Comment on Ng et al, page 1601
Using round-bottom wells, a brief centrifugation step of a defined number of human embryonic stem cells reproducibly yields embryoid bodies termed “spin EBs” and efficient hematopoiesis.
The potential of human embryonic stem cells (hESCs) for future use in the clinic has been emphasized extensively. In addition, these cells provide a model system for studying mechanisms of early human embryonic development, which, otherwise, would not be possible.1 Critical to exploiting the potential of this model is the reproducible formation of embryoid bodies (EBs); these are aggregates of hESCs that recapitulate many of the processes that occur in the early embryo, including the development of the primitive ectoderm giving rise to the primary germ layers and differentiated cell types including hematopoietic lineages. Thus far, the production of EBs using hESCs has been limited by the lack of reproducibility, owing to the fact that hESCs survive poorly as single cells.
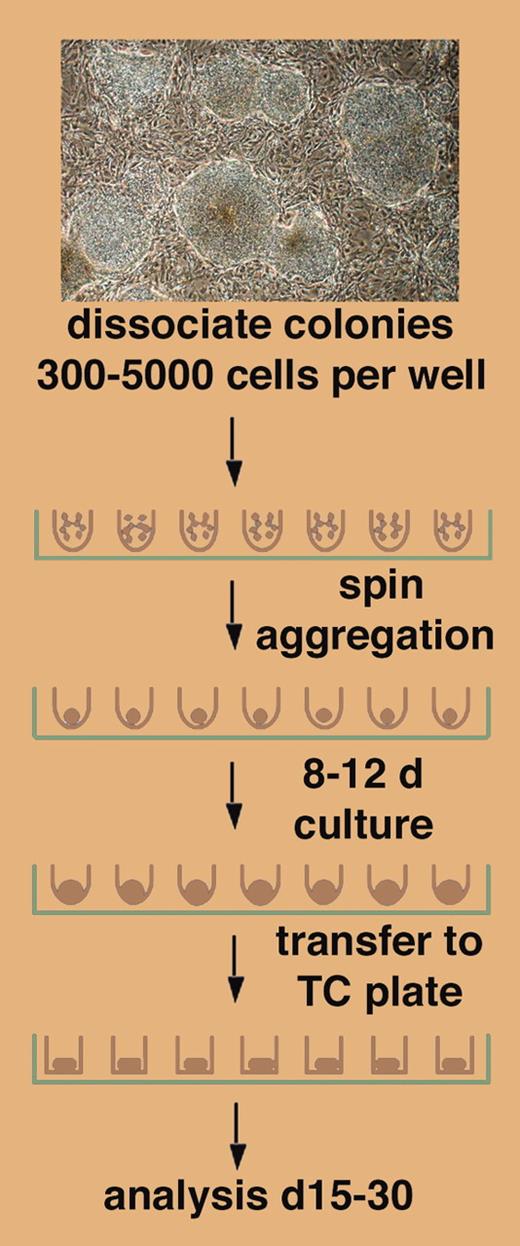
A novel method for EB formation from hESCs is presented by Ng and colleagues in this issue of Blood, demonstrating in a simple but elegant approach that a brief step of centrifugation of a defined number of hESCs in a round-bottom well of a 96-well plate leads to forced cell aggregation and EB formation (see figure). The success of this approach depends on the use of round-bottom, low-attachment wells, as “spin EBs” (as the authors have coined these aggregates) do not form in flat-bottom wells. Ng et al further show that spin EBs form in defined, serum-free medium, and that they can, following the initial aggregation phase, be exposed to specific cytokines to induce hematopoietic differentiation. Spin EBs form by forced aggregation of defined numbers of hESCs. The authors exploit this by evaluating hematopoiesis from spin EBs by showing that a minimal input number of 500 hESCs is required. Using 1000 hESCs in spin EBs, both myeloid and erythroid differentiation reach plateaus; but with 3000 hESCs, the myeloid lineage still is at plateau while the erythroid lineage has declined below detection.FIG1
Differentiation of defined numbers of hESCs as spin EBs. See the complete figure in the article beginning on page 1601.
Differentiation of defined numbers of hESCs as spin EBs. See the complete figure in the article beginning on page 1601.
The authors further use the reproducible properties of spin EBs to explore their gene expression profile over a period of 26 days. The genes analyzed include, among others, the pluripotent cell and germ line–specific transcription factor, Oct4, as well as the mesoderm genes, Brachy and Mixl1, and the hemangioblast kinase, Flk1. Based on the observed profile, Ng et al suggest that spin EBs pass through a transient phase of mesoderm induction followed by gastrulation, when hemangioblasts first appear at the posterior end of the primitive streak.2 Except for Oct4 expression, which in the embryo is limited to the inner cell mass and the germ line, the gene expression profile in spin EBs resembles the pattern described in the embryo. However, persistent Oct4 expression may suggest that pluripotent cells from the germ-line lineage continue to be present in these cellular aggregates, a finding that might be exploited in the future. In addition, more detailed gene expression profiling of spin EBs is likely to provide important information as to what culture conditions may be suitable for the differentiation of specific lineages. Finally, as spin EBs are generated by forced aggregation of defined numbers of cells, it will now be possible to assess how differentiation depends on hESC numbers and spin EB size. ▪