Abstract
Membrane-bound and soluble interleukin-15 (IL-15)/IL-15 receptor α (Rα) complexes trigger differential transcription factor activation and functions on human hematopoietic progenitors. Indeed, human spleen myofibroblasts (SMFs) are characterized by a novel mechanism of IL-15 trans-presentation (SMFmb [membrane-bound]-IL-15), based on the association of an endogenous IL-15/IL-15Rα complex with the IL-15Rβγc chains. SMFmb-IL-15 (1) induces lineage-specific signaling pathways that differ from those controlled by soluble IL-15 in unprimed and committed normal progenitors; (2) triggers survival and proliferation of leukemic progenitors expressing low-affinity IL-15R (M07Sb cells); (3) causes only an antiapoptotic effect on leukemic cells expressing high-affinity receptors (TF1β cells). This behavior is likely due to the IL-15Rα chain present on these cells that interact with the SMFmb-IL-15, inhibiting signal transducer and transcriptional activator 5 (STAT5) activation. On the other hand, the soluble IL-15/IL-15Rα complex (hyper IL-15) displays a dominant pattern of action, activating only those cells expressing low-affinity IL-15R (IL-15Rβγc). Thus, hyper IL-15 induces antiapoptotic effects on M075b cells and the up-regulation of STAT6 activation on adult peripheral blood (PB) pre-natural killer (NK) committed progenitors. The latter effect using 100-fold concentrations of recombinant (r)-IL-15. In conclusion, SMFmb-IL-15 and soluble IL-15Rα/IL-15 complexes seem to play a pivotal role in the control of the survival, proliferation and differentiation of both normal and leukemic circulating progenitors, highlighting new functions of IL-15 and of IL-15Rα.
Introduction
Interleukin-15 (IL-15) links innate and acquired immunity, transducing its signals through the β and γc chains of the IL-2 receptor (IL-2R) and through the specific IL-15Rα chain. IL-15 mRNA is constitutively found in several cell types and tissues, and IL-15 gene expression is up-regulated by a variety of stimuli. However, IL-15 has only been found to be secreted in pathologic conditions (eg, chronic inflammation).1-3 An alternate mechanism is represented by the expression of a biologically active, nonsoluble membrane-bound form of IL-15 that is expressed and/or acquired by several types of accessory cells.4-7 For instance, in murine T cells, exogenous IL-15 binds to IL-15Rα forming cell-surface complexes that, after trans-endosomal recycling, migrate to the cell surface, acquiring the property to activate bystander cells expressing the IL-15Rβγc low-affinity receptor.7
Moreover, analysis of IL-15 and IL-15R knockout mice has revealed that a membrane-bound IL-15/IL-15Rα complex present on accessory cells is essential in peripheral tissues for the development of natural killer (NK) cells, NKT cells, and CD8+ memory T cells.8-11
These data indicate that membrane-bound IL-15 is the major physiologic form of the cytokine involved in the cross-talk between accessory cells and lymphoid cells.6,7,10,11
IL-15 is the most powerful physiologic factor able to induce the differentiation of CD34+ hematopoietic progenitor cells (HPs) into functional CD56+/CD3- NK cells.12-14 Even if bone marrow (BM) is the main site where this process takes place,15 it has been suggested that the spleen could be a secondary alternate site. This hypothesis is based on the fact that human spleen-derived but not bone marrow-derived myofibroblasts constitutively express a mb-IL-15 (spleen myofibroblast [SMF] membrane-bound [mb]-IL-15) necessary and sufficient to trigger, upon coculture, the differentiation of circulating (but not BM) CD34+ progenitors into functional NK cells.16,17
The way IL-15 is anchored to the cell membrane seems to have a major effect on its presentation in trans and on its effects on target cells.4-7,10,11,18,19 For these reasons, we investigated (1) the mechanism of anchorage of SMFmb-IL-15 at the cell membrane; (2) the type of signal transduction triggered upon contact with normal and leukemic hematopoietic CD34+ cells; and (3) the biologic effects of SMFmb-IL-15 on leukemic cells. We chose to work with cord blood (CB) CD34+ cells, because these cells are a rich source of hematopoietic progenitors for therapeutic approaches.20 We also used 2 growth factor-dependent myeloid cell lines bearing low- or high-affinity IL-15 receptors,21 as this made it possible to dissect the properties of SMFmb-IL-15 according to the type of IL-15R involved; even so, caution should be used concerning the general significance of data obtained using leukemic cells.
Materials and methods
Cytokines, Abs, and fusion proteins
Recombinant (r-) IL-15, r-granulocyte macrophage-colony-stimulating factor (GM-CSF), r-Flt-3 ligand (r-Flt3-L), antibodies (Abs) against IL-15 (mAb247) or IL-15Rα (AF247) and the soluble r-IL-15Rα (147-IR-100) were purchased from R&D Systems (Abingdon, United Kingdom). R-stem-cell factor (SCF) is obtained from MABIO-International Laboratories (Tourcoing, France).
Anti-GM-CSFRβ and anti-γc (TUGh4) mAbs were from BD PharMingen (San Diego, CA). Abs against GM-CSFRα (S-20), signal transducer and transcriptional activator 6 (STAT6) (S-20), nuclear factor-κB (NF-κB) p65 (RelA), STAT3 (H-190), cyclin D1, suppressor of cytokine signaling 3 (SOCS3), and human IL-15 (p15) were purchased from Santa Cruz Biotechnology (Tebu, France), and Abs against phospho-STAT3 (pSTAT3, Tyr705), pSTAT5 (Tyr694), and pSTAT6 (Tyr641) were purchased from Cell Signaling, New England Biolabs (Beverly, MA). Abs against STAT5 and B-cell leukemia-X (Bcl-X) were obtained from BD Transduction Laboratories (Heidelberg, Germany), and anti-pIκBα and anti-IκBα were from Calbiochem (VWR International SAS, Fontenay sous Bois, France).
Goat anti-mouse and goat anti-rabbit AlexaFluor488 conjugates (Molecular Probes, Interchim, France), as well as goat anti-mouse, goat anti-rat, goat anti-rabbit, and rabbit anti-goat horseradish peroxidase (HRP) conjugates (Amersham Life Science, Little Chalfont, United Kingdom), were used as secondary Abs.
Anti-IL-15 (M111) and anti-IL-15Rα (M165) mAbs were generous gifts from Genmab (Utrecht, the Netherlands), and anti-IL-15Rβ (6E8, Mikβ1) mAbs were a generous gift from Dr Yannick Jacques (U463 INSERM, Nantes, France) and Dr Thomas Waldmann (National Institutes of Health [NIH], Bethesda, MD).
Purification of hematopoietic CD34+ progenitors
Mononuclear cells from cord blood (CB) and peripheral blood (PB) were isolated by an immunomagnetic method achieving a purity greater than 97%, as previously described.14 Commitment to the pre-NK pathway (NKP) was obtained treating CB CD34+ cells with 50 ng/mL of r-SCF and r-Flt3-L.2 Commitment to the pre-erythrocytic pathway was obtained treating CB CD34+ cells with STEMα AE medium (Stem Alpha France), which contains r-IL-3, r-IL-6, r-IL-9, r-SCF, and r-erythropoietin (EPO), and specifically induces erythrocytic differentiation.
Cell lines
Human TF1β (IL-15Rαβγc) and M07Sb (IL-15Rβγc) are 2 IL-15-dependent leukemic cell lines that display a proerythroid and promegakaryocytic differentiation potential, respectively. The growth characteristics and phenotypes of these cells have been reported elsewhere.22,23 The human stromal myofibroblast SMF, isolated in culture from the spleen of a healthy donor, was used as a feeder layer for hematopoietic cells. SMFs express a bioactive membrane-bound IL-15 that induces the NK-cell differentiation of CD34+ cells; they also secrete GM-CSF.16 TF1β cells were kindly provided by Dr Paul Sondel (University of Wisconsin, Madison, WI). Approval was obtained from the INSERM institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki.
Analysis of signal transduction in hematopoietic CD34+ cell lines progenitors by confocal microscopy
For signal transduction analysis, both unprimed and NKPs were deprived of growth factors for 3 hours at 37°C to reduce basal phosphorylation. Cells were then cocultured with SMFs for 30 minutes at 37°C. In some experiments, cells were initially treated for 1 hour with 10 μg/mL neutralizing antibody against IL-15Rα. To study STAT6 activation, NKPs and pre-erythroid cells were stimulated by incubation with 0.1 or 100 ng/mL of r-IL-15. Cells were then analyzed for the expression of activated transcription factors using confocal microscopy as described previously.21 The slides were analyzed by laser scanning confocal microscopy using a Leica TCS confocal system (Leica, Wetzlar, Germany).
