Abstract
T cells are important in the immune response to malaria, both for their cytokines and their help for antibody production. To look at the relative importance of these roles, a T-cell receptor (TCR) transgenic mouse has been generated carrying a TCR specific for an epitope of the merozoite surface protein 1 (MSP-1) of the malaria parasite, Plasmodium chabaudi. In adoptive transfer experiments, malaria-specific CD4+ T cells expand and produce interferon γ (IFN-γ) early in infection, but the population contracts quickly despite prolonged persistence of the parasite. MSP-1-specific CD4+ cells can protect immunodeficient mice from lethal infection; however, the parasite is only completely cleared in the presence of B cells showing that T helper cells are critical. Levels of malaria-specific antibody and the speed of their production clearly correlate with the time of resolution of infection, indicating that a critical threshold of antibody production is required for parasite clearance. Furthermore, T cells specific for a shed portion of MSP-1 are able to provide help for antibody to the protective region, which remains bound to the infected erythrocyte, suggesting that MSP-1 has all of the components necessary for a good vaccine. (Blood. 2005;106:1676-1684)
Introduction
T helper cells are essential for a protective immune response to the blood stages of the rodent malaria parasite, Plasmodium chabaudi chabaudi (AS).1 There is debate, however, over the extent to which T cells protect via Th1 cytokine-mediated mechanisms or by the antibodies that they help to produce. Both mechanisms dominate the T-cell response to P chabaudi in turn, with an early Th1-type cytokine response, which switches later in infection to one that provides effective help for malaria-specific antibody production and produces less interferon γ (IFN-γ).2,3 Increased T-helper cell activity in the later stages3 is clearly beneficial because experiments in B cell-deficient mice demonstrate that B cells and antibodies are required for complete clearance of parasites,4-6 although the requirements and specificity of T-cell help and antibody for rapid clearance or immunity to reinfection are not known.
To determine the precise role of malaria-specific T helper cells and the potential of an important vaccine antigen, merozoite surface protein 1 (MSP-1), in the protection from and clearance of malaria infection, we have generated a T-cell receptor (TCR) transgenic mouse with a TCR specific for P chabaudi MSP-1. This molecule is expressed on the invasive merozoite surface,7 and its C-terminal domain can induce a protective immune response.8-11 However, this domain is not efficiently processed by antigen-presenting cells and is a less effective inducer of CD4+ T-cell responses than other parts of MSP-1.10,12,13 For more effective vaccination strategies it would be important to know whether CD4+ T-cell help can be generated from other parts of MSP-1, which are more readily processed and presented on antigen-presenting cells. The MSP-1-specific TCR of our transgenic mouse, which recognizes a peptide within the soluble and readily processed 37/39-kDa fragment (Figure 2A) in the context of I-Ed,12,14 allows us to determine whether such help can be provided. Using this malaria-specific B5 TCR transgenic mouse, we show that anti-MSP-1 T cells can protect mice from a lethal P chabaudi infection. Furthermore, anti-MSP-137/39 T cells provide efficient help for the antibody response to the protective C-terminal fragment of MSP-1 even though they recognize an epitope within another region of MSP-1. Using this new model, we show that a critical level of protective antibody, as well as both effector T and B cells, are required for rapid control of parasitemia.
Materials and methods
Generation of transgenic mice
Transgenic mice were generated using V(D)J segments of the TCR-α and -β genes of a B5 CD4+ T-cell hybridoma, specific for P chabaudi MSP-1 (amino acids 1151-1171) in the context of I-Ed.15 Polymerase chain reaction (PCR) of cDNA from the hybridoma using a panel of primers to conserved regions of the Cα and Cβ TCR and TCR V genes16 revealed that Vβ8.1 and both Vα1 and Vα2 were transcribed. TCR Vβ8.1 Vα1 and Vα2 genes were identified using primers: Vα1 GATCGAATTCCACCATGAAATCCTTTA and TGAAGATATCTTGGCAGGTGA; Vα2, GATCGAATTCCACCATGGACAAGAT and TGAAGATATCTTGGCAGGTGA; and Vβ8.1, GATCCTCGAGCCACCATGGGTTCCAGACTCTTCTTTG and GGGTGAGCCCTCTGGCCACTT. The α chain 0.8-kb PCR product was cloned into pBluescript, with EcoRI and EcoRV, cloned into p14α2-AR,17 and then cloned into the BamHI/SalI site of transgenic expression vector, pHSE3′.18,19 XhoI and BanII sites were incorporated into the Vβ8.1 product and cloned into pBluescript. This fragment was moved to p142β8, and finally pHSE3′. Transgenic expression vectors were linearized with XhoI and injected into the pronucleus of fertilized eggs of (BALB/c × (CBA×C57Bl/6)) F1 mice as described.20 Both Vβ8/Vα2 and Vβ8/Vα1 lines were made and tested for specificity; only the T cells from Vβ8/Vα2 mice made any detectable response to MSP-1. Transgenic mice expressing Vα2/Vβ8 were backcrossed to BALB/c for 13 generations (B5 TCR Tg) and typed using the Vα2, Vβ8.1 primers mentioned.
Mice and parasites
Female BALB/c (MRC strain) and DO11.10 TCR Tg mice21 were maintained in the breeding facilities of the National Institute of Medical Research (NIMR). BALB/c Vβa mice22 were a kind gift from Dr Alexandra Livingstone (University of Rochester, Rochester, NY). BALB/c nu/nu mice were acquired from Harlan (Oxfordshire, United Kingdom), and BALB/c rag2-/- mice23 were a gift from Dr Anton Rolink (Basel Institute for Immunology, Basel, Switzerland). Mice, 5 to 8 weeks old, were infected with blood stages of P chabaudi chabaudi (AS) and monitored by Giemsa-stained blood films as described.24,25
Antibodies and flow cytometric analysis
Single-cell suspensions of thymus, spleen, and lymph nodes were incubated in phosphate-buffered saline (PBS) containing 2% fetal bovine serum (FBS) and 0.1% sodium azide with anti-CD16/32 followed by appropriate combinations of phycoerythrin (PE)-, fluorescein isothiocyanate (FITC)-, biotin-, or allophycocyanin (APC)-conjugated antibodies: Vα2 (B20.1), Vβ8.1/Vβ8.2 (MR5-2), Vα8.3 (KT50), CD4 (RM4-5), CD8 (53-6.7), CD45RB (16A), and CD69 (H1.2F3) from BD Biosciences, Cambridge Biosciences (Oxford, United Kingdom). The second-step reagent streptavidin-peridinin chlorophyll protein (PerCP; BD Biosciences) was used. For intracellular cytokine staining, cells were stimulated for 4 to 6 hours with phorbol 12,13-dibutyrate or phorbol myristate acetate (PMA; 50 ng/mL; Sigma, St Louis, MO), ionomycin (500 ng/mL; Sigma), and brefeldin A (10 μg/mL; Sigma). After surface labeling, cells were then fixed with 2% paraformaldehyde in PBS overnight, permeabilized in BD PharMingen (San Diego, CA) Perm/Wash buffer 15 minutes and washed twice, and then incubated for 40 minutes with anti-IFN-γ-APC (XMG1.2; BD Biosciences). Cells were washed three times in Perm/Wash buffer and resuspended in staining buffer. Data were analyzed on a FACScalibur using CellQuest Pro (BD Biosciences) or FloJo (Tree Star, Stanford, CA) software.
