron 2. The use of these 5 sets (1 to 5) enabled us to identify the normal allele (1 and 2), and the mutant alleles, skipping of exon 2 (3) and the retention of intron 2 (4 and 5). All PCR reactions were carried out using a Bio-Rad i Cycler (Bio-Rad, Hercules, CA) with the following program: 95°C for 30 seconds, 63°C for 2 minutes, and 72°C for 1 minute. SYBR green was used to monitor DNA synthesis. We assayed control cDNA as well as cDNA from the parents and the proband and normalized all values assuming the wild-type allele in control as 100%.
Red-cell membrane protein analysis
Red-cell ghosts were prepared according to the procedure of Dodge et al,27 except that 5 mM phenylmethylsulfonyl fluoride was added during the lysis step. Protein concentrations were determined by the procedure of Lowry et al,28 using a sodium carbonate solution containing 3% sodium dodecyl sulfate (SDS) and bovine serum albumin as a standard. Membrane proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).29,30 The gels were stained with Coomassie blue G. Erythrocyte membrane proteins were quantified by scanning of SDS-PAGE gels and through analysis of immunoblots as previously described.31,32 Band 3 was also quantified by labeling membranes with eosine maleimide followed by protein solubilization and fluorescence measurement (522 nm, excitation; 550 nm, emission).33
Band 3 purification
Band 3 monoclonal antibody (Sigma) directed against the 300 N-terminal amino acids was cross-linked to protein A-agarose essentially as described.34 Band 3 was solubilized by suspending the membranes in 1% (wt/vol) Triton X-100, 5 mM phosphate, pH 8, for 1 hour. After centrifugation at 30 000 × g for 30 minutes, the supernatant was diluted 10-fold and applied directly to the immunoaffinity column, previously equilibrated with 0.1% Triton X-100, 5 mM phosphate. The column was washed with 10 column volumes of 0.1% Triton X-100, 5 mM phosphate, and band 3 was eluted with 0.1 M glycine-HCl. Fractions of 300 μL were collected, and the protein composition of each of the collected fractions was analyzed by SDS-PAGE and Western blotting as previously reported.25 Fractions containing the pure band 3 protein were pooled and used for mass spectrometry analysis.
The N-terminal band 3 peptide of the control, heterozygous parents, and homozygous proband was prepared by digesting the membranes with trypsin (15 μg/mL) in 5 mM phosphate pH 8, 0.14 M NaCl for 2 hours at 4°C; the reaction was stopped by the addition of trypsin inhibitor. Digests were then centrifuged at 30 000 × g for 30 minutes, and the supernatant containing the N-terminal peptide was purified by affinity chromatography. Fractions containing the highest concentration of the peptide were analyzed by Western blotting to compare the apparent molecular weights of N-terminal peptides of normal and mutant band 3.
MALDI-TOF mass spectrometry analysis
Bands from SDS-PAGE were excised from the gel, triturated, and washed with water. Proteins in situ in the gel were reduced, S-alkylated with iodoacetamide, and digested with endoprotease AspN, as previously reported.35 On the basis of the primary structure of the protein, this protease was selected to be optimal for obtaining N-terminal sequence information on band 3. Digested aliquots were subjected to a desalting/concentration step using μZipTipC18 (Millipore, Bedford, MA) before MALDI-TOF mass spectrometry analysis. Peptide mixtures were loaded on the instrument target, using the dried droplet technique and α-cyano-4-hydroxy-cinnamic as matrix, and analyzed by using a Voyager-DE PRO mass spectrometer (Applied Biosystems, Framingham, MA). Spectra were acquired in the reflectron mode. Assignment of the recorded mass values to individual peptides was performed on the basis of their molecular mass and protease specificity.
Electron microscopy
An aliquot of proband red cells was fixed overnight in 25% glutaraldehyde and postfixed in 1% osmium tatroxide in 0.1 M sodium-cacodylate buffer for 2 hours. The sample was dehydrated in a graded ethanol series, dried at the critical point, coated with gold, and examined with a Cambridge Stereoscan 260 scanning electron microscope (SEM; Altran, Boston, MA) operating at 15 kV.
The image in Figure 3D was captured at 2170 ×, and the working distance (WD) was 8 mm. The total length of the images corresponds to 50 μm. The image was acquired by a ScanMaker 6800 scanner equipped with a transparency adapter (Microtek USA, Carson, CA), and the scanned images were processed using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Quantitation of anion transport
Washed erythrocytes were subjected to assays of unidirectional disodium [35 S] sulfate (Du Pont, Hertfordshire, United Kingdom) uptake in the absence or in the presence of 10 μM of the anion transport inhibitor di-isothiocyano-dihydrostilbene disulphonate (Sigma), as previously described.36 Each flux study using the proband's red cells was performed in parallel using red cells from the parents and from unrelated healthy subjects. The sulfate flux was expressed for a constant number of cells (1 × 1013 cells) in order to appropriately compare flux values for samples with variable mean corpuscular volume (MCV) values. The within and in-between run variation coefficients (CVs) for these measurements were less than 5%.