Analysis of signal transduction in hematopoietic CD34+ cell lines by immunoblotting
For signal transduction analysis, cells were serum and cytokine starved and then stimulated with 10 ng/mL of r-IL-15. In some experiments, cells were initially treated for 1 hour with either 10 μg/mL neutralizing antibody against IL-15, IL-15Rα, or IL-15Rβ.
Cytoplasmic lysates were prepared as described previously.21 Nuclear extracts from NKPs were prepared treating 2 × 106 cells with the NE-PER Nuclear and Cytoplasmic Extraction Kit (Pierce, Perbio, Brebieres, France) according to the manufacturer's instructions. The resolved proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (NEN Research Products, Boston, MA) and processed as described previously.21 The membranes were subjected to densitometry, including correction for background, with analysis using NIH Image software (Bethesda, MD). To correct for possible variations in the amount of protein loaded, values are expressed as pSTAT/STAT ratios. Results are expressed as increases (eg, 3 times) with respect to the results obtained for untreated cells.
Analysis of IL-15/IL-15R interactions
Plasma-membrane and cytosolic-fractions separation Cells were incubated or not overnight at 37°C with brefeldine A at 1 μg/mL. Cells were then washed twice with ice-cold phosphate-buffered saline (PBS) and subsequently homogenized with glass Dounce homogenizer in homogenization buffer (10 mM Tris [tris(hydroxymethyl)aminomethane], 0.25 mM sucrose, 2 mM EDTA [ethylenediaminetetraacetic acid], and 2 mM protease inhibitors). The homogenate was first centrifuged at 2500g for 30 minutes at 4°C to pellet nuclei and whole cells, and then at 100 000g for 30 minutes at 4°C to obtain the cytosolic fraction. The pellet containing the plasma membrane was resuspended in 10 mM Tris (pH 7.4), 150 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 1% digitonin, and 2 mM protease inhibitors. The detergent insoluble materials were removed by centrifugation for 15 minutes at 13 000g at 4°C to obtain the plasma-membrane fraction and immunoprecipitations were performed on both plasma-membrane and cytosolic fractions.
Immunoprecipitaion and immunoblotting For immunoprecipitation studies, lysates were first precleared with the anti-mouse immunoglobulin G (IgG) bound to protein A/G-agarose and immunoprecipitated overnight at 4°C by incubation in 1% digitonin buffer with antibodies against IL-15 or the chains of the IL-15 receptor. Immunocomplexes were captured on protein A/G-agarose, washed, resuspended in Laemmli buffer, and boiled. Immunocomplexes were then analyzed in 7.5% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), before transferring onto PVDF membranes. Membranes were blocked with 5% bovine serum albumin (BSA), probed with antibodies (anti-IL-15Rα, anti-IL-15Rβ, anti-IL-15Rγc, and anti-GM-CSFRβ), and revealed with peroxidase-conjugated antibodies. Bands were visualized by an electrochemiluminescence (ECL) system as described previously.21
Flow cytometry analysis
Nonpermeabilized human spleen myofibroblasts were labeled with a rabbit polyclonal IgG anti-IL-15 72 hours after seeding, as previously described.19 Treatment during the spreading period with neutralizing mAbs directed against IL-15 γc-chain epitope (mAb247, M111), IL-15Rβ chain (Mikβ1, 6E8), IL-15Rα (M165, AF247), or the γc chain (TUGh4, mAb284) was realized. IL-15 surface expression was analyzed using FACScalibur (Becton Dickinson, San Quentin Yvelines, France). Mean fluorescence intensity was calculated with CellQuest software (Becton Dickinson).
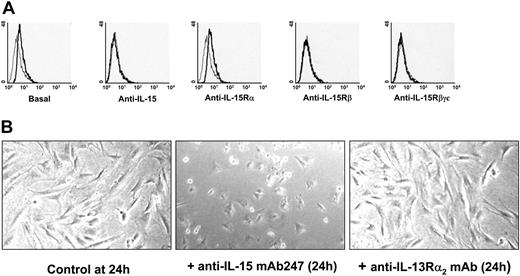
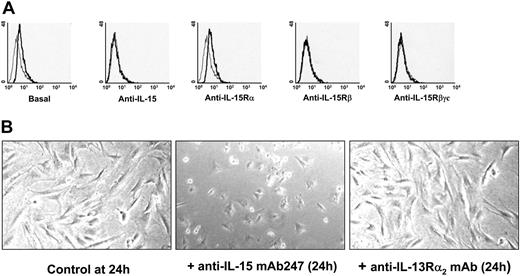
Flow cytometry analysis of mb-IL-15 expression on human spleen myofibroblasts. (A) Flow cytometry analysis using a rabbit polyclonal IgG anti-IL-15 revealed a mb-IL-15 form on human spleen myofibroblasts 72 hours after seeding (basal). Treatment during the spreading period with neutralizing mAbs directed against: IL-15 (10 μg/mL mAb247 recognizing the γc chain epitope); the IL-15Rβ chain (10 μg/mL mAb Mikβ1) and the γc chain (10 μg/mL mAb TUGh4) inhibited the expression of mb-IL-15. Use of neutralizing mAb against the IL-15Rα (10 μg/mL mAb M165) had no effect. The thin gray line defines IgG control; the bold black line defines mb-IL-15 expression. Treatment of spread cells had no effect. These data are representative of 3 different experiments. (B) Modulation of cell spreading in NHS7 cells treaded with 10 μg/mL of the neutralizing anti-IL-15 mAb247 or with a nonrelevant isotype-matched mAb recognizing the IL-13Rα2 subunit. These data are representative of 2 different experiments. Pictures were obtained with an Olympus microscope and a 40 ×/1.0 objective lens (Olympus, ArcuEil, France) using a Cool-Pix 995 numeric camera (Nikon, Paris, France).
Flow cytometry analysis of mb-IL-15 expression on human spleen myofibroblasts. (A) Flow cytometry analysis using a rabbit polyclonal IgG anti-IL-15 revealed a mb-IL-15 form on human spleen myofibroblasts 72 hours after seeding (basal). Treatment during the spreading period with neutralizing mAbs directed against: IL-15 (10 μg/mL mAb247 recognizing the γc chain epitope); the IL-15Rβ chain (10 μg/mL mAb Mikβ1) and the γc chain (10 μg/mL mAb TUGh4) inhibited the expression of mb-IL-15. Use of neutralizing mAb against the IL-15Rα (10 μg/mL mAb M165) had no effect. The thin gray line defines IgG control; the bold black line defines mb-IL-15 expression. Treatment of spread cells had no effect. These data are representative of 3 different experiments. (B) Modulation of cell spreading in NHS7 cells treaded with 10 μg/mL of the neutralizing anti-IL-15 mAb247 or with a nonrelevant isotype-matched mAb recognizing the IL-13Rα2 subunit. These data are representative of 2 different experiments. Pictures were obtained with an Olympus microscope and a 40 ×/1.0 objective lens (Olympus, ArcuEil, France) using a Cool-Pix 995 numeric camera (Nikon, Paris, France).
Proliferation assays and protection from apoptosis
For proliferation assays, M07Sb and TF1β cells were starved of GM-CSF for 24 hours and were subsequently cocultured for 96 hours with SMFs. Sister cultures were continuously incubated with neutralizing mAbs recognizing the GM-CSFRα, GM-CSFRβ, IL-15Rβ, and IL-15Rα chains, and IL-15. Cells were counted in an electronic Coulter counter (Beckman Coulter, Villepinte, France) and data are expressed as the percentage of difference in proliferative potential with respect to control untreated samples. The presented data are representative of 3 independent experiments. The statistical significance of differences was determined using Student t test, with a P value of .05 considered significant.
For protection from apoptosis, M07Sb and TF1β cells were starved of GM-CSF for 24 hours and were subsequently cocultured with SMFs in the presence or not of the same neutralizing mAbs. In addition, M07Sb cells were treated with r-IL-15 (10 and 0.1 ng/mL) and with 0.1 ng/mL r-IL-15 bound to decreasing concentrations of the soluble IL-15Rα chain (1-1000 ng/mL).
The percentage of apoptotic cells was evaluated by flow cytometry using the fluorescent DIOC63 probe (Molecular Probes, Leiden, the Netherlands) to detect cells with dissipated transmembrane mitocondrial potential (ΔΨm). Cells were collected, resuspended with culture medium to 106 cells/mL, and incubated at 37°C for further 15 minutes, then DIOC63 fluorescence was immediately recorded with flow cytometry. For each sample, 10 000 cells were acquired for data analysis. Three independent experiments are presented and the statistical significance of differences was determined using Student t test, with a P value of .05 considered significant.