Recombinant malaria proteins
MSP-1 fragments (Figure 2A) in the vector pMalCR1 (New England Biolabs, Bishops Stortford, United Kingdom) were expressed and purified as described.26 MSP-11658-1746/MSP-119/21 was cloned into pPIC9K, expressed in Pichia pastoris SMD1168, and purified as described.10
MSP11658-1746 was linked to MSP1900-1507 by incubating 24 μM MSP11658-1746 with 8 μM MSP1900-1507 in 5 mM glutaraldehyde for 30 minutes at 4°C. The reaction was stopped by addition of 1 M ethanolamine, pH 10, to a final concentration of 1% and dialyzed into PBS. Linked protein was observed on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel. Recombinant P chabaudi adami apical membrane antigen 1 (AMA-127 was kindly provided by Robin Anders (La Trobe University, Melbourne, Victoria, Australia).
In vitro stimulation of transgenic CD4+ T cells
Lymph node cells or CD4+ T cells (4 × 104) isolated from spleens and lymph nodes of B5 Tg or BALB/c mice using magnetic-activated cell sorting (MACS) columns (Miltenyi Biotec, Surrey, United Kingdom), were cocultured with different antigens and either 5 × 103 bone marrow-derived dendritic cells (DCs) derived as described,28,29 or 1 × 105 irradiated (3000 rad) normal BALB/c splenocytes in a total volume of 225 μL Iscove medium (Sigma) containing 10% FBS, 2 mM l-glutamine, 0.5 mM sodium pyruvate, 100 U penicillin, 100 μg streptomycin, and 50 μM 2-mercapto-ethanol (Gibco, Invitrogen, Paisley, Scotland) in round-bottom 96-well plates (Nunc, Roskilde, Denmark). The following were used as antigens: (1) the specific (B5) peptide, MSP-11157-1171 (ISVLKSRLLKRKKYI); (2) an irrelevant peptide (B7), MSP-11690-1709 (RCEKDTEATCSINKGGCDPS); (3) recombinant protein fragments of MSP-1; (4) P chabaudi-infected erythrocytes prepared described previously30 ; or (5) uninfected erythrocytes. CFSE (5-(and-6)-carboxyfluorescein diacetate) (Molecular Probes, Eugene, OR) was used at 1 μM, and cells were labeled for 15 minutes at 37°C and cultured for 5 days. The B5 T-cell hybridoma12 was used as a positive control for the response to MSP-11151-1171.
T-cell proliferation was measured by incorporation of 3H-thymidine for 12 hours after 4 days, and interleukin 2 (IL-2) production was measured in the supernatants of the cultures using CTLL-2 cells.31 Results were expressed as stimulation indices (SIs) where SI = cpm obtained in presence of test antigens/cpm obtained in presence of medium, maltose-binding protein, or normal erythrocytes at equivalent concentrations as indicated. IFN-γ was measured in 48-hour supernatants by enzyme-linked immunosorbent assay (ELISA).32
Adoptive transfer into Vβa, nu/nu, and RAG-/- mice
Splenic CD4+ T and CD19+ B cells were purified using anti-CD4 or anti-CD19 microbeads on the autoMACS (> 95% purity, Miltenyi Biotec). For RAG-/- cell transfers, a further purification (> 99%) was performed on a high-speed cell sorter (Cytomation MoFlo, Fort Collins, CO) gating on PE-labeled CD4+ T cells and CD19+ B cells. Cells were washed thrice and resuspended in 0.09% saline at 5 × 107/mL. CD4+ cells (5 × 106) were injected intravenously into mice that were infected with 104 to 105P chabaudi-infected erythrocytes intraperitoneally. Mice receiving DO11.10 cells were immunized with 50 μg chicken ovalbumin (OVA; Sigma) intraperitoneally at the time of cell transfers. Immune B cells for RAG-/- transfers were generated by infecting BALB/c mice with 105P chabaudi twice 2 months apart and then, 1 week before transfer immunizing with MSP11658-1746. RAG-/- mice received CD19+ B cells or CD4+ purified cells (or both) intravenously. Some groups were immunized 1 to 2 days later, with 100 μg MSP11658-1746-MSP1900-1507 in 200 μL monophosphoryl lipid A (MPL)+trehalose dicorynomycolate (TDM) RIBI adjuvant (Sigma). Mice were infected with 104 parasites intravenously the next day. Mice were humanely killed at intervals during the infection and spleen and lymph node cells were analyzed by flow cytometry. Survival curves were tested for significance using the log-rank comparison (Prism, GraphPad, San Diego, CA).
Malaria-specific and MSP-1-specific ELISA
Malaria-specific and MSP-1-specific antibodies were measured by ELISA as described.15 A lysate of P chabaudi blood-stage parasites, recombinant MSP11-672, MSP1581-921, MSP1900-1507, or MSP11508-1766, and recombinant AMA-1 were used as the coating antigens (2-5 μg/mL in PBS).15 Maltose-binding protein at the same concentration was used as a negative control. Results are expressed as arbitrary units (AU) of specific antibody based on a standard hyperimmune plasma (1000 AU).15 Results are plotted as geometric means, with SEM of 7 animals at each point. Statistics were performed in Prism (GraphPad) using the Student t test, with P equal to .05 considered significant.
Results
Generation of TCR transgenic mice recognizing an epitope of MSP-1
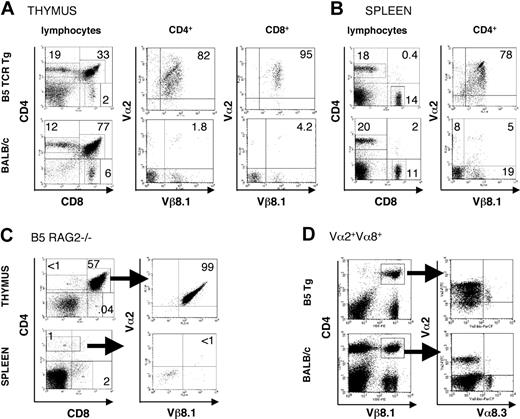
Because the 37/39-kDa region of P chabaudi MSP-1 was found to stimulate a strong CD4+ T-cell response in a primary infection of BALB/c mice,15 we selected a T-cell hybridoma specific for a peptide in this region with I-Ed for the generation of a CD4+ TCR transgenic mouse. The MSP1-specific TCR of the B5 hybridoma15 is composed of Vβ8.1 and Vα2 chains. Thymii and spleens of BALB/c B5 Tg mice were analyzed for the expression of the transgenic TCR (Figure 1A-B). Thymic and splenic cellularity of transgenic BALB/c mice were similar in B5 TCR Tg and BALB/c mice. As is commonly seen in CD4+ TCR Tg mice,21 the ratio of single-positive CD4+/CD8+ is greatly increased (from 2 to 9.5) in the CD4+ B5 TCR Tg mice, whereas increased numbers of thymocytes are negative for both CD4 and CD8, suggesting a challenge to thymic selection (Figure 1A). In the spleen, CD4+ and CD8+ patterns of B5 Tg were similar to wild type (Figure 1B). Approximately 80% of spleen and thymic CD4 and CD8 T cells were positive for both transgenic TCR chains, whereas BALB/c mice had only 5% of CD4+ T cells with this TCR.
