Comment on Yang et al, page 584
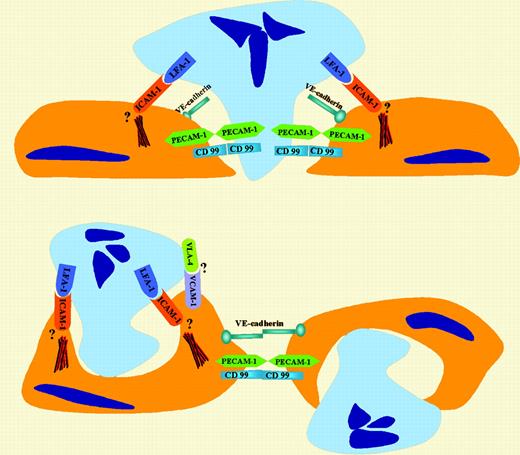
ICAM-1 controls neutrophil transendothelial migration (TEM) via both transcellular (nonjunctional) and paracellular (junctional) pathways.
Extravasation of blood leukocytes is critical for immune surveillance and a first step in the development of inflammation and atherosclerosis. While several events involved in neutrophil TEM, such as selectin-mediated rolling and integrin-mediated firm adhesion, are well characterized, there is controversy over the route taken by polymorphonuclear leukocytes (PMNs) as they migrate across the endothelium. As summarized in the figure, two major models for leukocyte TEM have been proposed. One involves a traditional paracellular pathway that has been the focus of the majority of transmigration studies using in vitro systems.1 By this pathway, neutrophils migrate across the endothelial monolayer at cell-cell junctions. This model envisions a combination of localized endothelial-presented chemoattractants and adhesion molecules, such as platelet endothelial cell adhesion molecule-1 (PECAM-1), CD99, and junctional adhesion molecules, playing critical roles in driving diapedesis. The other route of migration is the transcellular pathway that involves TEM of leukocytes at nonjunctional locations, or “through” the endothelial cell. This model is supported by previous electron microscopic observations of neutrophil transmigration in in vivo models of inflammation.2 Interestingly, as highlighted in this paragraph, intercellular adhesion molecule-1 (ICAM-1) plays a key role in regulating PMN transmigration by both pathways. In this issue, Yang and colleagues demonstrate that neutrophils can use both transcellular and paracellular pathways to migrate across cultured endothelial monolayers under conditions of flow. In their study, Yang et al measured neutrophil adhesion and migration directly under live-cell fluorescence microscopy using tumor necrosis factor-α (TNF-α)–activated human umbilical vein endothelium (HUVEC) or a HUVEC cell line transfected with ICAM-1GFP (green fluorescent protein). The authors clearly demonstrate that ICAM-1 concentrated at PMN-endothelial contacts, forming ringlike structures around cell-cell contacts. This observation was largely in agreement with the results of Carman and Springer,3 who reported high densities of ICAM-1 and vascular cell adhesion molecule-1 (VCAM-1) in “cuplike” structures surrounding migrating cells. Both of these studies highlight the importance of high-density ICAM-1–lymphocyte function-associated antigen-1 (LFA-1) interactions as providing the driving force that promotes PMN transcellular migration. It appears that LFA-1– ICAM-1 interactions facilitate PMN extension of pseudopods into endothelial cells (ECs) at nonjunctional locations and initiate diapedesis. By mutating ICAM-1, Yang et al further demonstrate that the cytoplasmic tail ofFIG1 ICAM-1 is necessary for its function in modulating neutrophil-endothelial interactions. Although the mechanism is still not clear, ligation of endothelial ICAM-1 by LFA-1 likely results in ICAM-1–meditated signaling that leads to cytoskeleton changes and leukocyte TEM.4
Role of ICAM-1 in regulating both paracellular and transcellular pathways of neutrophil TEM.
Role of ICAM-1 in regulating both paracellular and transcellular pathways of neutrophil TEM.
Not only did the authors find that the surface density of ICAM-1 is important in regulating transcellular migration, but they observed that the shape of endothelial cells played a role in determining the migration route of PMNs. Comparison of PMN migration across ICAM-1GFP–transfected and control endothelial monolayers revealed that endothelial cells with polygonal morphology appeared to promote nonjunctional arrest and transcellular migration. Interestingly, polygonal endothelial cells with high-density expression of ICAM-1 and VCAM-1 have been identified in locations with high predilection for atherosclerosis in both rabbit and mouse models of atherogenesis.5 It is thus likely that these conditions (high density of ICAM-1 and polygonal endothelial cell shape) might support a high ratio of transcellular to paracellular TEM and contribute to disease pathogenesis. Another interesting point from this study was that T lymphocytes, unlike neutrophils, do not migrate via transcellular mechanisms even under conditions of increased ICAM-1 expression. Future in vitro studies are needed to determine if TEM of monocytes is similar to that observed for PMNs or T lymphocytes. ▪