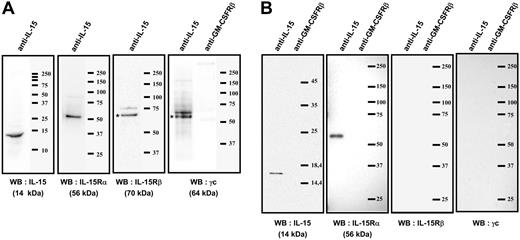
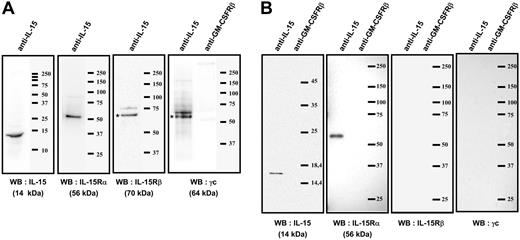
Immunoprecipitations analysis of the mb-IL-15 present on human spleen myofibroblasts. (A) Coimmunoprecipitation with an anti-IL-15 on plasma-membrane fraction. The membrane was then subsequently reprobed by Western blotting (WB) with anti-IL-15Rα, β, γc, or anti-IL-15 Ab: a single 56-kDa specific band was detected by anti-IL-15Rα and a single 14-kDa band with anti-IL-15 Ab. The anti-IL-15Rβ and γc Ab detected specific bands at 70 and 64 kDa, respectively. The isotype control (anti-GM-CSFRβ chain Ab) did not detect any band. (B) Coimmunoprecipitation with an anti-IL-15 on brefeldin A-treated cytosolic fraction. The membrane was then subsequently reprobed by Western blotting with anti-IL-15Rα, β, γc, or anti-IL-15 Ab: a single 14-kDa specific band was detected by anti-IL-15 and a single 56-kDa band with anti-IL-15Rα Ab. No specific bands were detected with the anti-IL-15Rβ and γc Ab. The isotype control (anti-GM-CSFRβ chain Ab) did not detect any band. These results are representative of 3 different experiments. Our data suggest that an IL-15/IL-15Rα complex is assembled intracellularly (B) and then migrates to the cellular membrane, where it associates with the IL-15Rβγc chains (A).
Immunoprecipitations analysis of the mb-IL-15 present on human spleen myofibroblasts. (A) Coimmunoprecipitation with an anti-IL-15 on plasma-membrane fraction. The membrane was then subsequently reprobed by Western blotting (WB) with anti-IL-15Rα, β, γc, or anti-IL-15 Ab: a single 56-kDa specific band was detected by anti-IL-15Rα and a single 14-kDa band with anti-IL-15 Ab. The anti-IL-15Rβ and γc Ab detected specific bands at 70 and 64 kDa, respectively. The isotype control (anti-GM-CSFRβ chain Ab) did not detect any band. (B) Coimmunoprecipitation with an anti-IL-15 on brefeldin A-treated cytosolic fraction. The membrane was then subsequently reprobed by Western blotting with anti-IL-15Rα, β, γc, or anti-IL-15 Ab: a single 14-kDa specific band was detected by anti-IL-15 and a single 56-kDa band with anti-IL-15Rα Ab. No specific bands were detected with the anti-IL-15Rβ and γc Ab. The isotype control (anti-GM-CSFRβ chain Ab) did not detect any band. These results are representative of 3 different experiments. Our data suggest that an IL-15/IL-15Rα complex is assembled intracellularly (B) and then migrates to the cellular membrane, where it associates with the IL-15Rβγc chains (A).
Detection of STAT5-dependent molecular targets
Five-day-old CB NKPs and MO7Sb cells were cytokine starved for 4 hours in the presence of the Janus kinase 2 (JAK2)/STAT5 inhibitor AG490 (Calbiochem). Subsequently, control and treated cells were cocultured for 24 to 48 hours with SMFs. Finally, cells were washed and permeabilized with ORTHOpermeafix (Ortho Diagnostic Systems, Raritan, NJ) for 45 minutes at room temperature, and were analyzed by flow cytometry for the expression of the 3 genes—Bcl-X, cyclin D1, and SOCS 324,25 —the expression of which depends on STAT5 activation.
Results
Human spleen myofibroblasts express a novel form of IL-15 that is anchored to the cell membrane through the IL-15Rβγc complex
Using flow cytometry and immunoprecipitation we investigated the expression and cell-membrane anchorage of the mb-IL-15 form expressed on human spleen myofibroblasts (SMFs). Flow cytometry revealed that 72 hours after seeding, human spleen myofibroblasts expressed a membrane-bound form of IL-15 (Figure 1A), the expression of which was inhibited by the anti-IL-15 mAb247, which recognizes the γc chain-binding epitope on IL-15 molecules,26 by anti-IL-15Rβ (Mikβ1, 6E8) and anti-γc chain (TUGh4, mAb284) neutralizing mAbs. No inhibition was observed using anti-IL-15Rα ones (M165, AF247) or the soluble r-IL-15Rα chain at 1 μg/mL (data not shown) or when the neutralizing mAbs were added 24 hours after seeding, when the cells were totally spread (data not shown). These data strongly suggest that spleen myofibroblasts express a mb-IL-15 form (SMFmb-IL-15) that is anchored to the cell surface through the IL-15Rβγc complex.
Membrane-bound IL-15 can also function as an activating receptor, participate in reverse signaling, and modify several properties of trans-presenting cells.27,28 Herein, we extend these observations, showing that neutralization of SMFmb-IL-15 with the anti-IL-15 mAb247 causes in the first 24 hours a delay in cells spreading (Figure 1B, middle panel), whereas this property is not affected by the use of a nonrelated isotype-matched mAb (Figure 1B, left panel).
To confirm the existence and the composition of the IL-15/IL-15R association, we performed coimmunoprecipitation experiments with an anti-IL-15 mAb on plasma-membrane fraction (Figure 2A) using mild detergent lysis conditions (digitonin). The membrane was subsequently reprobed by Western blotting with anti-IL-15Rα, β, γc, or anti-IL-15 Abs: a single 56-kDa specific band was detected by an anti-IL-15Rα Ab and a single 14-kDa band with an anti-IL-15 Ab. The anti-IL-15Rβ and γc Abs detected specific bands at 70 kDa and 64 kDa, respectively. The isotype control (anti-GM-CSFRβ Ab) did not detect any band.
In order to understand the mechanisms leading to the association of IL-15Rα chain to this complex, we treated cells with brefeldin A to block intracellular trafficking, and immunoprecipitated the cytosolic fraction with anti-IL-15 mAb. Western blotting with anti-IL-15Rα Ab (Figure 2B) revealed a single 56-kDa band, whereas Western blotting with anti-IL-15Rβ and γc Abs did not reveal any band. These coimmunoprecipitation experiments strongly suggest the intracellular assembly of IL-15 with the IL-15Rα p56 isoform, and the subsequent association of this high affinity heterodimer with the IL-15Rβ and γc chains present on the cell membrane.
The interaction between SMFmb-IL-15 and CB progenitors triggers lineage-specific activation of transcription factors
In the absence of exogenous cytokines, long-term contact between SMFmb-IL-15 and circulating CD34+ HPs leads to the generation of functional NK cells.18,19 To study the molecular pathways activated by this type of interaction, we used confocal microscopy to analyze the transcription factors activated by short-term contact between SMFmb-IL-15 and unprimed or lineage-committed CB progenitors.