Intrathymic and peripheral expression of Vα2/Vβ8.1 Tg TCR chains and a second Vα chain. Thymocytes (A) or splenocytes (B) from B5 TCR Tg mice or BALB/c were analyzed by flow cytometry for expression of CD4, CD8, Vβ8.1, and Vα2. Expression of CD4 and CD8 on gated lymphocytes within thymus and spleen is shown as well as expression of the transgenic TCR chains on CD4+ cells and CD8+ cells (thymus) and CD4+ cells (spleen). The numbers indicate the percentage of positive cells within the regions shown. Data are representative of 5 experiments. (C) Thymocytes and splenocytes of the B5 TCR Tg mice on RAG2-/- background were stained for CD4, CD8, Vα2, and Vβ8.1, Vβ8.2. Expression of the transgenic TCR is shown on CD4+CD8+ thymocytes and on CD4+ splenic T cells. CD4+ cells in the spleen were stained for Vα2/Vβ8. (D) Splenocytes of B5 TCR Tg and BALB/c control mice were stained for CD4, Vβ8.1. Expression of the transgenic α chain Vα2, as well as another TCR α, Vα8.3 is shown on gated CD4, Vβ8.1 cells. The numbers indicate the percent of positive gated cells. Arrows indicate gate shown in following plot. The data are representative of 3 independent experiments.
Intrathymic and peripheral expression of Vα2/Vβ8.1 Tg TCR chains and a second Vα chain. Thymocytes (A) or splenocytes (B) from B5 TCR Tg mice or BALB/c were analyzed by flow cytometry for expression of CD4, CD8, Vβ8.1, and Vα2. Expression of CD4 and CD8 on gated lymphocytes within thymus and spleen is shown as well as expression of the transgenic TCR chains on CD4+ cells and CD8+ cells (thymus) and CD4+ cells (spleen). The numbers indicate the percentage of positive cells within the regions shown. Data are representative of 5 experiments. (C) Thymocytes and splenocytes of the B5 TCR Tg mice on RAG2-/- background were stained for CD4, CD8, Vα2, and Vβ8.1, Vβ8.2. Expression of the transgenic TCR is shown on CD4+CD8+ thymocytes and on CD4+ splenic T cells. CD4+ cells in the spleen were stained for Vα2/Vβ8. (D) Splenocytes of B5 TCR Tg and BALB/c control mice were stained for CD4, Vβ8.1. Expression of the transgenic α chain Vα2, as well as another TCR α, Vα8.3 is shown on gated CD4, Vβ8.1 cells. The numbers indicate the percent of positive gated cells. Arrows indicate gate shown in following plot. The data are representative of 3 independent experiments.
Transgenic T cells have a second TCR but are specific for MSP-1
When B5 TCR Tg mice were crossed onto a RAG2-/- background to eliminate endogenous TCR rearrangement, virtually no single-positive thymocytes or peripheral T cells expressing the transgene were found (Figure 1C), suggesting that the Vα2/Vβ8.1 TCR was not sufficient for positive selection in the thymus. Because the TCRα chain is incompletely allelically excluded,33 and because the B5 hybridoma transcribed 2 α chains, we investigated the possibility that a second α chain was required for thymic selection, as described previously.34-36 The Vα2/Vβ8+ Tg T cells in the periphery of the Tg RAG+/+ mice were therefore analyzed for a second Vα chain using a Vα8.3 antibody as representative of the Vα repertoire, because no antibodies are available that recognize other TCR Vα expressed on BALB/c T cells. In B5 Tg spleens, all Vα8+ T cells were also Vα2+, whereas in BALB/c, there were very few double positives. This indicates that for the transgenic cells the Vα8/Vβ8 TCR may have supported positive selection for these cells (Figure 1D), and that each transgenic T cell selected to survive in the B5 TCR Tg thymus is equipped with a second TCR α.
To confirm that B5 Tg cells respond to the same epitopes as the precursor hybridoma, and that expressing a second TCR does not change their specificity, we stimulated them in vitro with specific peptide in several forms and measured cytokines in the supernatant (Figure 2B-E). B5 TCR Tg CD4+ cells and B5 hybridoma responded specifically to the B5-specific peptide (MSP-11157-1171; Figure 2B,E), a recombinant fragment of MSP-1900-1507 containing the epitope (Figure 2C), and to P chabaudi (AS)-infected red blood cells (RBCs; Figure 2D). Control stimulations with irrelevant peptide (B7, MSP11690-1709), recombinant fragment (MSP11508-1766), and uninfected RBCs (Figure 2B-E) showed minimal responses, and naive BALB/c T cells had only a low response to P chabaudi-infected erythrocytes (Figure 2B-E; BALB/c).We also observed (Figure 2F) B5 TCR Tg cells proliferating by flow cytometry in cells loaded with the fluorescent dye CFSE, which showed dilution of the dye over a period of 5 days' exposure to MSP-1 B5 peptide. The cells also up-regulated CD25 and CD44 and down-regulated CD45RB and CD62L as expected for activated T cells.
In vivo response of transgenic T cells to P chabaudi infection
With this transgenic mouse, we were able to follow the immune response of malaria-specific CD4+ cells in vivo. CD4+ T cells from B5 Tg mice were transferred into BALB/c Vβa mice, which have a natural deletion in the TCR Vβ locus eliminating expression of all Vβ8 family members, thus facilitating detection of the transgenic Vβ8+ T cells by flow cytometry (fluorescence-activated cell sorting [FACS]) using anti-Vβ8 antibody. The adoptive hosts were infected with P chabaudi. This infection in BALB/c Vβa mice was similar to that in normal BALB/c mice (see Hensmann et al10 ) and transfer of either B5 Tg or polyclonal BALB/c CD4+ cells did not significantly affect the course of infection (data not shown).