Phosphorylation of band 3
[γ32P] adenosine triphosphate (ATP) was purchased from Amersham (Arlington Heights, IL). Anti-P-Tyr antibody was purchased from ICN Biotechnology (Irvine, CA). Syk and Lyn were isolated from human red cells as previously described.11 Erythrocyte stimulation by diamide, cell-membrane preparation, and the sequential phosphorylation of band 3 catalyzed in vitro by Syk and Lyn tyrosine kinases were performed as previously described.11,37
Plasmodium falciparum invasion of red cells
Plasmodium falciparum invasion of red cells was measured in the first and second cycle of parasite growth essentially as reported in Cappadoro et al.38
Results
A new SLC4A1 mutation, band 3 Neapolis, causes aberrant mRNA splicing
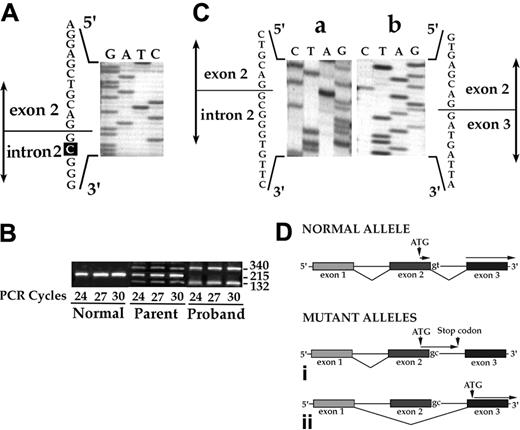
A 21-month-old child, the second son of a consanguineous marriage, was referred for the evaluation of a life-threatening nonimmune hemolytic anemia. The patient was transfusion dependent prior to undergoing splenectomy in 1999. At the present time no major clinical complications or thromboembolic events have been noted. The liver is enlarged, and the red blood cell indices are: red blood cell (RBC) count 3.0 × 109/mL, Hb 90 g/L, Htc 27%; MCV 91 fL, MCH 31 pg, MCHC 34 g/dL; and reticulocytes 10%. The blood smear shows a wide range of abnormal red-cell morphologies, including stomatocytes, spherocytes, and microcytes. Since a mild form of autosomal dominant HS due to band 3 deficiency was noted in both parents, the proband was analyzed for a possible SLC4A1 homozygous mutation. Direct nucleotide sequence of SLC4A1 gene showed a single base substitution (T → C) at position + 2 in the donor splice site of intron 2 (Figure 1A). The observed mutation abolishes a BspMI restriction site. The novel variant is designated as band 3 Neapolis.
RT-PCR analysis of reticulocyte mRNA was performed using primers located in SLC4A1 exons 1 and 3. A 215-bp product was obtained in the control, while 2 amplified fragments of 340 bp and 132 bp were noted in the patient (Figure 1B). Sequencing analysis revealed that the 340 bp resulted from the retention of intron 2, while the 132-bp fragment is the result of skipping of exon 2 (Figure 1C). All 3 amplified fragments (340, 215, and 132 bp) were noted in the parents (Figure 1B). Quantitative evaluation of the various transcripts using real-time PCR of reticulocyte RNA showed that in the proband, as expected, no normal mRNA was present, and each of the 2 mutant mRNAs was present at 11% to 13% of normal levels. In the heterozygous parents 52% of the message was of wild type, with 6% each of the 2 mutant transcripts. The retention of intron 2 leads to a premature termination of translation after the 19th triplet, while skipping of exon 2 results in the loss of the normal translation start site for erythroid band 3 (Figure 1D). Thus, the first alternative splicing event can generate only a very truncated form of altered erythroid band 3, while the second splicing event will generate a mutant form of band 3 with an altered N-terminus. Due to the location of the mutation, kidney band 3 expression will be unaffected and, consistent with this expectation, no defect in the distal urinary acidification in the homozygous child was noted (data not shown).
Genetic characterization of band 3 Neapolis mutation. (A) Genomic analysis. Sequence of PCR-amplified genomic DNA of proband band 3. The sequence shown encompasses the donor splice site of intron 2. Note that the second nucleotide of intron 2 was changed from T to C. (B) RT-PCR analysis. Reticulocyte cDNAs were PCR amplified using primers localized in exons 1 and 3. cDNA of a healthy subject generated a 215-bp band. Two bands of 340 bp and 132 bp were noted in the cDNA of the proband. In the heterozygous parent (the mother), all 3 amplified products were seen. A similar pattern also was seen using cDNA from the father (data not shown). (C) Sequencing of PCR products. The 2 abnormal amplified products obtained in the proband (panel 1B, 340 bp and 132 bp) were sequenced. The 340-bp fragment corresponds to mRNA in which intron 2 is retained, and the 132-bp fragment corresponds to mRNA in which exon 2 is skipped. (D) Schematic diagram of the different patterns of splicing. In contrast to normal splicing shown in the top panel, 2 aberrant splicing patterns are seen in band 3 Neapolis. Retaining of intron 2 leads to a premature termination of translation after the 19th triplet (i), and skipping of exon 2 leads to a loss of the normal translation start site for erythroid AE1 protein (ii).