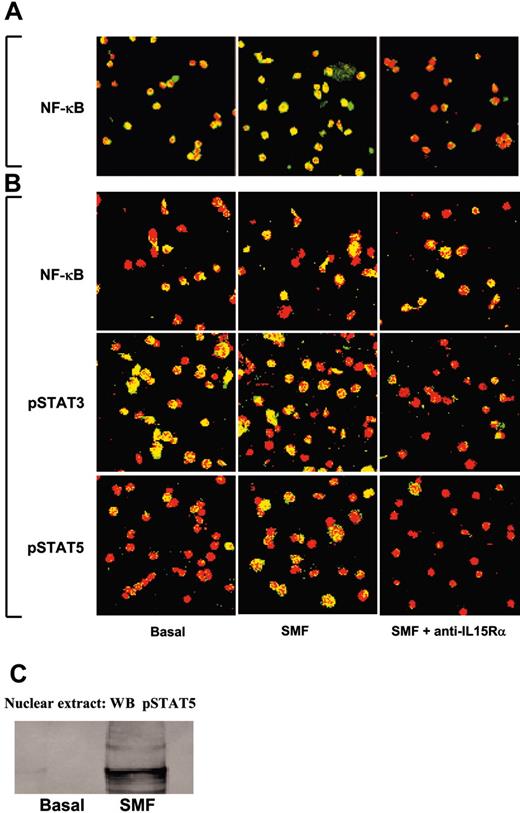
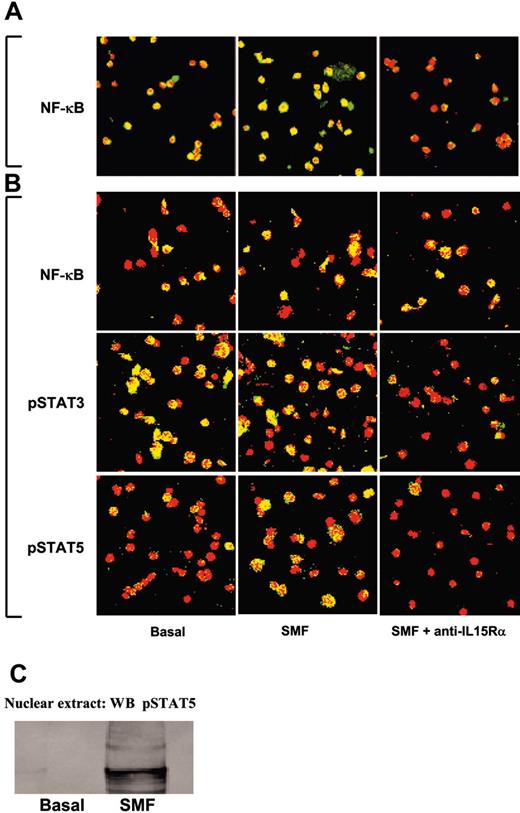
In 50% of unprimed CD34+ CB progenitors, the p65 subunit of the NF-κB transcription factor (green staining) was constitutively located in the nuclear compartment (yellow staining) (Figure 3A, left panel). Short-term contact with SMF (Figure 3A, central panel) increased the translocation efficiency (88% yellow nuclei) of the cytoplasm p65 (green staining) into the nucleus (red staining). No p65 was detected in the nuclei after preincubation with neutralizing anti-IL-15Rα mAb (Figure 3A, right panel). Treatment of CB HPs with r-SCF and r-Flt3-L for 10 days causes the expansion of CB CD34+/CD56- progenitors committed to the pro-NK pathway (NKPs).12,13 At this stage, several transcription factors displayed a constitutive nuclear localization in CB progenitors: p65 subunit of NF-κB (50%), pSTAT3 (85%), and pSTAT5 (10%) (Figure 3B, left panels). Short-term contact with SMFs did not modify the locations of p65 or STAT3, whereas it triggered the nuclear translocation of STAT5 in 50% of nuclei (Figure 3B, middle panels). Preincubation with neutralizing anti-IL-15Rα mAb did not modify the pattern of NF-κB, but totally inhibited the constitutive pSTAT3 and the induced pSTAT5 nuclear localization (Figure 3B, right panels). STAT5 activation was further confirmed showing, by Western blot, the presence of pSTAT5 only in the nuclear extracts of NKPs stimulated by 45 minutes of contact with SMFmb-IL-15 (Figure 3C).
Confocal microscopy analysis of NF-κB p65, STAT3, and STAT5 nuclear localization in unprimed and pro-NK-committed CB progenitors induced by SMFmb-IL-15. (A) CB hematopoietic progenitors were analyzed soon after immunopurification (day 0, panel A) or after 5 days in the presence of r-SCF/r-Flt3-L (day 5, panel B). Overlay pictures (original magnification, ×63). Left panels show basal conditions; central panels, 30 minutes of contact with SMF at 37°C; and right panels, 60 minutes of preincubation with 10 μg/mL neutralizing anti-IL-15Rα mAbs, followed by 30 minutes with SMF. CB progenitors were labeled with anti-NF-κB p65, anti-pSTAT3, and anti-pSTAT5 antibodies, and then incubated with Alexa Fluor488-GARa (goat antirabbit) antibody (green staining). Propidium iodide stained the nuclei red. Nuclear p65, STAT3, and STAT5 are indicated by nuclear yellow staining. As negative controls, cells were incubated with only the second reagent and propidium iodide. These data are representative of 3 different experiments. Images were obtained with a 63 ×/1.30 objective lens. (C) Western blot analysis of STAT5 phosphorylation using anti-pSTAT5 Ab in nuclear extracts of 5-day-old CB pro-NK progenitors stimulated or not by 45 minutes of contact with SMFmb-IL-15.
Confocal microscopy analysis of NF-κB p65, STAT3, and STAT5 nuclear localization in unprimed and pro-NK-committed CB progenitors induced by SMFmb-IL-15. (A) CB hematopoietic progenitors were analyzed soon after immunopurification (day 0, panel A) or after 5 days in the presence of r-SCF/r-Flt3-L (day 5, panel B). Overlay pictures (original magnification, ×63). Left panels show basal conditions; central panels, 30 minutes of contact with SMF at 37°C; and right panels, 60 minutes of preincubation with 10 μg/mL neutralizing anti-IL-15Rα mAbs, followed by 30 minutes with SMF. CB progenitors were labeled with anti-NF-κB p65, anti-pSTAT3, and anti-pSTAT5 antibodies, and then incubated with Alexa Fluor488-GARa (goat antirabbit) antibody (green staining). Propidium iodide stained the nuclei red. Nuclear p65, STAT3, and STAT5 are indicated by nuclear yellow staining. As negative controls, cells were incubated with only the second reagent and propidium iodide. These data are representative of 3 different experiments. Images were obtained with a 63 ×/1.30 objective lens. (C) Western blot analysis of STAT5 phosphorylation using anti-pSTAT5 Ab in nuclear extracts of 5-day-old CB pro-NK progenitors stimulated or not by 45 minutes of contact with SMFmb-IL-15.
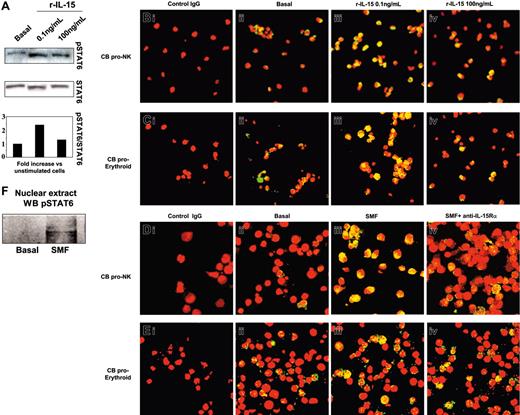
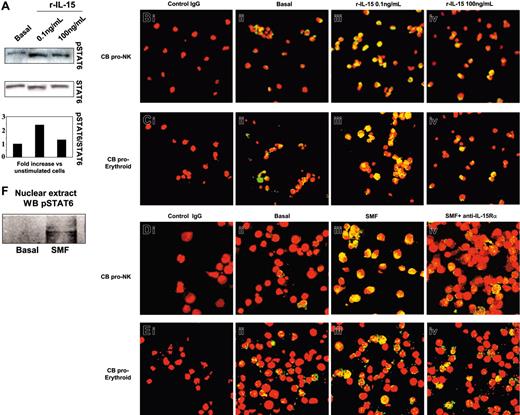
We next analyzed the activation pattern of STAT6 in NKPs or in the erythrocytic progenitors (Figure 4). In 5-day-old progenitors, we found that 0.1 ng/mL but not 100 ng/mL of human r-IL-15 significantly increased (2.4-fold) the phosphorylation of STAT6 (Figure 4A). This was followed by its massive nuclear translocation (yellow staining) in both types of progenitor using 0.1 ng/mL (Figure 4Biii, 4Ciii) but not 100 ng/mL of r-IL-15 (Figure 4Biv, 4Civ) compared with the low basal expression (Figure 4Bii, 4Cii). In contrast, 30 minutes of contact with SMFs induced STAT6 nuclear translocation in NKPs (Figure 4Diii). This effect was totally inhibited in the presence of neutralizing anti-IL-15Rα mAb (Figure 4Div). In proerythrocytic progenitors, the basal level of STAT6 activation (17% ± 2.1%) was not significantly modified by contact with SMFs (19% ± 1.9%) (Figure 4Eiii), or by neutralizing anti-IL-15Rα mAb (Figure 4Eiv).