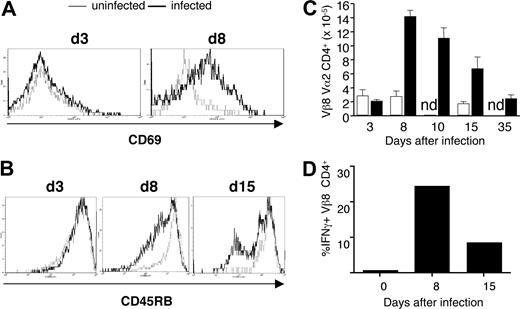
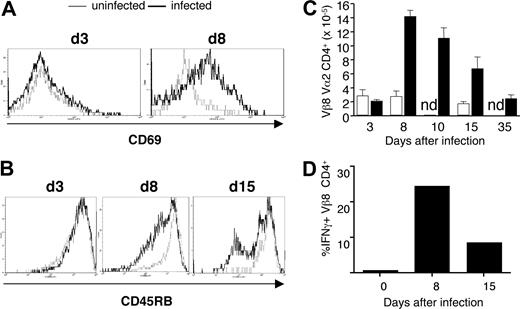
The activation marker, CD69, was up-regulated on the majority of transgenic CD4+ cells early in infection at day 8 (Figure 3A), as was the activation marker IL-2 receptor α (IL-2Rα; CD25; data not shown). The expression of CD45RB was down-regulated later in infection (Figure 3B). By day 8, the population of transferred transgenic T cells had expanded approximately 7-fold and contracted to original levels by day 35 after infection (Figure 3C). No changes in activation markers or cell numbers were seen on transgenic cells in uninfected recipient Vβa mice (Figure 3A-C). Activation and proliferation of B5 Tg T cells were accompanied by production of IFN-γ (Figure 3D). A relatively large proportion of malaria-specific CD4+ T cells secreted IFN-γ on day 8 (25%) and day 15 (15%).
Transgenic CD4+ T cells respond to MSP-1 on the parasite. (A-D) In vitro stimulation of anti-MSP-1 transgenic T cells with peptide, recombinant protein, and parasites. (A) A diagrammatic representation of MSP-1 showing its proteolytic cleavage into 6 fragments during invasion of RBCs. The fragments shed on invasion of the RBC are indicated by the arrow. The 4 recombinant fragments, which cover the full length of the protein, are shown below. The location of the B5 peptide, to which the Tg TCR is specific, and a control peptide, B7, are indicated by black bars. (B-F) CD4+ T cells from B5 TCR transgenic mice. Results are shown as counts per minute of 3H-thymidine incorporation measured for proliferation of CTLL-2 cells incubated with supernatant of various combinations of B5 CD4+ cells with antigen-presenting cells. B5 Tg and BALB/c T cells were incubated with (B) bone marrow dendritic cells (BMDCs) and specific MSP-1 B5 peptide or nonspecific B7 peptide (1024 nM), (C) irradiated splenocytes and recombinant fragments of MSP1 (44 pmol), and (D) BMDCs and parasitized RBCs at the schizont stage (50:1 RBC/DC). The B5 hybridoma was also incubated with the protein or parasite as a positive control and BALB/c CD4+ T cells are shown as a negative control. (E) BMDCs and B5 or control B7 peptide (100 nM) were added to CD4+ B5 or littermate control T cells. Forty-eight hours later, the concentration of IFN-γ in the supernatant was measured by ELISA. Means and SEM of triplicate wells are shown. These data are representative of at least 5 independent experiments. (F) Proliferation and activation of B5 TCR Tg cells is shown after 5 days in culture. Collagenase-treated B5 spleens were labeled with CFSE and incubated with B5 peptide for 5 days. The cells were then stained for activation markers CD25, CD44, CD45RB, and L-selectin (CD62L).
Transgenic CD4+ T cells respond to MSP-1 on the parasite. (A-D) In vitro stimulation of anti-MSP-1 transgenic T cells with peptide, recombinant protein, and parasites. (A) A diagrammatic representation of MSP-1 showing its proteolytic cleavage into 6 fragments during invasion of RBCs. The fragments shed on invasion of the RBC are indicated by the arrow. The 4 recombinant fragments, which cover the full length of the protein, are shown below. The location of the B5 peptide, to which the Tg TCR is specific, and a control peptide, B7, are indicated by black bars. (B-F) CD4+ T cells from B5 TCR transgenic mice. Results are shown as counts per minute of 3H-thymidine incorporation measured for proliferation of CTLL-2 cells incubated with supernatant of various combinations of B5 CD4+ cells with antigen-presenting cells. B5 Tg and BALB/c T cells were incubated with (B) bone marrow dendritic cells (BMDCs) and specific MSP-1 B5 peptide or nonspecific B7 peptide (1024 nM), (C) irradiated splenocytes and recombinant fragments of MSP1 (44 pmol), and (D) BMDCs and parasitized RBCs at the schizont stage (50:1 RBC/DC). The B5 hybridoma was also incubated with the protein or parasite as a positive control and BALB/c CD4+ T cells are shown as a negative control. (E) BMDCs and B5 or control B7 peptide (100 nM) were added to CD4+ B5 or littermate control T cells. Forty-eight hours later, the concentration of IFN-γ in the supernatant was measured by ELISA. Means and SEM of triplicate wells are shown. These data are representative of at least 5 independent experiments. (F) Proliferation and activation of B5 TCR Tg cells is shown after 5 days in culture. Collagenase-treated B5 spleens were labeled with CFSE and incubated with B5 peptide for 5 days. The cells were then stained for activation markers CD25, CD44, CD45RB, and L-selectin (CD62L).
MSP1-specific transgenic T cells respond to P chabaudi in vivo after adoptive transfer into BALB/c Vβa mice. CD4+ T cells from B5 TCR Tg (5 × 106) were purified and transferred into Vβa mice (which lack endogenous Vβ8) and infected with 1 × 105P chabaudi parasites. Histogram overlays represent CD69 (A) and CD45RB (B) expression on gated CD4+ Vα2/Vβ8+ splenocytes from uninfected (thin line) and infected (thick line) recipient mice on the indicated days after infection. (C) Expansion and contraction of anti-MSP-1 T cells during infection. The histogram shows the number of anti-MSP-1 B5 CD4+ cells in the spleen, determined from the percentage of CD4+Vα2+Vβ8+ cells and the number of total viable splenocytes. (D) B5 Tg cells make IFN-γ during infection. The mean percentage (of 3 mice) of CD4+Vβ8+Vα2+ cells staining positive for IFN-γ on stimulation. SEMs are less than 10% of the means.
MSP1-specific transgenic T cells respond to P chabaudi in vivo after adoptive transfer into BALB/c Vβa mice. CD4+ T cells from B5 TCR Tg (5 × 106) were purified and transferred into Vβa mice (which lack endogenous Vβ8) and infected with 1 × 105P chabaudi parasites. Histogram overlays represent CD69 (A) and CD45RB (B) expression on gated CD4+ Vα2/Vβ8+ splenocytes from uninfected (thin line) and infected (thick line) recipient mice on the indicated days after infection. (C) Expansion and contraction of anti-MSP-1 T cells during infection. The histogram shows the number of anti-MSP-1 B5 CD4+ cells in the spleen, determined from the percentage of CD4+Vα2+Vβ8+ cells and the number of total viable splenocytes. (D) B5 Tg cells make IFN-γ during infection. The mean percentage (of 3 mice) of CD4+Vβ8+Vα2+ cells staining positive for IFN-γ on stimulation. SEMs are less than 10% of the means.
