Comment on Zaza et al, page 1778
Accumulation of TGN by ALL cells may be affected by other drugs (methotrexate) and by gene expression profiles. Rational pharmacology is becoming more complex.
Cure of children with acute lymphoblastic leukemia (ALL) depends upon complex interactions between the leukemia cells, host factors, and treatments. One approach to improve prognosis would increase intensity of treatment for all patients until everyone is cured. The alternative is to dissect these variables in order to better understand what is necessary to cure each individual.FIG1
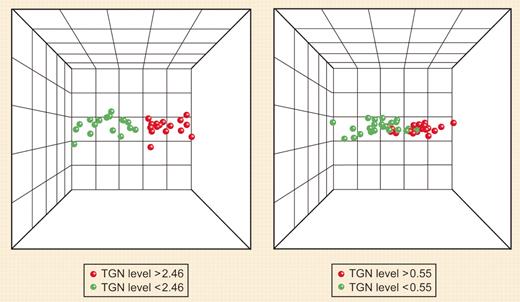
PCA of ALL cells with high (red) and low (green) TGN concentrations based on the selected probe sets. The left-hand side represents treatment with MP alone, and the right-hand side, treatment with MP + MTX. See the complete figures in the article beginning on page 1778.
PCA of ALL cells with high (red) and low (green) TGN concentrations based on the selected probe sets. The left-hand side represents treatment with MP alone, and the right-hand side, treatment with MP + MTX. See the complete figures in the article beginning on page 1778.
Thiopurines have been a key component of ALL treatment for 50 years. Factors that alter their effectiveness include drug dose, drug schedule, concomitant medications, patient compliance, drug absorption, and genetic polymorphisms.
In this issue of Blood, Zaza and colleagues present elegant analyses of factors that affect accumulation of thioguanine nucleotides (TGNs) in leukemia cells of children with newly diagnosed ALL after in vivo exposure to intravenous mercaptopurine (MP) alone or MP plus methotrexate (MTX). This enabled the authors to bypass several problems inherent in previous studies: in vitro drug exposure and use of red cell TGN levels as a surrogate for blast cell accumulation of TGNs. TGN levels were higher in patients who received MP alone than in those treated with MP plus MTX (note, however, that there was substantial overlap between these 2 groups). Thus, the clinical synergy (or additive effects) of MP plus MTX cannot be explained simply by intracellular drug levels.
Zaza et al then identified genes that predicted accumulation of TGNs in leukemia cells; a set of 60 genes predicted TGN accumulation after MP alone and a nonoverlapping set of 75 genes predicted TGN accumulation after MP plus MTX (see figure). The relevance of several of these genes was further documented by in vitro studies.
These data raise as many questions as answers. MP and MTX were given sequentially: would results have been the same if they were given concurrently? TGN accumulation was measured in naive cells: how would uptake be affected by prior MP exposure? Why was there no overlap of genes predicting TGN accumulation after MP alone and MP plus MTX? What fraction of the accumulation of TGNs is explained by each gene (or groups of genes)? What is the contribution of each identified gene to TGN accumulation in a specific patient as opposed to an overall group of patients with ALL? How well will accumulation of TGN (or a specific gene profile) correlate with event-free survival?
We are beginning to learn more and more about less and less. What are we to do with this wealth of detail? Is it science in a bottle or can it be applied to patients in the clinic? This challenge, though daunting, is better than stumbling in the dark. ▪