Western blot and confocal microscopy analysis of STAT6 phosphorylation and nuclear localization in committed CB progenitors induced by r-IL-15 and SMFmb-IL-15. CB progenitors were analyzed after 5 days in the presence of r-SCF/r-Flt3-L (pro-NK commitment) or in Stemα AE medium (proerythroid commitment). (A) Western blot analysis of STAT6 phosphorylation using anti-pSTAT6 Ab in CB proerythroid progenitors incubated or not (basal) with 0.1 and 100 ng/mL human r-IL-15. Membrane was reprobed with antibody recognizing the native protein. Confocal microscopy of pro-NK (B) and proerythroid cells (C) treated with r-IL-15. Overlay pictures (original magnification, ×63). Panels Bi and Ci indicate negative control; panels Bii and Cii, basal conditions; panels Biii and Ciii, 30 minutes of incubation at 37°C with 0.1 ng/mL human r-IL-15; and panels Biv and Civ, 30 minutes of incubation at 37°C with 100 ng/mL human r-IL-15. Confocal microscopy of pro-NK (D) and proerythroid cells (E) in contact with SMFs. Overlay pictures (original magnification, × 63). Panels Di and Ei indicate negative control; panels Dii and Eii, basal conditions; panels Diii and Eiii, 30 minutes of contact at 37°C with SMF; and panels Div and Eiv, 60 minutes of preincubation with 10 μg/mL neutralizing anti-IL-15Rα mAbs, followed by 30 minutes of contact with SMFs. As negative controls, cells were incubated with rabbit IgG, the second reagent, and propidium iodide. These data are representative of 3 different experiments. (F) Western blot analysis of STAT6 phosphorylation using anti-pSTAT6 Ab in nuclear extracts of 5-day-old CB pro-NK progenitors stimulated or not by 45 minutes of contact with SMFmb-IL-15. Images were acquired with a 63 ×/1.30 objective lens.
Western blot and confocal microscopy analysis of STAT6 phosphorylation and nuclear localization in committed CB progenitors induced by r-IL-15 and SMFmb-IL-15. CB progenitors were analyzed after 5 days in the presence of r-SCF/r-Flt3-L (pro-NK commitment) or in Stemα AE medium (proerythroid commitment). (A) Western blot analysis of STAT6 phosphorylation using anti-pSTAT6 Ab in CB proerythroid progenitors incubated or not (basal) with 0.1 and 100 ng/mL human r-IL-15. Membrane was reprobed with antibody recognizing the native protein. Confocal microscopy of pro-NK (B) and proerythroid cells (C) treated with r-IL-15. Overlay pictures (original magnification, ×63). Panels Bi and Ci indicate negative control; panels Bii and Cii, basal conditions; panels Biii and Ciii, 30 minutes of incubation at 37°C with 0.1 ng/mL human r-IL-15; and panels Biv and Civ, 30 minutes of incubation at 37°C with 100 ng/mL human r-IL-15. Confocal microscopy of pro-NK (D) and proerythroid cells (E) in contact with SMFs. Overlay pictures (original magnification, × 63). Panels Di and Ei indicate negative control; panels Dii and Eii, basal conditions; panels Diii and Eiii, 30 minutes of contact at 37°C with SMF; and panels Div and Eiv, 60 minutes of preincubation with 10 μg/mL neutralizing anti-IL-15Rα mAbs, followed by 30 minutes of contact with SMFs. As negative controls, cells were incubated with rabbit IgG, the second reagent, and propidium iodide. These data are representative of 3 different experiments. (F) Western blot analysis of STAT6 phosphorylation using anti-pSTAT6 Ab in nuclear extracts of 5-day-old CB pro-NK progenitors stimulated or not by 45 minutes of contact with SMFmb-IL-15. Images were acquired with a 63 ×/1.30 objective lens.
STAT6 activation was also confirmed showing, by Western blot, the presence of pSTAT6 only in the nuclear extracts of NKPs stimulated by 45 minutes of contact with SMFmb-IL-15 (Figure 4F).
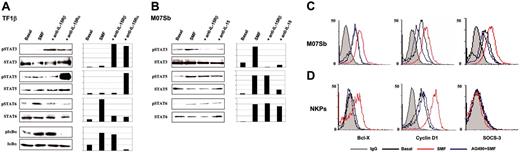
SMFmb-IL-15 triggers differential signal transduction in growth factor-dependent CD34+ leukemic cell lines with high- or low-affinity IL-15R
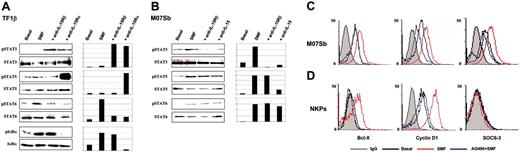
In TF1β cells, 15 minutes of contact with SMFs (Figure 5A) causes the phosphorylation of IκBα and STAT6, but not of STAT3 and STAT5. Preincubation of TF1β cells with neutralizing IL-15Rα chain mAb prevented the activation of IκBα and STAT6, whereas it triggered the phosphorylation of STAT3 and STAT5. STAT3 phosphorylation was also induced by neutralizing anti-IL-15Rβ mAb, suggesting that SMFmb-IL-15 plays a negative control in STAT3 activation in these cells.
Western blot analysis of transcription factor phosphorylation in TF1β (IL-15Rαβγc) and M07Sb (IL-15Rβγc) cells after short-term contact with SMFs. Leukemic TF1β (A) and M07Sb (B) progenitors were untreated (basal) or incubated with SMFs for 30 minutes. Parallel cultures were preincubated with neutralizing anti-IL-15Rα, IL-15Rβ, or anti-IL-15 mAbs for 1 hour and then with SMFs for 1 hour. Leukemic progenitors were then detached from myofibroblasts by gentle shaking and centrifuged with 10 mL of growth medium. Cell extracts were analyzed by Western blotting using anti-IκBα (pIκBα), anti-phospho-STAT3 (pSTAT3), anti-phospho-STAT5 (pSTAT5), and anti-phospho-STAT6 (pSTAT6) Abs (top blots). Each membrane was reprobed with antibodies recognizing the native proteins or β-actin (bottom blots). β actin was used as a loading control. These data are representative of 3 different experiments. Flow cytometry analysis of intracellular Bcl-X, cyclin D1, and SOCS3 expression in permeabilized 5-day-old CD34+ CB progenitors (C) and MO7Sb cells (D). Cytokine-deprived cells were incubated for 4 hours with the JAK2/STAT5 inhibitor AG490, and then control and treated cells were cocultured for 24 to 48 hours with SMFs. These data are representative of 3 different experiments. In C and D, gray line indicates IgG; black line, Basal; red line, SMF; blue line, AG490+SMF.
Western blot analysis of transcription factor phosphorylation in TF1β (IL-15Rαβγc) and M07Sb (IL-15Rβγc) cells after short-term contact with SMFs. Leukemic TF1β (A) and M07Sb (B) progenitors were untreated (basal) or incubated with SMFs for 30 minutes. Parallel cultures were preincubated with neutralizing anti-IL-15Rα, IL-15Rβ, or anti-IL-15 mAbs for 1 hour and then with SMFs for 1 hour. Leukemic progenitors were then detached from myofibroblasts by gentle shaking and centrifuged with 10 mL of growth medium. Cell extracts were analyzed by Western blotting using anti-IκBα (pIκBα), anti-phospho-STAT3 (pSTAT3), anti-phospho-STAT5 (pSTAT5), and anti-phospho-STAT6 (pSTAT6) Abs (top blots). Each membrane was reprobed with antibodies recognizing the native proteins or β-actin (bottom blots). β actin was used as a loading control. These data are representative of 3 different experiments. Flow cytometry analysis of intracellular Bcl-X, cyclin D1, and SOCS3 expression in permeabilized 5-day-old CD34+ CB progenitors (C) and MO7Sb cells (D). Cytokine-deprived cells were incubated for 4 hours with the JAK2/STAT5 inhibitor AG490, and then control and treated cells were cocultured for 24 to 48 hours with SMFs. These data are representative of 3 different experiments. In C and D, gray line indicates IgG; black line, Basal; red line, SMF; blue line, AG490+SMF.
In M07Sb cells, contact with SMFs causes the phosphorylation of STAT3 and STAT5, and a very faint induction of STAT6 (Figure 5B). Preincubation with neutralizing IL-15 mAb inhibits the phosphorylation of STAT3 and STAT5, but not of STAT6. Neutralizing anti-IL-15Rβ mAb inhibits the phosphorylation of STAT3, but not of STAT5 and STAT6.
Because SMFmb-IL-15 triggers STAT5 activation both in normal NKPs, and in MO7Sb leukemic progenitors, we checked whether the gene repertoire controlled by STAT5 was the same in these 2 types of cells. Control and AG490 (inhibitor of the JAK2/STAT5 pathway29 )-treated cells were stimulated by coculture with SMFmb-IL-15 and then analyzed for the expression of 3 major STAT5-dependent molecular targets (cyclin D1, Bcl-X, and SOCS3) that play a pivotal role in the control of proliferation, apoptosis, and STAT signalling.24,25
In MO7Sb cells, 24 hours of contact with SMFmb-IL-15 up-regulates intracellular expression of cyclin D1, Bcl-X, and SOCS3, whereas preincubation with AG490 inhibits these effects (Figure 5C). In NKP cells, SMFmb-IL-15 displays a more restricted pattern of action, increasing only cyclin D1 and Bcl-X expression; preincubation with AG490 counteracts these IL-15 effects (Figure 5B).