Genetic characterization of band 3 Neapolis mutation. (A) Genomic analysis. Sequence of PCR-amplified genomic DNA of proband band 3. The sequence shown encompasses the donor splice site of intron 2. Note that the second nucleotide of intron 2 was changed from T to C. (B) RT-PCR analysis. Reticulocyte cDNAs were PCR amplified using primers localized in exons 1 and 3. cDNA of a healthy subject generated a 215-bp band. Two bands of 340 bp and 132 bp were noted in the cDNA of the proband. In the heterozygous parent (the mother), all 3 amplified products were seen. A similar pattern also was seen using cDNA from the father (data not shown). (C) Sequencing of PCR products. The 2 abnormal amplified products obtained in the proband (panel 1B, 340 bp and 132 bp) were sequenced. The 340-bp fragment corresponds to mRNA in which intron 2 is retained, and the 132-bp fragment corresponds to mRNA in which exon 2 is skipped. (D) Schematic diagram of the different patterns of splicing. In contrast to normal splicing shown in the top panel, 2 aberrant splicing patterns are seen in band 3 Neapolis. Retaining of intron 2 leads to a premature termination of translation after the 19th triplet (i), and skipping of exon 2 leads to a loss of the normal translation start site for erythroid AE1 protein (ii).
Band 3 Neapolis gene encodes a protein lacking the 11 N-terminal amino acids
As the aberrant splicing pattern of mutant gene suggested the generation of an altered protein in the red cells of the proband, we biochemically characterized band 3 from the proband's red cells. SDS-PAGE immunoblotting experiments showed that the mutant band 3 migrated slightly faster than the normal protein, suggesting a decreased molecular weight and that the band 3 content of the membrane was significantly reduced (data not shown). To substantiate the altered mobility of the mutant band 3, isolated red-cell membranes were digested with trypsin for 2 hours, and the supernatant of the digests was passed over a column of protein A sepharose cross-linked to a monoclonal antibody raised against the first 300 amino acids of band 3 to obtain purified N-terminal fragments. Western blot analysis of the various purified fragments clearly showed that the apparent molecular weight of the N-terminus of mutant band 3 was smaller than that of the normal (Figure 2A). Interestingly, the red-cell membranes from the heterozygous parents showed only the normal N-terminal fragment of the protein, implying an apparent lack of assembly of the mutant band 3 in these membranes (Figure 2A).
Although the DNA analysis precisely identified the mutation in the band 3 Neapolis gene, it did not provide definitive information on the nature of the truncated protein product. While the normal translation initiation ATG is deleted due to skipping of exon 2, there are at least 3 different ATG triplets in exon 3, which could function as the starting codon for the synthesis of mutant band 3 (Figure 2B). The first 2 ATGs are located at codons 11 and 12, and their use should result in the lack of either 10 or 11 amino acids at the N-terminus, respectively, while the use of the third ATG located at codon 31 would result in the loss of 30 amino acids at the N-terminus (Figure 2B).
To characterize the N-terminus of the mutant protein, normal and mutant band 3 were purified to homogeneity by immunoaffinity techniques, reduced, alkylated, and digested with endoprotease AspN. The resulting digests were subjected to peptide mapping analysis by MALDI-TOF mass spectrometry. As shown in Figure 2C, the spectra obtained from normal band 3 showed distinct peaks at m/z 3335.3031, 3351.3044, and 3367.3101 that correspond to the expected peptide10-37 and its oxidized products. While these signals are absent in the spectra of mutant protein, unique signals at m/z 1484.6521, 1696.8653, 3131.2913, and 3147.2927 were noted instead. On the basis of their molecular mass and protease specificity, these peaks can be assigned to a protein with a modified N-terminus and, in particular, to peptides (12-22), (12-24), (12-37), and oxidized (12-37) all bearing acetylated Met12 as the N-terminal residue. These findings enabled us to definitively demonstrate that the truncated mutant band 3 is devoid of the first 11 amino acids and that it is generated through the use of the secondATG in exon 3. Interestingly, the resulting N-terminal residue Met12 is acetylated, as is the case for normal Met1.Adefinitive confirmation for the absence of 11 N-terminal amino acids in the mutant band 3 was obtained by Western blot analysis using a monoclonal antibody directed against this region. As shown in Figure 2D, while Western blot analysis readily detected normal band 3 in membranes of red cells from healthy subjects and the parents, it failed to detect mutant band 3 in membranes of red cells from the proband.