Both B and T cells are required to control and reduce parasitemia
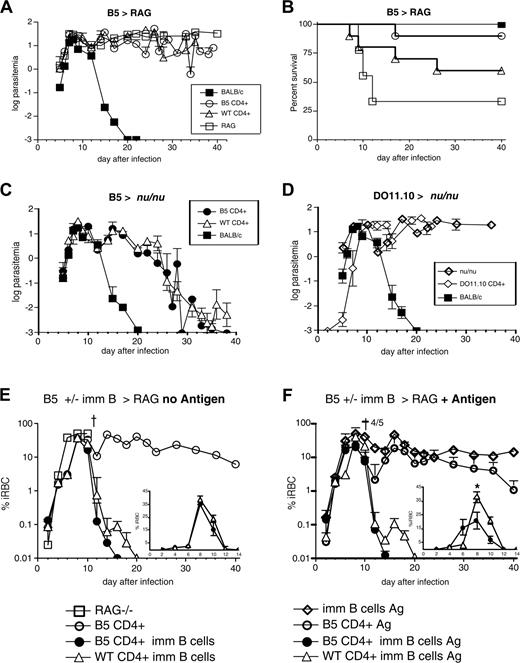
To determine whether MSP-1-specific transgenic CD4+ cells could control a P chabaudi infection, purified CD4+ B5 Tg cells were transferred into T cell-deficient BALB/c nu/nu mice or B and T cell-deficient RAG2-/- mice, which were infected with P chabaudi. Unreconstituted RAG2-/- mice were not able to clear parasitemia, unlike BALB/c wild-type control mice, which cleared infection by day 20 (Figure 4A).
Although neither wild-type nor MSP-1-specific CD4+ cells brought about clearance of parasites, transfer of T cells into RAG2-/- mice improved their survival. Ninety percent of the RAG-/- mice that received B5 T cells survived compared with 60% of the recipients of BALB/c T cells, whereas 60% of unreconstituted RAG2-/- mice died by day 15 of infection (Figure 4B). Although the difference between B5 and wild-type CD4+ cells does not reach significance, the survival curves of RAG mice with or without B5 T cells are significantly different using the log-rank comparison of the curves (P < .05).
To investigate whether transgenic T cells together with naive B cells reduced parasitemia, B5 Tg and BALB/c CD4+ T cells were transferred into T cell-deficient BALB/c nu/nu mice, which were infected with P chabaudi 1 day later. In contrast to RAG-/- mice, all nu/nu mice receiving B5 Tg CD4+ cells survived and reduced parasitemias to subpatent levels on a par with recipients of polyclonal BALB/c CD4+ T cells (Figure 4C). Unreconstituted nu/nu mice showed a chronic parasitemia throughout the course of infection and a variable proportion (approximately 40%) died between day 9 and day 35 (Figure 4D).
Control of infection was not due to nonspecific downstream effects of priming CD4+ T cells in vivo because OVA-specific DO11.10 TCR Tg CD4+ cells that were transferred into nu/nu mice and primed in vivo with OVA protein 1 day before infection with P chabaudi had no effect on the clearance of parasite, despite their activation, and all recipient mice died within 24 days (Figure 4D).
Activation of T and B cells reduces parasitemia and increases rate of clearance of infection
Transfer of CD4+ T cells into nu/nu mice demonstrated the requirement for T cells in reducing parasitemia. However, parasites were not cleared until 28 to 30 days after infection and there was no effect on magnitude of the peak parasitemia. To determine whether immune B cells would reduce the parasitemia earlier or improve the rate of clearance, naive B cells of the nu/nu mouse were replaced by immune B cells, which contain a higher frequency of malaria-specific cells, as well as activated and memory B cells. In these experiments, B cells isolated from mice that had recovered from 2 P chabaudi infections and been additionally primed with MSP119/21 were cotransferred with naive CD4+ T cells from either B5 transgenic or BALB/c mice into RAG-/- mice, which were infected 2 days later with P chabaudi (Figure 4E).
MSP-1 TCR Tg cells protect RAG-/- mice from death and clear P chabaudi parasitemia only when B cells are present. (A) B5 and BALB/c CD4+ cells were purified (> 99%CD4+) by high-speed flow cytometry and transferred into RAG-/- mice, which were then infected with 104P chabaudi parasites. Parasitemia is shown as percent infected RBCs on a logarithmic (log10) scale. (B) Mortality is shown as percent survival. RAG mice survive significantly better than RAG mice with B5 T cells P < .05 (log-rank test). (C) B5 and BALB/c CD4+ cells were purified by high-speed flow cytometry and transferred into nu/nu mice, which were then infected with 104P chabaudi parasites. (D) A total of 5 × 106 anti-OVA TCR Tg, DO11.10, CD4+ cells were purified and transferred into nu/nu mice; 50 μg OVA was given at the same time and the mice were infected with 104P chabaudi. The courses of infection and mortality in BALB/c mice are shown for comparison. The infections shown are the mean parasitemias of 5 to 7 mice and are representative of 2 to 3 independent experiments. Error bars represent SEs of geometric means. (E-F) B5 Tg and BALB/c CD4+ cells were purified (> 99%) by high-speed flow cytometry and transferred into RAG2-/- mice, which were then infected with 104P chabaudi parasites. (F) Some mice were additionally immunized with the T-cell antigen (MSP1900-1507) covalently linked to a protective B-cell antigen (MSP11658-1746 or MSP121) the day after T-cell transfer and 2 days before infection. Parasitemia is shown on a logarithmic (log10) scale, and data are shown as geometric means of 5 to 8 mice and SEMs. Inset graphs in panels E and F represent the same parasitemia curves on a linear scale to day 14, with maximum of 45% iRBC on y-axis. * indicates significance of P < .05 by the Student t test.
