Two independent observations published in this issue of Blood confirm the validity of using strategies that manipulate apoptotic programs in the treatment of hematologic malignancies.
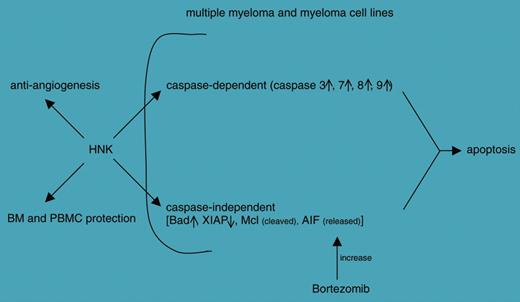
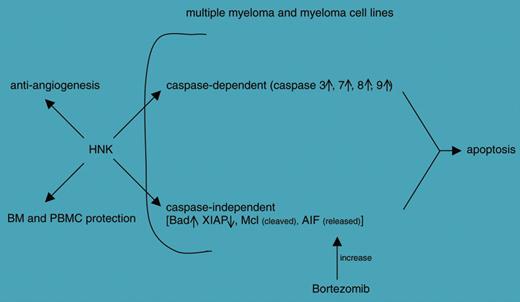
The paper by Ishitsuka and colleagues explores the therapeutic potential of honokiol (HNK), a compound purified from the root of Magnolia grandiflora and a weapon used in traditional Chinese and Japanese medicine. Their analysis concludes that HNK induces apoptosis in multiple myeloma (MM) lines as well as in cells purified from patients. The apoptotic effects are induced through caspase-dependent and -independent pathways. Furthermore, HNK is clearly endowed with antiangiogenic properties and apparently spares bone marrow and derived cells from its cytotoxic effects. Another characteristic of HNK identified by this study is the ability to operate (eg, via release of apoptosis-inducing factor [AIF]) in synergy with the proteasome inhibitor bortezomib, suggesting that combination therapies may be successful (see figure).
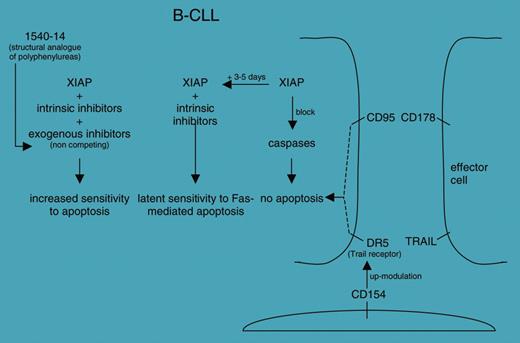
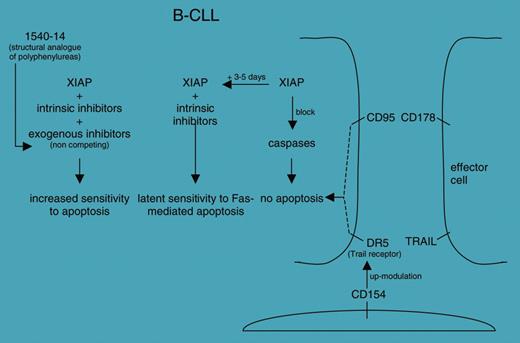
The second paper, by Kater and colleagues, summarizes the results obtained with reagents that selectively bind and inactivate the X-linked inhibitor of apoptosis protein (XIAP). The group used an innovative approach (ie, mixture-based combinatorial libraries) to select polyphenylurea analogs with good potential in inhibiting XIAP but with improved drug-like characteristics. The work is part of an ongoing effort aimed at identifying the mechanisms responsible for the early resistance to CD95-mediated apoptosis frequently observed in vitro in chronic lymphocytic leukemia (CLL) cells (see figure).
These papers share the common view of the induction of apoptosis as a fundamental step in the eradication of MM and CLL. In both instances, the authors stress the wide efficacy of the compounds, their safety, and the appealing possibility of combining them with other known and effective therapies to maximize clinical benefits. However, what has clearly surfaced in hematology is the need for a more accurate molecular characterization of patients affected by diseases grouped under the same name. And CLL is a clear example of how more accurate prognosis estimates and therapeutic plans can be made if the patients are stratified according to biologic and molecular parameters.1 Hence, it would clinically and culturally add to the Kater et al paper to see whether there are different effects of the 1540-14 compound on the different subgroups of patients, in order to identify those who will benefit most from these therapies. It is likely that this issue also holds true for MM.FIG1,FIG2
Schematic representation of the effects induced by honokiol (HNK) on different targets; see Ishitsuka et al.
Schematic representation of the effects induced by honokiol (HNK) on different targets; see Ishitsuka et al.
Proposed roles of the 1540-14 compound in finely tuning the increased sensitivity to apoptosis in B-CLL cells; see Kater et al.
Proposed roles of the 1540-14 compound in finely tuning the increased sensitivity to apoptosis in B-CLL cells; see Kater et al.
These papers differ in the approach that led to the identification of HNK and of the selective XIAP inhibitors and are conceptually different. The observations made by Ishitsuka et al are the last in a stream of literature that has picked up a great deal from the armamentarium of Chinese traditional medicine: the most successful example is differentiation therapy, which changed the fate of acute promyelocytic leukemia patients. Kater et al used de novo–synthesized compounds designed to inactivate specific proteins involved in the modulation of the apoptotic cascade.
A limit might be represented by the notion that the models used may not accurately mirror the effects on tumor cells in vivo. Indeed, in addition to the issue of genetic drift in vitro, one may accurately consider the additional influence provided by the surrounding environment in terms of cells and soluble factors. It has now become quite clear that anticancer agents should be tested on target tumor cells as well as on normal effectors that orchestrate the antitumoral defense.2 It would be of interest for basic and clinical scientists working on MM and CLL to determine the potential effects on innate and specific immunity at different time intervals when these drugs will enter preclinical trials. Special attention should be given to the ability to modulate the intricate network of surface molecules regulating cell-to-cell contacts.3
The help and assistance of S. Deaglio, T. Vaisitti, E. Ferrero, and E. V. Tibaldi are gratefully acknowledged. ▪