Band 3 Neapolis protein analysis. (A) Immunoblotting of tryptic fragments of band 3. Red-cell membranes were digested by trypsin, and band 3 in the supernatant fraction was purified using an immunoaffinity column. The fraction eluted with 1 M glycine, pH 3.0 was analyzed by immunoblotting, using antibodies directed against band 3. The top panel shows the results from a short exposure of film, while the bottom panel represents a longer exposure. (B) The potential ATG start sites in exon 3. (C) Mass spectrometric analysis of normal and mutant band 3. Each of the derived signals was characterized on the basis of its molecular mass and protease specificity. Asterisks indicate peptides generated from endoprotease AspN autoproteolysis. (D) Immunoblotting of band 3. Equivalent amounts of red-cell membranes were analyzed by immunoblotting, using monoclonal antibodies directed against N-terminal amino acids (clone CDB3). Note complete absence of the band 3 epitope in red-cell membranes of the proband.
Band 3 Neapolis protein analysis. (A) Immunoblotting of tryptic fragments of band 3. Red-cell membranes were digested by trypsin, and band 3 in the supernatant fraction was purified using an immunoaffinity column. The fraction eluted with 1 M glycine, pH 3.0 was analyzed by immunoblotting, using antibodies directed against band 3. The top panel shows the results from a short exposure of film, while the bottom panel represents a longer exposure. (B) The potential ATG start sites in exon 3. (C) Mass spectrometric analysis of normal and mutant band 3. Each of the derived signals was characterized on the basis of its molecular mass and protease specificity. Asterisks indicate peptides generated from endoprotease AspN autoproteolysis. (D) Immunoblotting of band 3. Equivalent amounts of red-cell membranes were analyzed by immunoblotting, using monoclonal antibodies directed against N-terminal amino acids (clone CDB3). Note complete absence of the band 3 epitope in red-cell membranes of the proband.
Red cells of the homozygous band 3 Neapolis contain a reduced amount of the truncated protein and show altered membrane function
Following the detailed biochemical characterization of the mutant band 3, we investigated the effect of this genetic alteration on the various red-cell membrane and cellular functions. Band 3 content of red-cell membranes of the proband and parents was determined by 3 different methodologies, namely SDS-PAGE analysis, immunoblotting analysis, and eosine maleimide labeling of band 3 in intact red cells. Band 3 content of the membrane proteins assayed by SDS-PAGE or immunoblotting analysis was normalized to protein 4.1 and GPC contents of the membrane since the GPC complex (GPC, protein 4.1 and p55) is thought to be distinct and independent on the band 3 complex. Band 3 and protein 4.2 content of red-cell membranes of the proband was 12% ± 4% of normal, while that of red cells from the heterozygous parents was 82% ± 6% of normal (Figure 3A,B and data not shown). Eosine maleimide labeling showed that the band 3 content of proband red cells was 13% ± 3% of normal. GPA and CD47 content of the membrane also were markedly reduced to 11% ± 5% and 42% ± 10% of normal, respectively (Figure 3C). The membrane content of α-spectrin, β-spectrin, and ankyrin also was reduced but to a much lesser extent, 79% ± 3%, 81% ± 4.%, and 75% ± 6.% of normal, respectively (Figure 3A). A marked reduction in the membrane content of GAPDH (corresponding to band 6) also was noted (Figure 3A).
Studies on erythrocytes of the homozygous band 3 Neapolis. (A) SDS-PAGE analysis of red-cell membrane proteins. Membrane proteins of red cells were separated by SDS-PAGE41 and the gel stained by Coomassie blue G. (B) Immunoblotting of band 3. The membrane content of band 3 was determined by immunoblotting, using an anti-band 3 monoclonal antibody. Equivalent amounts of membrane proteins were loaded, based on the GPC content. (C) Immunoblotting of GPA and GPC and CD47. Red-cell membrane content of GPA, GPC, and CD47 was evaluated by immunoblotting, using monospecific antibodies. (D) Scanning electron micrograph of red cells of the proband. (E) Quantitation of anion transport. DIDS-sensitive unidirectional disodium [35S] sulfate uptake in red cells of normal and proband red cells.
Studies on erythrocytes of the homozygous band 3 Neapolis. (A) SDS-PAGE analysis of red-cell membrane proteins. Membrane proteins of red cells were separated by SDS-PAGE41 and the gel stained by Coomassie blue G. (B) Immunoblotting of band 3. The membrane content of band 3 was determined by immunoblotting, using an anti-band 3 monoclonal antibody. Equivalent amounts of membrane proteins were loaded, based on the GPC content. (C) Immunoblotting of GPA and GPC and CD47. Red-cell membrane content of GPA, GPC, and CD47 was evaluated by immunoblotting, using monospecific antibodies. (D) Scanning electron micrograph of red cells of the proband. (E) Quantitation of anion transport. DIDS-sensitive unidirectional disodium [35S] sulfate uptake in red cells of normal and proband red cells.