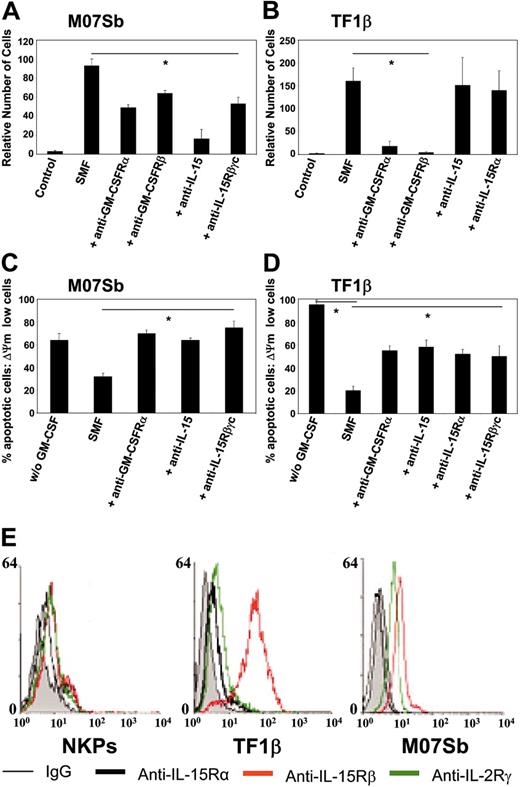
SMFmb-IL-15 triggers the proliferation of M07Sb cells expressing low-affinity IL-15R
We analyzed whether the differential effects of SMFmb-IL-15 on leukemic progenitors bearing low- and high-affinity IL-15R was associated with the induction of different biologic effects.
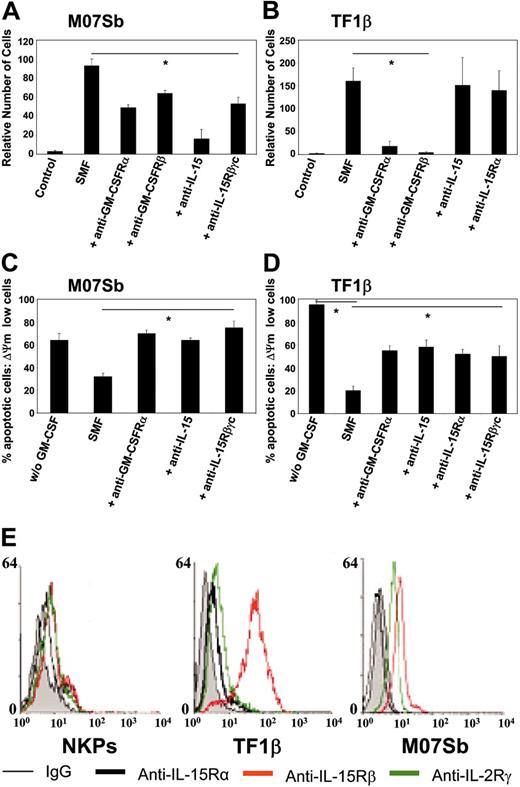
M07Sb (Figure 6A) and TF1β (Figure 6B) cells proliferated when cultured with SMFs, with a doubling time of 4 days. The proliferation of M07Sb cells was inhibited by 50% following the addition of neutralizing anti-GM-CSFR α and β chains or anti-IL-15Rβγc mAbs and by 90% following the addition of anti-IL-15 mAb. In contrast, the proliferation of TF1β cells was totally inhibited by neutralizing anti-GM-CSFR α and β chain mAbs, but not by anti-IL-15 or anti-IL-15Rα mAbs.
In cytokine-starved cultures, we found that 24 hours of r-GM-CSF deprivation caused the apoptosis of 60% of MO7Sb cells (Figure 6C) and 90% of TF1β cells (Figure 6D). In these conditions, 24 hours of coculture with SMFs decreased the percentage of apoptotic cells to 30% (M07Sb) and 20% (TF1β). Neutralizing anti-GM-CSFRα, anti-IL-15, and anti-IL-15Rβγc mAbs totally restored apoptosis in M07Sb cells, whereas anti-GM-CSFRα, anti-IL-15, anti-IL-15Rα, and anti-IL-15Rβγc mAbs caused a 50% decrease in the number of TF1β cells protected from apoptosis.
Thus, the proliferation of M07Sb cells and their protection from apoptosis are dependent both on GM-CSF produced by SMF16 and on mb-IL-15. In contrast, TF1β-cell proliferation is completely dependent on GM-CSF and is not affected by SMFmb-IL-15, whereas both SMF cytokines contribute to TF1β survival.
An increased surface expression of the IL-15Rβ chain improves the efficiency of the response to soluble IL-15,13,30 but no data are available concerning the response of this type of cells to membrane-bound IL-15.
Since NKP, TF1β, and M07Sb cells show different types of response to SMFmb-IL-15, we tried to correlate this behavior to their surface expression of the 3 IL-15R subunit levels (Figure 6E). NKPs express low but detectable levels of the 3 chains, whereas MO7Sb cells do not express the IL-15Rα chain but display surface levels of the β and γc subunits similar to those detected on NKPs. Finally, TF1β cells express the 3 subunits, but the surface level of the IL-15Rβ chain is 1 order of magnitude higher than that found on NKPs or M07Sb cells. Therefore, an optimal response to SMFmb-IL-15 is not dependent on a strong surface expression of the β chain.
Soluble IL-15Rα/IL-15 complex (hyper IL-15) exerts biologic effects on leukemic and normal hematopoietic progenitors
The forms of mb-IL-15 expressed by monocytes7 and by spleen myofibroblasts both form IL-15Rα/IL-15 complexes, enabling the membrane-bound cytokine to activate in trans target cells bearing low-affinity IL-15 receptors. Thus, we investigated whether soluble IL-15Rα/IL-15 complexes, which have no biologic activity on lymphoid cells bearing high-affinity receptors,26 were able to activate cells expressing low-affinity IL-15R.
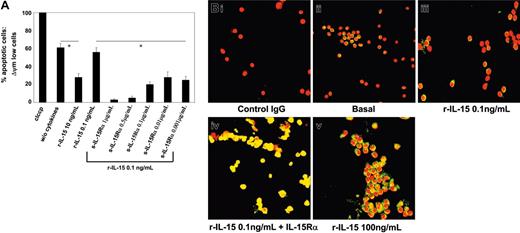
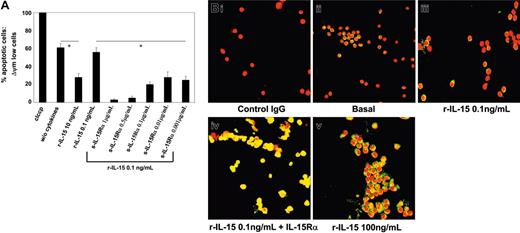
In M07Sb cultures starved of r-GM-CSF for 48 hours, 60% of cells were apoptotic (Figure 7A). In M07Sb cultures treated with 10 ng/mL of r-IL-15 for 24 hours, only 30% of cells were apoptotic, whereas 0.1 ng/mL of r-IL-15 did not have a protective effect. In contrast, starved M07Sb cultures treated with 0.1 ng/mL r-IL-15 preassociated with decreasing concentrations of soluble r-IL-15Rα chain (1 μg-1 ng/mL) displayed between 10% and 30% of apoptotic cells.
We next analyzed the activation pattern of STAT6 in PB NKPs. These cells behave differently from CB NKPs, since they do not express detectable surface or cytoplasmic amounts of the IL-15Rα chain (data not shown), and 25% to 30% of them display the constitutive nuclear localization of pSTAT6 (Figure 7Bii; nuclear yellow staining). This basal STAT6 activation is not modified by low concentrations of r-IL-15 (0.1 ng/mL; Figure 7Biii), but is significantly increased (90%-95% of yellow nuclei) by high concentrations of the cytokine (100 ng/mL) or by the soluble IL-15Rα/IL-15 complexes (Figure 7Bv and 7Biv, respectively). Thus, soluble IL-15Rα/IL-15 complexes (hyper IL-15) may have the same biologic effects on cells bearing low-affinity IL-15R that are obtained only using 100-fold concentrations of r-IL-15 or their membrane-bound counterpart.