MSP-1 TCR Tg cells protect RAG-/- mice from death and clear P chabaudi parasitemia only when B cells are present. (A) B5 and BALB/c CD4+ cells were purified (> 99%CD4+) by high-speed flow cytometry and transferred into RAG-/- mice, which were then infected with 104P chabaudi parasites. Parasitemia is shown as percent infected RBCs on a logarithmic (log10) scale. (B) Mortality is shown as percent survival. RAG mice survive significantly better than RAG mice with B5 T cells P < .05 (log-rank test). (C) B5 and BALB/c CD4+ cells were purified by high-speed flow cytometry and transferred into nu/nu mice, which were then infected with 104P chabaudi parasites. (D) A total of 5 × 106 anti-OVA TCR Tg, DO11.10, CD4+ cells were purified and transferred into nu/nu mice; 50 μg OVA was given at the same time and the mice were infected with 104P chabaudi. The courses of infection and mortality in BALB/c mice are shown for comparison. The infections shown are the mean parasitemias of 5 to 7 mice and are representative of 2 to 3 independent experiments. Error bars represent SEs of geometric means. (E-F) B5 Tg and BALB/c CD4+ cells were purified (> 99%) by high-speed flow cytometry and transferred into RAG2-/- mice, which were then infected with 104P chabaudi parasites. (F) Some mice were additionally immunized with the T-cell antigen (MSP1900-1507) covalently linked to a protective B-cell antigen (MSP11658-1746 or MSP121) the day after T-cell transfer and 2 days before infection. Parasitemia is shown on a logarithmic (log10) scale, and data are shown as geometric means of 5 to 8 mice and SEMs. Inset graphs in panels E and F represent the same parasitemia curves on a linear scale to day 14, with maximum of 45% iRBC on y-axis. * indicates significance of P < .05 by the Student t test.
Immune B cells clearly accelerated the clearance of parasites, and transgenic CD4+ T cells were more effective in promoting this B cell-dependent clearance of parasites. RAG-/- mice receiving T cells along with immune B cells cleared their infections 10 to 14 days faster than was observed in the nu/nu mice. Strikingly, those RAG-/- mice receiving B5 TCR Tg CD4+ cells and immune B cells cleared parasitemia 4 days earlier than recipients of wild-type T cells (Figure 4E). However, the replacement of naive B cells by immune B cells still did not reduce the magnitude of peak parasitemia, suggesting that further activation of CD4+ T and B cells might be required to bring about overall reduction of the challenge infection.
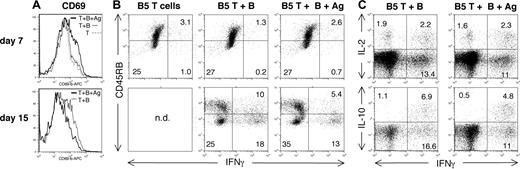
When RAG-/- recipients were additionally immunized at the time of transfer of T and B cells with MSP1900-1507 (contains B5) covalently linked to MSP11658-1746 (induces protective B-cell responses) (antigen, Figure 4F), the time taken to clear parasitemia was further reduced in mice receiving transgenic B5 cells (Figure 4F), and parasitemia was controlled 6 days sooner than in mice receiving wild-type T cells. Activation of transgenic cells and immune B cells after transfer not only reduced parasitemia faster, but also significantly reduced peak parasitemia in recipient RAG-/- mice (Figure 4F, inset), suggesting that an effective immune response was accelerated by the activation step, such that it could now inhibit early parasite growth as well as enhance the clearance. In fact, it can be seen in Figure 5A with CD69 up-regulation that immunization with antigen induces early activation of the B5 T cells by day 7, whereas both groups down-regulate CD45RB by day 15 in RAG mice. Because cytokines are critical in parasite clearance and regulation of pathology in the infection, we have analyzed cytokine production of anti-MSP-1 CD4+ T cells in infection by intracellular cytokine staining and flow cytometry. Transferred B5 T cells can make IFN-γ, IL-2, and IL-10 (Figure 5B-C) at a very high level by day 15.
Antibody is produced faster by immune B cells, and its production is enhanced by activation of malaria-specific T-cell help
The requirement for B cells for elimination of parasites by B5 Tg CD4+ cells infers that these anti-MSP-1 Tg T cells are functional T helper cells for malaria antibody production. However, the epitope recognized by B5 Tg T cells is not within the protective C-terminal fragment of MSP-1 (MSP-119/21). The B5 peptide is part of the p37/39 fragment, which is cleaved off the merozoite surface prior to invasion of the erythrocyte and released into the serum.7 It was therefore important to determine whether B5 Tg CD4+ cells could provide help not only to B cells specific for the cleaved fragment containing the B5 epitope but also for the membrane-bound C-terminal region or other malaria proteins.
B5 T cells make cytokines to parasite infection in RAG mice. B5 and BALB/c CD4+ cells were purified (> 99% CD4+) by high-speed flow cytometry and transferred into RAG-/- mice, which were then infected with 104P chabaudi parasites. Some mice were immunized with the T-cell antigen (MSP1900-1507) covalently linked to a protective B-cell antigen (MSP121). (A-C) On days 7 and 15, splenocytes were analyzed by flow cytometry for surface expression of activation markers CD45RB and CD69 and intracellular IFN-γ, IL-2, and IL-10. Cells shown are lymphocyte gated CD4+Vβ8+ B5 T cells. n.d. indicates not determined.
B5 T cells make cytokines to parasite infection in RAG mice. B5 and BALB/c CD4+ cells were purified (> 99% CD4+) by high-speed flow cytometry and transferred into RAG-/- mice, which were then infected with 104P chabaudi parasites. Some mice were immunized with the T-cell antigen (MSP1900-1507) covalently linked to a protective B-cell antigen (MSP121). (A-C) On days 7 and 15, splenocytes were analyzed by flow cytometry for surface expression of activation markers CD45RB and CD69 and intracellular IFN-γ, IL-2, and IL-10. Cells shown are lymphocyte gated CD4+Vβ8+ B5 T cells. n.d. indicates not determined.
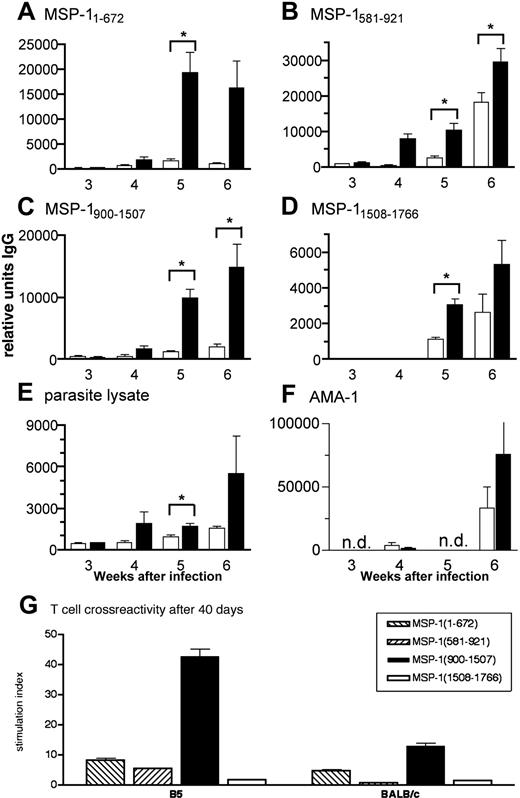
To determine the ability of anti-MSP-137/39 T cells to provide help for malaria-specific antibodies, we analyzed the serum antibody production. Immunoglobulin G (IgG) antibodies specific for all fragments of MSP-1 and for crude parasite lysate were generally detected sooner and at higher levels in infected nu/nu mice receiving Tg CD4+ cells than in those receiving BALB/c CD4+ cells (Figure 6A-E). Mice with B5 TCR Tg cells produced significant levels of anti-MSP1 and antiparasite IgG by week 4 of infection compared with mice receiving BALB/c CD4+ cells, which still had significantly lower antibody levels at week 6 (P < .05 for fragments MSP11-672, 581-921, and 900-1507). This suggests that transgenic T cells specific for the B5 epitope in MSP1900-1507 provide T-cell help to B cells producing antibody not only to that fragment but to the other 3 MSP-1 recombinant fragments and whole parasite lysate containing many malarial antigens as well as to another P chabaudi protein, AMA-1, expressed in the micronemes of the merozoite37 (Figure 6F).