The consequences of the marked deficiency of band 3 on red-cell morphology were significant. Stomatocytes and spherocytes were the dominant morphologic features revealed by scanning electron microscopy of mutant red cells (Figure 3D). In addition, there were also a number of microspherocytes and membrane protrusions similar to those reported in band 3 null mouse red cells.15,16,39 Osmotic gradient ektacytometry40 confirmed a marked increase in osmotic fragility of the red cells as a consequence of a significant reduction in membrane surface area of mutant red cells (data not shown).
Using 4,4′-di-isothiocyano-dihydrostilbene-2,2-disulphonate (DIDS), a specific inhibitor of the anion transporter, we evaluated the anion transport activity of red cells with mutant band 3. The DIDS sensitive sulfate influx was reduced to 10% of normal in red cells from the proband and to 78% of normal in the heterozygous parents (Figure 3E and data not shown). As the extent of reduction in anion transport activity was proportional to the extent of decrease in membrane content of band 3, this finding implies that the mutant band 3 retained its ability to transport anions.
The 11 N-terminal amino acids of band 3 are critical for the binding of glycolytic enzymes and for the in vivo protein phosphorylation
The cytoplasmic domain of band 3 is the anchoring site for several red-cell glycolytic enzymes such as aldolase, GAPDH, and phosphofructokinase and is also a substrate for the protein tyrosine kinase, Syk.11,37 Several nuclear magnetic resonance (NMR) studies provided important insights into the structure and function of the 11 N-amino terminal amino acids in solution. It is inferred that the structure of this region forms a loop consisting of residues 4 through 9 in which the phenolic ring of Y8 is sandwiched between the methyl groups of L4 and M12.41,42 Phosphorylation of tyrosine 8 destabilizes the intermolecular protein-protein association, particularly between band 3 and glycolytic enzymes.43
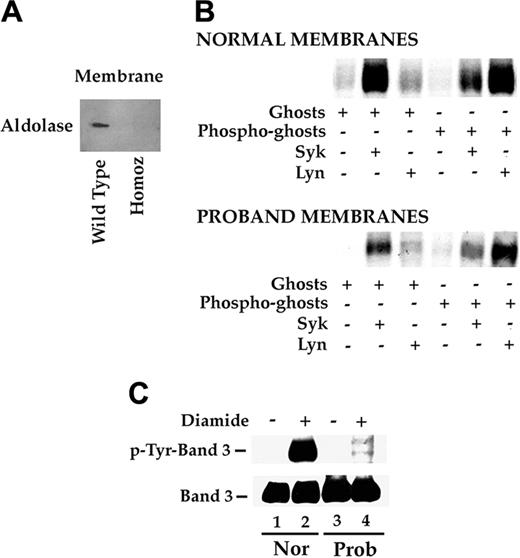
Since the naturally occurring band 3 Neapolis assembled on to the red-cell membrane lacks these N-terminal 11 amino acids, we explored the altered function of this domain in the context of intact red-cell membrane. The association of aldolase with band 3 in the membranes of red cells from the proband and from healthy subjects was assessed. In contrast to the strong association of aldolase with intact normal membranes, no aldolase could be detected in association with membranes of red cells from the proband (Figure 4A). This lack of association was not due to absence of aldolase in red cells, since the total aldolase content of cells was similar in red cells from healthy subjects and from proband (data not shown). This finding implies that N-terminal 11 amino acids are critical for association of aldolase with band 3 in intact red-cell membranes.
Band 3 Neapolis functions: binding of glycolytic enzymes and protein phosphorylation. (A) Aldolase binding to normal and mutant band 3. Red-cell membrane proteins from a healthy subject and the proband were separated by SDS-PAGE, transferred to a nitrocellulose paper, and analyzed by immunoblotting using an anti-aldolase antibody. (B) Sequential phosphorylation of band 3 in vitro. Red-cell membrane proteins were subjected to various treatments as outlined. Specifically, ghosts were incubated with Syk (lane 2) or with Lyn (lane 3) or without the enzymes (lane 1). Phosphate incorporation was evaluated on the basis of 32P signal (determined by exposing dried gels to x-ray films). In lanes 4, 5, and 6, membranes that were previously treated with Syk in the presence of unlabeled phosphate were incubated with Syk (lane 5) or with Lyn (lane 6) in the presence of labeled phosphate. Lane 4 is a control without addition of either Syk or Lyn. (C) Phosphorylation of band 3 in vivo. Red blood cells were incubated in the absence or presence of diamide. Cell membranes were isolated, solubilized, and submitted to SDS-PAGE followed by transfer to nitrocellulose. The upper part of blot shows immunostaining of band 3 with antiphosphotyrosine antibody. The filter was stripped and reprobed with anti-band 3 antibody (bottom panel). Nor indicates control membranes; Prob, proband membranes.