Proliferation and protection from apoptosis in M07Sb and TF1β cells following incubation with SMFs. M07Sb (A) and TF1β (B) cells that had been starved of GM-CSF for 24 hours were cocultured for 96 hours with SMFs and subjected to proliferation analysis. Parallel cultures were continuously incubated with neutralizing mAbs recognizing the GM-CSFRα, GM-CSFRβ, IL-15Rβ, IL-15Rα chains, and IL-15. Cells were counted in an electronic Coulter counter and the data are expressed as percentages of difference in proliferative potential with respect to control-untreated samples. The data presented are representative of 3 independent experiments. Treatment with the different neutralizing mAbs significantly reduced SMF-induced proliferation in M07Sb cells (*P < .05 compared with the SMF group). In contrast, in TF1β cells, only treatment with anti-GM-CSFRα and β neutralizing mAbs decrease their proliferation (*P < .05 compared with the SMF group). M07Sb (C) and TF1β (D) cells that had been starved of GM-CSF starved for 24 hours were cocultured for 24 hours with SMFs and analyzed for protection from apoptosis. Parallel cultures were continuously incubated with neutralizing mAbs recognizing the GM-CSFRα, IL-15Rβ, IL-15Rα chains, and IL-15. The percentage of apoptotic cells was determined by flow cytometry using the fluorescent DIOC63 probe to detect cells with a dissipated transmembrane mitochondrial potential (ΔΨm). The data presented are representative of 3 independent experiments. In both cell lines, treatment with the different neutralizing mAbs significantly reduced SMF-antiapoptotic effects (*P < .05 compared with the SMF group). (E) Flow cytometry analysis of IL-15R α, β, and γc subunit surface expressions in NKPs and TF1β and MO7Sb cells. These data are representative of 3 different experiments. Values are means ± SD (n = 3).
Proliferation and protection from apoptosis in M07Sb and TF1β cells following incubation with SMFs. M07Sb (A) and TF1β (B) cells that had been starved of GM-CSF for 24 hours were cocultured for 96 hours with SMFs and subjected to proliferation analysis. Parallel cultures were continuously incubated with neutralizing mAbs recognizing the GM-CSFRα, GM-CSFRβ, IL-15Rβ, IL-15Rα chains, and IL-15. Cells were counted in an electronic Coulter counter and the data are expressed as percentages of difference in proliferative potential with respect to control-untreated samples. The data presented are representative of 3 independent experiments. Treatment with the different neutralizing mAbs significantly reduced SMF-induced proliferation in M07Sb cells (*P < .05 compared with the SMF group). In contrast, in TF1β cells, only treatment with anti-GM-CSFRα and β neutralizing mAbs decrease their proliferation (*P < .05 compared with the SMF group). M07Sb (C) and TF1β (D) cells that had been starved of GM-CSF starved for 24 hours were cocultured for 24 hours with SMFs and analyzed for protection from apoptosis. Parallel cultures were continuously incubated with neutralizing mAbs recognizing the GM-CSFRα, IL-15Rβ, IL-15Rα chains, and IL-15. The percentage of apoptotic cells was determined by flow cytometry using the fluorescent DIOC63 probe to detect cells with a dissipated transmembrane mitochondrial potential (ΔΨm). The data presented are representative of 3 independent experiments. In both cell lines, treatment with the different neutralizing mAbs significantly reduced SMF-antiapoptotic effects (*P < .05 compared with the SMF group). (E) Flow cytometry analysis of IL-15R α, β, and γc subunit surface expressions in NKPs and TF1β and MO7Sb cells. These data are representative of 3 different experiments. Values are means ± SD (n = 3).
Discussion
IL-15 presented in trans as a membrane-bound cytokine, associated to IL-15Rα, represents a dominant form displaying functions that mimic those obtained using high concentrations of soluble r-IL-15.7 Herein, we describe a novel form of biologically active mb-IL-15 constitutively expressed by spleen myofibroblasts and presented in trans associated with the IL-15Rβγc heterodimer. SMFmb-IL-15 activates both normal and leukemic circulating HPs. However, the transcription factor used and the biologic effects induced differ according to the type of progenitor involved and the type of IL-15R expressed by the target cells.
Endogenous IL-15 associates intracellularly to the p56 isoform of the IL-15Rα chain. This complex subsequently migrates to the cell membrane, where it anchors to IL-15Rβγc heterodimers. There are several lines of evidence implying that the 3 chains are involved in the presentation of the SMFmb-IL-15: (1) coimmunoprecipitation experiments on the cytosolic fraction show that, intracellularly, only the p56 isoform of IL-15Rα chain binds to endogenous IL-15; whereas (2) coimmunoprecipitation experiments on the plasma-membrane fraction show the association of the 3 IL-15Rα, IL-15Rβ, and γc chains with IL-15; (3) the expression of this mb-IL-15 is inhibited by adding, during the early phases of cell spreading, neutralizing mAbs directed against the β and γc subunits as well as mAb247 and M111, which recognize the γc chain binding epitope on IL-15.24 Changes in cell shape and architecture, during mitosis or following enzymatic detachment or viral transformation, may unmask some epitopes that are not accessible to antibodies when the cells are spread on a substratum.31-33 Therefore, the transient unmasking of the γc epitope of the endogenous IL-15/IL-15Rα complex as well as of the IL-15-binding epitopes present on the β and γc chains may explain the inhibition of mb-IL-15 caused by the aforementioned neutralizing mAbs during cell spreading. The lack of inhibition observed with the neutralizing anti-IL-15Rα mAb may be explained by the high affinity of the preformed IL-15/IL-15Rα complex that cannot be dissociated by saturating concentrations of any of the neutralizing mAbs used.
In CB HPs, the activation pattern of IL-15-dependent transcription factors suggests the existence of a complex cross-talk, which characterizes the signaling of the different forms of IL-15. Indeed, in freshly isolated unprimed CB progenitors, soluble endogenous IL-1534 only activates NF-κB, the nuclear translocation of which is strongly increased by short-term contact with SMFmb-IL-15.
In the same cells cultured for 5 days in vitro in the presence of r-SCF and r-Flt3-L, and therefore committed to the pro-NK pathway,12,13 endogenous IL-15 triggers the activation of STAT3, whereas short-term contact with SMFmb-IL-15 activates STAT5 and STAT6.
STAT6 has long been considered to be a specific target of the T helper 2 (Th2) cytokines, IL-4 and IL-13.35 However, STAT6 can also be induced by IL-15 in murine mast cells displaying a restricted expression of IL-15Rα isoforms competent for this type of signalling.36,37
We have previously reported that unprimed CD34+ HPs, soon after immunopurification, in the absence of exogenously added cytokines, constitutively secrete low amounts of IL-15, which controls, by autocrine loops, the activation of different transcription factors but not of STAT6.34 Here, we show that STAT6 can be activated by IL-15 in normal proliferating HPs committed to the NK and to the erythroid pathways. The activation pattern, however, depends on the type of progenitors involved and on the form of IL-15. Indeed, soluble IL-15 activates STAT6 (phosphorylation and nuclear translocation) both in NKPs and proerythroid progenitors at very low concentrations (0.1 ng/mL), but not at high concentrations (100 ng/mL). In contrast, in adult PB NKPs, we detected an opposite pattern of STAT6 activation, since a subset of these progenitors display a constitutive activation of STAT6 that is up-regulated only using high concentrations of soluble r-IL-15. Moreover, SMFmb-IL-15 activates STAT6 in NKPs but not in proerythroid progenitors. This indicates that the membrane-bound form of IL-15, but not the soluble form, may exert a selective activation of STAT6.
Protection of M07Sb cells from apoptosis and STAT6 activation in PB progenitors committed to the pro-NK lineage by r-IL-15/s-IL-15Rα complex. (A) Protection of M07Sb cells from apoptosis by r-IL-15/s-IL-15Rα complex. M07Sb cells that had been starved of GM-CSF for 24 hours were treated for 24 hours with r-IL-15 (10 and 0.1 ng/mL) and with 0.1 ng/mL r-IL-15 bound to decreasing concentrations of the soluble IL-15Rα chain (1000-1 ng/mL). The percentage of apoptotic cells was determined by flow cytometry as described in the legend to Figure 6. As a control of apoptosis, cells were also treated with a membrane uncoupler, carbonyl cyanide m-chlorophenylhydrazone (ClCCP; Sigma Chemical), at a final concentration of 50 μM, for 15 minutes at 37°C under 5% CO2. The data presented are representative of 3 independent experiments. R-IL-15 at 10 ng/mL but not at 0.1 ng/mL induced significant protection from apoptosis (*P < .05 compared with the group without cytokine). Association of decreasing concentrations of soluble IL-15Rα with 0.1 ng/mL r-IL-15 induced powerful antiapoptotic effects (*P < .05 compared with the group treated with 0.1 ng/mL r-IL-15). Values are means ± SD (n = 3). Horizontal bars identify the sample on which statistic significance has been observed. (B) STAT6 activation analysis by confocal microscopy of PB progenitors committed to the pro-NK lineage using r-SCF/r-Flt3-L medium. Overlay pictures (original magnification, ×63). (Bi) Negative control. (Bii) basal conditions. (Biii) 30 minutes of incubation at 37°C with 0.1 ng/mL human r-IL-15. (Biv) 30 minutes of incubation at 37°C with 0.1 ng/mL human r-IL-15 bound to 1 μg/mL of the soluble IL-15Rα chain. (Bv) 30 minutes of incubation at 37°C with 100 ng/mL human r-IL-15. As negative controls, cells were incubated with rabbit IgG, the second reagent, and propidium iodide. The data presented are representative of 3 independent experiments. Images were acquired with a 63 ×/1.30 objective lens.