Because transgenic T cells express 2 TCRα chains, it was possible that the endogenous α chain allowed expression of a TCR repertoire that could recognize peptides in different parts of MSP-1, or AMA-1, and that this interaction was responsible for the other antibody responses. We therefore tested the specificity of transferred cells 6 weeks after transfer and infection. Lymph node cells from the infected nu/nu mice reconstituted with transgenic cells still primarily responded to MSP-1900-1507, and only marginally to the other 3 fragments (Figure 6G), similar to naive Tg cells. Lymph node cells taken from similarly infected mice reconstituted with BALB/c wild-type cells responded slightly to MSP-1900-1507 and MSP-11-672 and lymph node cells from uninfected nu/nu mice reconstituted with BALB/c cells did not respond perceptibly to any of the recombinant fragments of MSP-1 (data not shown).
Because RAG-/- mice receiving transgenic CD4+ cells and immune B cells recovered from infection faster than transferred nu/nu mice, and transgenic CD4+ cells and immune B cells cleared parasites faster than wild-type cells, it was important to determine whether improvement in parasite clearance rate was due to a faster and greater antibody response. The differences were indeed reflected in antibody level and speed; at 3 weeks of infection, antibodies to MSP1900-1507, MSP11508-1766, and crude parasite lysate in RAG-/- mice receiving both T and B cells were already more than 10- to 30-fold higher than those observed at the same time in the transferred nu/nu mice (compare units in Figure 7 with Figure 6). Furthermore, antibody responses to MSP1900-1507, and MSP11508-1766 of immune B cells transferred with the B5 transgenic cells were significantly greater than those of immune B cells cotransferred with wild-type T cells. It appears that a threshold level of protective antibodies may have been reached sooner in the infection, which led to faster parasite clearance.
Immunization of mice receiving B5 transgenic T cells and B cells activated with coupled MSP-1 antigens resulted in a further 10-fold increase in antibody level. At 2 and 4 weeks after infection there were significantly higher levels of antibodies to MSP1900-1507, MSP11508-1766, and parasite lysate when the cells were primed in vivo (Figure 7D-F; note different scale in panels A,C and B,D). This was not the case for the transferred wild-type T and B cells except the antibody response to MSP11508-1766 at week 4.
In addition to higher antibody levels after immunization, antibody production was much faster. Malaria-specific antibody was already present in plasma at the earliest time point measured, at 2 weeks after transfer and infection. This was significantly higher with the transfer of B5 Tg cells than wild-type cells (Figure 7; P < .05). Thus, faster clearance of parasites by activated B5 Tg cells and immune B cells is accompanied by a more rapid and greater antibody response.
Discussion
We have generated a TCR transgenic mouse carrying CD4+ T cells specific for a peptide within P chabaudi MSP-1, a highly expressed surface molecule of the malaria parasite, and an important vaccine candidate. These transgenic CD4+ T cells respond specifically in vivo with early production of IFN-γ, are effective helper cells for antibody production, and together with B cells bring about elimination of a blood-stage P chabaudi infection.
MSP1-specific TCR Tg T cells help B cells produce quicker and greater levels of malaria-specific antibody. (A-F) B5 (▪) and BALB/c CD4+ cells (□) were purified by high-speed flow cytometry and transferred into nu/nu mice, which were then infected with 104P chabaudi parasites. Antibodies specific for (A) MSP11-672, (B) MSP1581-921, (C) MSP1900-1507, (D) MSP11508-1766, (E) whole parasite lysate, and (F) recombinant AMA-1 were measured in the plasma of samples taken weekly during the course of a P chabaudi infection. Specific IgG antibody is expressed as arbitrary units relative to a standard hyperimmune serum (1000 AU). The values shown are the geometric means and SEs of units of antibody measured in plasma of 5 to 7 mice. The asterisk indicates significant differences (P < .05, Student t test). (G) B5 TCR Tg T cells are still specific to MSP1900-1507 after 40 days of exposure to a P chabaudi infection in vivo. Lymph node cells were taken 6 weeks after infection from nu/nu mice reconstituted with B5 and BALB/c T cells and stimulated with 4 MSP-1 recombinant fragments in vitro (from left to right, MSP11-672, MSP1581-921, MSP1900-1507, and MSP11508-1766). Proliferation was determined by 3H-thymidine incorporation for the last 12 hours of culture.
MSP1-specific TCR Tg T cells help B cells produce quicker and greater levels of malaria-specific antibody. (A-F) B5 (▪) and BALB/c CD4+ cells (□) were purified by high-speed flow cytometry and transferred into nu/nu mice, which were then infected with 104P chabaudi parasites. Antibodies specific for (A) MSP11-672, (B) MSP1581-921, (C) MSP1900-1507, (D) MSP11508-1766, (E) whole parasite lysate, and (F) recombinant AMA-1 were measured in the plasma of samples taken weekly during the course of a P chabaudi infection. Specific IgG antibody is expressed as arbitrary units relative to a standard hyperimmune serum (1000 AU). The values shown are the geometric means and SEs of units of antibody measured in plasma of 5 to 7 mice. The asterisk indicates significant differences (P < .05, Student t test). (G) B5 TCR Tg T cells are still specific to MSP1900-1507 after 40 days of exposure to a P chabaudi infection in vivo. Lymph node cells were taken 6 weeks after infection from nu/nu mice reconstituted with B5 and BALB/c T cells and stimulated with 4 MSP-1 recombinant fragments in vitro (from left to right, MSP11-672, MSP1581-921, MSP1900-1507, and MSP11508-1766). Proliferation was determined by 3H-thymidine incorporation for the last 12 hours of culture.
Activated anti-MSP-1 T cells help immune B cells make a faster and greater antibody response, which is critical for clearance. B5 (▪) and BALB/c (□) CD4+ cells were purified by high-speed flow cytometry and transferred into RAG-/- mice, which were then infected with 104P chabaudi (AS) parasites (A-C). Some mice were immunized with the T-cell antigen (MSP1900-1507) covalently linked to the B-cell antigen (MSP121) intraperitoneally 2 days after T-cell transfer and a day before infection (D-F). Antibodies recognizing MSP1900-1507 (A-B), MSP11508-1766 (B,E) and whole parasite lysate (C,F) were measured by ELISA. The results are expressed as arbitrary units of antibody relative to a standard hyperimmune serum. The values shown are the geometric means and SEs of IgG responses of 5 to 7 mice. The asterisk indicates that the differences are significant (P < .05, Student t test). (G) T cells protect from lethal infection but a threshold of antibody is critical in clearance of P chabaudi. A schematic representation of the influence of transferred transgenic CD4+ T cells and immune B cells in the presence and absence of immunization on the rate of clearance of P chabaudi infection (▪), mortality of RAG-/- mice (□), and the speed and magnitude of a malaria-specific antibody response (gray shaded area).
Activated anti-MSP-1 T cells help immune B cells make a faster and greater antibody response, which is critical for clearance. B5 (▪) and BALB/c (□) CD4+ cells were purified by high-speed flow cytometry and transferred into RAG-/- mice, which were then infected with 104P chabaudi (AS) parasites (A-C). Some mice were immunized with the T-cell antigen (MSP1900-1507) covalently linked to the B-cell antigen (MSP121) intraperitoneally 2 days after T-cell transfer and a day before infection (D-F). Antibodies recognizing MSP1900-1507 (A-B), MSP11508-1766 (B,E) and whole parasite lysate (C,F) were measured by ELISA. The results are expressed as arbitrary units of antibody relative to a standard hyperimmune serum. The values shown are the geometric means and SEs of IgG responses of 5 to 7 mice. The asterisk indicates that the differences are significant (P < .05, Student t test). (G) T cells protect from lethal infection but a threshold of antibody is critical in clearance of P chabaudi. A schematic representation of the influence of transferred transgenic CD4+ T cells and immune B cells in the presence and absence of immunization on the rate of clearance of P chabaudi infection (▪), mortality of RAG-/- mice (□), and the speed and magnitude of a malaria-specific antibody response (gray shaded area).
Adoptive transfer experiments allowed us to observe the expansion and contraction of a malaria-specific T-cell population during infection. The transgenic CD4+ T-cell population expands and contracts in a manner similar to that described for model antigen systems.38,39 Contraction of T-cell populations is a common feature of CD4+ and CD8+ T-cell responses to nonreplicating antigens and infectious agents, as reviewed by Marsden and Strasser.40 The kinetic shown here is fairly similar to that reported for CD4+ cells in Salmonella infection, which is cleared up more quickly than P chaubadi.41 It would be important to determine whether this early contraction plays any role in immune evasion and persistent parasitemia as proposed for malaria and other infections.40,42-44
The TCR used for generating the transgenic mouse was cloned from an MSP1-specific hybridoma15 that transcribed 2 α chains. Because T cells carrying the transgenic Vβ8.1 and Vα2 chains were not positively selected in the RAG-/- thymus, the transgenic T cells must have been selected by a second α chain. This is relatively common and has been well studied in other models.34,45 However, our data provide the first evidence that dual TCR cells can effectively extend the TCR repertoire to infectious agents, by allowing the rescue of functional TCRs that could not have been selected in thymus on T cells carrying a single TCR.34,46
CD4+ T cells have 2 functional roles in the control or elimination of blood-stage malaria. One of these is a cytokine-dependent effector role that controls but does not eliminate Pchabaudi,25,47-51 and the other is as a helper cell for the production of antibodies by B cells, which eliminate the parasite.4,48 Our data provide evidence for both of these roles. Naive and activated Tg CD4+ T cells transferred into RAG2-/- recipients, although not eliminating the parasite, prevent mortality. However, it is clear that the most important role of CD4+ T cells is to act as helper cells for the production of protective antibodies, because parasitemia is cleared only when B cells are included. In adoptively transferred nu/nu mice, this clearance took 28 days. Cotransfer of transgenic CD4+ T cells with immune B cells, which included a higher frequency of malaria-specific B cells, into RAG-/- mice, increased the rate of clearance of parasites and resulted in a significantly faster and several-fold greater antibody response than that seen in the nu/nu, or in RAG-/- mice with transferred BALB/c T cells.
Addition of antigen to the B5 CD4+ T and B cells at the time of transfer, activating both cell populations, enhanced the antibody response still further with the kinetics of a classic secondary response. Antigen generates effector cells, which respond quickly and strongly enough that the antibody induced is sufficient not only to decrease the time of clearance but also to reduce the peak parasitemia. Interestingly, in the liver stages of malaria, where CD8+ T cells are the predominant effector mechanism, it has been found that they also must be activated specifically before infection to improve protection.52
Transgenic B5 CD4+ T cells were effective helper cells for malaria-specific antibodies, and they not only provided help for an antibody response to the fragment containing their specific peptide, but also for antibodies to other regions of MSP-1 including the protective C-terminal region. Because MSP-1 is cleaved by the parasite prior to invasion of the erythrocyte into 5 noncovalently associated fragments and one merozoite-bound fragment, which remains attached during erythrocyte invasion,7 it was possible that the various fragments could be taken up and processed by different antigen-presenting cells. Linked recognition is a requirement for specific T-cell help,53 and therefore CD4+ T cells specific for epitopes within soluble fragments may not have been very effective helper cells for antibody production for domains that remain attached to the parasite. This was clearly not the case; recognition of the same MSP-1 fragment by the Tg T cell was not necessary for a specific antibody response. The most obvious explanation for these results is that the MSP-1 protein complex is not always completely cleaved or that it associates noncovalently54 but tightly enough for the fragments to be taken up by the same B cell; however, it is also possible that the transgenic cells provide noncognate help to B cells of diverse specificities. Thus, our data indicate that microbial products, which may only be noncovalently associated, meet the requirements for linked recognition necessary for T-cell help and antibody production. In the case of MSP-1, this means that CD4+ T helper cells specific for epitopes outside of the protective region can still provide help for a protective antibody response. This has distinct advantages for vaccination with MSP-1. The protective region of this molecule (MSP119/21) is difficult for antigen-presenting cells to process,10,12 thus limiting the production of T-helper epitopes. An alternative source of CD4+ T-cell epitopes from more readily processed forms of MSP-1, such as that recognized by B5 T cells,12 would provide a more effective source of T cell help for a protective vaccine.
In summary, our data indicate that in malaria, as in a variety of virus infections, high-titer, neutralizing antibody can effectively control a challenge infection.55 To achieve protection, a critical threshold of protective antibody is required, and effector B cells, activated T-cell help, and antigen are necessary for enhanced and rapid clearance (Figure 7G). Thus, vaccination strategies may need to strive for long-term survival or continuous restimulation of effector B cells and T-cell help.
Prepublished online as Blood First Edition Paper, May 12, 2005; DOI 10.1182/blood-2004-10-4047.
Supported by the Medical Research Council, United Kingdom. S.Q. was supported by a Wellcome Trust Prize studentship, and B.J.P. by a Fulbright Scholarship. R.S. and F.R.A. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Andre Boonstra and Alexandre Potocnik for critical reading and constructive comments on this manuscript. We would also like to thank Anna Sponaas for experimental support.