Band 3 Neapolis functions: binding of glycolytic enzymes and protein phosphorylation. (A) Aldolase binding to normal and mutant band 3. Red-cell membrane proteins from a healthy subject and the proband were separated by SDS-PAGE, transferred to a nitrocellulose paper, and analyzed by immunoblotting using an anti-aldolase antibody. (B) Sequential phosphorylation of band 3 in vitro. Red-cell membrane proteins were subjected to various treatments as outlined. Specifically, ghosts were incubated with Syk (lane 2) or with Lyn (lane 3) or without the enzymes (lane 1). Phosphate incorporation was evaluated on the basis of 32P signal (determined by exposing dried gels to x-ray films). In lanes 4, 5, and 6, membranes that were previously treated with Syk in the presence of unlabeled phosphate were incubated with Syk (lane 5) or with Lyn (lane 6) in the presence of labeled phosphate. Lane 4 is a control without addition of either Syk or Lyn. (C) Phosphorylation of band 3 in vivo. Red blood cells were incubated in the absence or presence of diamide. Cell membranes were isolated, solubilized, and submitted to SDS-PAGE followed by transfer to nitrocellulose. The upper part of blot shows immunostaining of band 3 with antiphosphotyrosine antibody. The filter was stripped and reprobed with anti-band 3 antibody (bottom panel). Nor indicates control membranes; Prob, proband membranes.
We next explored whether Syk tyrosine kinase could recognize the truncated band 3 as a substrate in vitro by assessing the ability of Syk purified from normal erythrocytes to phosphorylate band 3. As shown in Figure 4B, in spite of the absence of 1 of 2 phosphorylation sites (Tyr8), the mutant protein was phosphorylated in vitro by the exogenously added purified enzyme (lane 2). The extent of Syk-phosphorylation, determined using equivalent amounts of normal and mutant band 3, showed that the phosphorylation of the mutant protein was approximately 50% of normal (data not shown), consistent with the presence of a single phosphorylation site in the mutant protein at Tyr21. Furthermore, we were able to document that the presence of the single phosphotyrosine docking site at Tyr 21 is sufficient for the interaction of band 3 with the SH2 domain of Lyn and subsequent phosphorylation of the truncated band 3 (Figure 4B, lanes 3, 6). To establish whether these in vitro phosphorylation events can be recapitulated in intact cells, we stimulated red cells from the proband and from healthy subjects with diamide to induce tyrosine phosphorylation of band 3.37 As shown in Figure 4C, while band 3 was tyrosine phosphorylated in normal red cells, no phosphorylation could be detected in proband's red cells. These findings imply an absolute requirement for Tyr8 for band 3 phosphorylation in vivo in intact membranes.
Malaria parasite life cycle is altered in band 3 Neapolis erythrocytes
Previous studies using various peptides corresponding to the extracellular loops of band 3 have suggested an important role for this surface protein in red-cell invasion by P falciparum.44 To further substantiate a requirement for band 3 in parasite invasion, we assayed the ability of 2 different strains of P falciparum to invade red cells of the proband with markedly reduced membrane content of band 3. As shown in Table 1, invasion and maturation of the parasites were markedly inhibited in red cells of the proband. The decreased invasion and maturation could be seen at 24 hours (early stage during first cycle of invasion), at 48 hours (late stage during first cycle of invasion), and at 72 hours (early stage during second cycle of invasion). The ability of parasites to invade red cells of the parents was not significantly different from that of normal red cells (Table 1). These findings establish an important role for band 3 in red-cell invasion by P falciparum.
P falciparum invasion of band 3 Neapolis red cells
. | Parasitemia (%) . | . | . | ||
|---|---|---|---|---|---|
. | Invasion, 24 h . | Maturation, 48 h . | Reinvasion, 72 h . | ||
| Control | 5.80 ± 0.71 (100) | 3.65 ± 1.63 (100) | 5.20 ± 1.27 (100) | ||
| Mother | 4.35 ± 1.48 (75) | 2.55 ± 1.34 (70) | 4.20 ± 0.42 (81) | ||
| Proband | 0.75 ± 0.07 (13) | 0.20 ± 0.00 (5.5) | 0.10 ± 0.00 (1.9) | ||
. | Parasitemia (%) . | . | . | ||
|---|---|---|---|---|---|
. | Invasion, 24 h . | Maturation, 48 h . | Reinvasion, 72 h . | ||
| Control | 5.80 ± 0.71 (100) | 3.65 ± 1.63 (100) | 5.20 ± 1.27 (100) | ||
| Mother | 4.35 ± 1.48 (75) | 2.55 ± 1.34 (70) | 4.20 ± 0.42 (81) | ||
| Proband | 0.75 ± 0.07 (13) | 0.20 ± 0.00 (5.5) | 0.10 ± 0.00 (1.9) | ||
Cultures were inoculated by the addition of purified schizont-stage parasitized normal red cells (purity more than 95%) to test red cells suspended in growth medium (hematocrit, 0.5%). Two strains of parasite (Palo alto and FCR3) were used in each case. After 24 hours (ring stage of first cycle), 48 hours (trophozoite stage of the first cycle), and 72 hours (ring stage of second cycle) of culture, slides were prepared and stained with Diff-Quik parasite stain, and stage-dependent parasitemia was assessed by microscopic inspection. Values are expressed as percent of parasitized red cells during the course of the experiment. In parentheses, growth is normalized assuming 100% growth for the control.