Protection of M07Sb cells from apoptosis and STAT6 activation in PB progenitors committed to the pro-NK lineage by r-IL-15/s-IL-15Rα complex. (A) Protection of M07Sb cells from apoptosis by r-IL-15/s-IL-15Rα complex. M07Sb cells that had been starved of GM-CSF for 24 hours were treated for 24 hours with r-IL-15 (10 and 0.1 ng/mL) and with 0.1 ng/mL r-IL-15 bound to decreasing concentrations of the soluble IL-15Rα chain (1000-1 ng/mL). The percentage of apoptotic cells was determined by flow cytometry as described in the legend to Figure 6. As a control of apoptosis, cells were also treated with a membrane uncoupler, carbonyl cyanide m-chlorophenylhydrazone (ClCCP; Sigma Chemical), at a final concentration of 50 μM, for 15 minutes at 37°C under 5% CO2. The data presented are representative of 3 independent experiments. R-IL-15 at 10 ng/mL but not at 0.1 ng/mL induced significant protection from apoptosis (*P < .05 compared with the group without cytokine). Association of decreasing concentrations of soluble IL-15Rα with 0.1 ng/mL r-IL-15 induced powerful antiapoptotic effects (*P < .05 compared with the group treated with 0.1 ng/mL r-IL-15). Values are means ± SD (n = 3). Horizontal bars identify the sample on which statistic significance has been observed. (B) STAT6 activation analysis by confocal microscopy of PB progenitors committed to the pro-NK lineage using r-SCF/r-Flt3-L medium. Overlay pictures (original magnification, ×63). (Bi) Negative control. (Bii) basal conditions. (Biii) 30 minutes of incubation at 37°C with 0.1 ng/mL human r-IL-15. (Biv) 30 minutes of incubation at 37°C with 0.1 ng/mL human r-IL-15 bound to 1 μg/mL of the soluble IL-15Rα chain. (Bv) 30 minutes of incubation at 37°C with 100 ng/mL human r-IL-15. As negative controls, cells were incubated with rabbit IgG, the second reagent, and propidium iodide. The data presented are representative of 3 independent experiments. Images were acquired with a 63 ×/1.30 objective lens.
STAT3 and STAT5 are involved in the control of early hematopoietic development, promoting multilineage differentiation,38 whereas STAT6 displays a more restricted role, controlling the committed myeloid progenitors but not the primitive stem/progenitor cells.39 Thus, the combined action of IL-15 produced by HPs34 and that of SMFmb-IL-15 suggests that this cytokine plays a central role in hematopoietic functions, controlling both primitive and committed hematopoietic compartments. In addition, this combined activation of different transcription factors is probably involved in triggering survival and proliferation of circulating CD34+ NKPs as suggested by the SMFmb-IL-15-induced, STAT5-dependent up-regulation of the antiapoptotic factor Bcl-X25 and the key cell-cycle regulator cyclin D1.25
As macrophagic mb-IL-15 fully activates cells expressing the IL-15 low-affinity receptor IL-15Rβγc,7 we compared the effects of SMFmb-IL-15 on 2 growth factor-dependent human CD34+ hematopoietic cells lines: M07Sb and TF1β, which express low- and high-affinity IL-15R, respectively. SMFmb-IL-15 promoted the proliferation and survival of M07Sb cells, but only promoted the survival of TF1β cells. This apparently paradoxic effect clearly indicates that SMFmb-IL-15 exerts specialized functions, modulating its biologic effects according to not only the type of progenitor it interacts with (Figures 3,4) but also the type of IL-15R present on target cells.
The differential behavior reported herein is due to the specific role of the IL-15Rα chain present on TF1β cells, which, upon contact with SMFmb-IL-15, favors the phosphorylation of NF-κB and STAT6, but inhibits that of STAT3 and STAT5. In contrast, in M07Sb cells, SMFmb-IL-15 activates the STAT3 and STAT5 pathways, which seem to be necessary and sufficient to promote IL-15-induced survival and proliferation likely through the STAT5-dependent up-regulation of Bcl-X and cyclin D1.
Our previous results indicate that human spleen myofibroblasts, through the action of SMFmb-IL-15, constitute a specialized subset able to influence the behavior of both normal and pathologic progenitors. Indeed, SMFs allow the proliferation and the differentiation into NK cells of circulating CD34+ cells from healthy donors, whereas they favor the expansion but not the differentiation of progenitors from patients with myelodysplasia.18,19 SMFs can also favor the survival and expansion of myeloid leukemic clones expressing low- (survival and expansion) or high-affinity IL-15R (survival), probably mediating the activation of aberrant signaling pathways. For instance, SMFmb-IL-15 up-regulates, through STAT5 activation, Bcl-X, cyclin D1, and SOCS3 expression in MO7Sb cells, whereas there is no SOCS3 induction in normal NKP cells. Suppressor of cytokine signaling (SOCS) proteins are induced by STATs and inhibit signaling through various negative-feedback mechanisms.24 In this respect, SOCS3 is a powerful inhibitor of STAT3 and STAT5 activation,24 the efficiency of which is largely increased by the presence of an activated IL-2Rβ chain,40 and may act as major negative regulator of different hematopoietic differentiation pathways.41-43 Thus, the STAT5-dependent up-regulation of SOCS3 in MO7Sb cells may be part of the molecular mechanisms that block the differentiation potential of these leukemic cells, whereas lack of SOCS3 IL-15-dependent induction in NKP cells could favor their differentiation potential.
SMFmb-IL-15 and macrophagic mb-IL-15 share the capacity to activate target cells expressing a low-affinity IL-15 receptor (IL-15Rβγc). This property is probably due to the IL-15/IL-15Rα complex present in both mb-IL-15 forms. However, it is not clear whether this dominant effect is strictly dependent on the membrane-bound presentation or not. Our data show that low concentrations (0.1 ng/mL) of soluble IL-15, which are unable to protect GM-CSF-starved M07Sb cells from apoptosis or to induce STAT6 activation in adult PB pro-NK progenitors, can do so when the cytokine is preassociated with picomolar concentrations of the soluble r-IL-15Rα chain. Therefore, this dominant pattern is a property of the IL-15/IL-15Rα complex (hyper IL-15), regardless of whether it is in its soluble or membrane-bound form. This has important bearings, because several human cell lines constitutively secrete picomolar concentrations of the soluble IL-15Rα chain, able to block the IL-15-induced proliferation of T cells bearing a high-affinity receptor.44 These data highlight the dual potential of the soluble IL-15Rα chain: on the one hand, it can inhibit the action of physiologic concentrations of soluble IL-15 on cells expressing a high-affinity IL-15R; but on the other hand, it is able to amplify its effects on cells bearing a low-affinity IL-15 receptor. However, unlike the mb-IL-15 form, which has long-lasting effects, the soluble complex only has short-term effects.
In conclusion, IL-15 is a multifaceted cytokine that, through the combined action of its different forms (soluble, membrane-bound, soluble IL-15/IL-15Rα complex), appears to play a major role in the hematopoietic development controlling the survival, proliferation, and differentiation of both normal and leukemic progenitors.
Prepublished online as Blood First Edition Paper, June 23, 2005; DOI 10.1182/blood-2005-01-0064.
Supported by grants from the Association pour la Recherche sur le Cancer (ARC; no. 3206), Nouvelles Recherches Scientifiques (NRB)-Vaincre le Cancer, Fondation de France (no. 2003005187), Italian Association for Cancer Research (AIRC), and the Italian Ministry of Health and Consiglio Nazionale Ricerca (CNR) Functional Genomics. J.G-M. is a recipient of ARC and Istituto G. Gaslini Fellowships, M.F. is a recipient of an NRB-Vaincre le Cancer Fellowship, M.G. is a recipient of a Clinical and Experimental Immunology Doctoral School of the University of Genoa fellowship, and D.D. is a recipient of NRB-Vaincre le Cancer and ARMIA Fellowships.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank the Centre Hospitalier de Longjumeau and the Clinique Ambroise-Paré of Bourg-la-Reine for providing us with cord-blood samples. We also thank Drs Paul Sondel, Yannick Jacques, and Thomas Waldmann for providing reagents.