Discussion
In the present study, we have identified and characterized a new variant of human red-cell AE1 protein, designated band 3 Neapolis, that provides novel insights into the role of the 11 N-terminal amino acids in regulating protein function. A single base substitution (T → C) at position + 2 in the donor splice site of intron 2 was identified as the mutation responsible for band 3 Neapolis. The mutation causes altered splicing of AE1 gene with the consequent generation of 2 different mature band 3 mRNAs, one that includes intron 2 and the other that skips exon 2. Both transcripts are produced, since the GU to GC mutation does not completely abrogate its ability to function as a donor splice site. In fact, a minority of genes (approximately 1%) use the GC donor splice site instead of the canonical GU donor splice site.45 Only the shorter mRNA that skips exon 2 is translated into a protein that is assembled into the red-cell membrane. Mass spectrometric analysis showed that the membrane-assembled mutant band 3 lacked the first 11 N-terminal amino acids due to elimination of the normal start site in exon 2 and the use of an alternate start site in exon 3. Importantly, the red-cell membrane content of the mutant band 3 is dramatically decreased to 12% of normal. As such, the red cells of the proband exhibit both a qualitative defect and a quantitative deficiency of band 3. These band 3 defects thus account for the severe transfusion-dependent hemolytic anemia noted in the proband prior to splenectomy. Following splenectomy, the proband became transfusion independent with a moderately compensated hemolytic anemia. The quantitative deficiency of band 3 can account for the observed reduction in anion transport and loss of red-cell membrane surface area. As a result of the location of the mutation, only the synthesis of red-cell band 3 protein was affected, while the synthesis of the kidney form of band 3 was uncompromised, accounting for the normal renal function noted in the proband.
The mechanism responsible for the marked deficiency of mutant band 3 is not completely clear. Quantitative PCR showed a marked decrement in the number of mutant transcripts and a strong correlation between the amount of mutant mRNA and the membrane content of truncated protein. Thus, either a lower rate of transcription of the mutant gene or an intrinsic instablity of the aberrant mRNA is likely to account for marked deficiency of the mutant protein in red cells. It is interesting, in this context, that red cells of heterozygous parents do not express detectable levels of band 3 Neapolis protein, in spite of the fact that they synthesize measurable amounts of altered mRNA. Similar findings have been reported for other eAE1 mutations in the heterozygous state.46,47
A major advantage of our analysis compared to earlier studies on red cells with quantitative deficiencies of band 3 is that for the first time we are able to assess the function of the N-terminus of band 3 in the context of an intact membrane. Our in vivo demonstration that the 11 N-terminal amino acids form the major binding site of aldolase and that other glycolitic enzymes (eg, GAPDH) employ different band 3 binding domains validates that this domain of band 3 is critical for recruiting aldolase to the red-cell membrane. These findings lend strong support to studies using normal red cells.48
A second significant finding is the validation of a critical role for the 11 N-terminal amino acids in the sequential Tyr-phosphorylation of band 3 triggered by diamide stimulation.11,37 The observation that the truncated band 3 can be phosphorylated by Syk and thereby promote the recruitment of Lyn tyrosine kinase in vitro but not in vivo has major structural and functional implications. Our findings provide strong evidence to support a critical role for Tyr8 and its neighboring amino acids in the overall control of the reversible band 3 phosphorylation in intact membranes. The observation also implies that the structural alterations involving both band 3 and the overall membrane architecture play a key role in phosphorylation of band 3 in vivo.
The finding that the red cells of the proband are resistant to malaria infection supports a pivotal role for band 3 in parasite invasion and maturation. As band 3 has been identified to be an important receptor for the merozoite surface protein MSP1, it is likely that deficiency of band 3 is responsible for the reduced invasion. However, we cannot rule out the potential contribution of an altered metabolic and/or redox state of red cells in defective invasion and maturation of the parasites in band 3-deficient red cells.
In addition to marked deficiency of band 3, red cells from the proband also exhibit deficiencies in protein 4.2 (10% of normal), glycophorin A (11% of normal), and CD47 (47% of normal). These findings are similar to those previously reported for the only case of complete band 3 deficiency in humans, band 3 Coimbra,20 in which there is complete deficiency of protein 4.2 and CD47, while the membrane content of glycophorin A is 29% of normal.14 As the major interacting site for CD47 in mature human red cells is protein 4.2,49 the presence of 10% of protein 4.2 could account for the significant difference in CD47 of the proband's red cells compared with band 3 Coimbra erythrocytes. The presence of similar amounts of band 3 and protein 4.2 in membranes of red cells of the proband implies that the membrane content of protein 4.2 is exclusively determined by its interaction with band 3 and that the 11 N-terminal amino acids of band 3 are not involved in the binding of protein 4.2.50 Furthermore, the presence of significant amounts of ankyrin in band 3 Neapolis membranes implies that the N-terminal 11 amino acids of band 3 are not required for band 3-ankyrin interaction.12
Despite the marked deficiency of band 3, spectrin, ankyrin, and protein 4.1, the content of red cells from proband is very similar to normal, and the membrane skeletal architecture also appears to be normal. These findings further support the notion that band 3 is not essential for the stable biogenesis of membrane skeleton.15-17
In summary, we identified a novel mutant band 3 (band 3 Neapolis) that lacks the first 11 N-terminal amino acids in association with hereditary spherocytosis. Detailed studies have enabled us to show that the mutant band 3 fails to bind aldolase but does bind other glycolitic enzymes and that the 11 N-terminal amino acids are critical for tyrosine phosphorylation of membrane-associated band 3. Furthermore, we established a key role for band 3 in malarial parasite invasion of human red cells.
Prepublished online as Blood First Edition Paper, August 25, 2005; DOI 10.1182/blood-2005-07-2806.
Supported by grants from PRIN (Progetti di Rilevante Interesse Nazionale), FIRB (Fund for Investments in Basic Research), “Progetto di Ricerca di Ateneo,” II Università di Napoli, Regione Campania, Italy, and Associazione Italiana Ricerca sul Cancro (AIRC).
S.P. and F.D.R. designed all the phases of the research, analyzed the data, and wrote the manuscript. A.B. purified band 3 and performed several biochemical and immunochemical experiments. A.S. performed the MALDI-TOF mass spectrometry studies. L.D.F. carried out the analysis of anion transport activity. A.M.B. and A.D.-D. performed band 3 phosphorylation experiments. F.T. performed the malaria transport experiments. V.N. carried out the real-time PCR investigations. B.N. was involved in the conception of the study and in the design of some genetic experiments. E.M.d.G., M.L.C., F.R., and A.I. contributed to the genetic characterization of band 3 Neapolis. V.Z. critically reviewed and revised the manuscript. V.P. followed the clinical aspect of the study. W.A. performed some of the biochemical and immunochemical studies. P.L. supervised some of the biochemical and immunochemical studies and revised the manuscript. M.N. supervised the osmotic gradient ektacytometric experiments, critically reviewed all the experiments, and wrote the paper.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Figure 3. Studies on erythrocytes of the homozygous band 3 Neapolis. (A) SDS-PAGE analysis of red-cell membrane proteins. Membrane proteins of red cells were separated by SDS-PAGE41 and the gel stained by Coomassie blue G. (B) Immunoblotting of band 3. The membrane content of band 3 was determined by immunoblotting, using an anti-band 3 monoclonal antibody. Equivalent amounts of membrane proteins were loaded, based on the GPC content. (C) Immunoblotting of GPA and GPC and CD47. Red-cell membrane content of GPA, GPC, and CD47 was evaluated by immunoblotting, using monospecific antibodies. (D) Scanning electron micrograph of red cells of the proband. (E) Quantitation of anion transport. DIDS-sensitive unidirectional disodium [35S] sulfate uptake in red cells of normal and proband red cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/13/10.1182_blood-2005-07-2806/2/m_zh80240588270003.jpeg?Expires=1765908326&Signature=lebHKfGwOXtqAkflzhYeGG~82AqSfQ97JKPaVSMRqvzjjM0mnr~7RQQf4MobLpPLvY4nY7HmNtzxMUQF4sQPUfHepS0fbxYYfCSM8n8Wv6Qt1a358sJPJa6cqGORN4Z~~3~2RU5ll9pBuUu2FN1fEPepJwIaXTiDLDF7Y-nhY6F0T2F71HL4VkjqV~v-DuOC266fdM5UhPFtswHJmhsc-ye7Ro9UoeGw3eWxlwXHnVTzvB5SK5WGNLboyt86KHLDZjJsYa9NM04Ydct4dAMYDhEnSjmUWQ6j58uz9mDjARVvloTy-4h9Ajnkm2cgVIBjHKbH2tjpWujgCE~k3nbr0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal