Abstract
Immunostimulants represent an emerging class of drugs for the treatment of infectious disorders and cancer. CpG oligonucleotides and imiquimod, prototypic drugs in this category, are now known to activate dendritic cells (DCs). Here we report the development of a highly sensitive, unbiased functional screen to detect DC-stimulatory signals. Because interleukin-1β (IL-1β) mRNA expression is closely associated with DC activation, we engineered DCs to stably express a fluorescent marker gene under the control of IL-1β promoter. By screening about 3000 compounds with the resulting DC biosensor clone, we identified DC-stimulatory potentials of topoisomerase I inhibitors (camptothecin derivatives) and microtubule depolymerizing drugs (colchicine and podophyllotoxin). In response to treatment with each agent, bone marrow–derived DC preparations exhibited characteristic phenotypic and/or functional changes associated with DC activation. All of these agents also triggered nuclear factor–κB (NFκB) activation in DCs, suggesting a common pharmacologic mechanism of action. Furthermore, locally administered colchicine induced in situ maturation and migration of DCs and augmented both humoral and cellular immune responses. These results support the practical utility of the DC-based biosensor system to discover novel DC-targeted immunostimulants and unveil previously unrecognized (and totally unexpected) pharmacologic activities of several drugs that are commonly used for the treatment of various disorders.
Introduction
Dendritic cells (DCs) are special subsets of professional antigenpresenting cells that play pivotal roles in the induction of protective immunity against potentially harmful antigens. DCs in peripheral epithelial tissues are “immature” and, upon encountering microbial products or proinflammatory mediators, they undergo dynamic and coordinated reprogramming of gene expression, surface phenotype, and cellular function, the process known as DC maturation. For example, DCs differentiate into fully potent T-cell stimulators by elevating surface expression of major histocompatibility complex class II (MHC II) molecules and costimulatory molecules, produce a wide variety of cytokines and chemokines, and migrate to draining lymph nodes (LNs), where antigen presentation takes place. In essence, DC maturation is the initial and key event for triggering both innate and adaptive immune responses.1 Partial or full maturation of DCs is inducible by many agents, including Toll-like receptor (TLR) ligands, cytokines, various molecules released from necrotic cells (eg, adenosine triphosphate [ATP], adenosine diphosphate [ADP], uric acid, and high-mobility group B1 protein), CD40 ligand, and eicosanoids.2-8
Immunostimulants (also known as immune response modifiers) represent an emerging class of drugs that are designed to amplify naturally occurring immune responses against infectious pathogens and tumor cells. The unmethylated CpG motif in bacterial DNA was originally discovered as a B-cell–stimulating adjuvant,9 and synthetic oligodeoxynucleotides (ODNs) containing the CpG motifs have been shown to exhibit potent therapeutic activities in many infection and cancer models in experimental animals.10,11 Imiquimod, which was first identified as a small molecular antiviral agent, is now applied topically to patients with anogenital warts, one of the most common sexually transmitted viral infections, as well as basal-cell carcinoma.12,13 With respect to pharmacologic mechanisms of action, CpG ODNs and imiquimod serve as synthetic ligands for TLR9 and TLR7, respectively,14,15 and both induce DC maturation efficiently.16-18 Thus, DCs appear to serve as a relevant target of immunostimulants.
Most of the currently known DC-stimulatory agents were identified by testing rationally selected compounds for their in vitro capacities to induce expected phenotypic and functional changes that accompany DC maturation or by testing a limited array of natural products for their in vivo capacities to augment protective immune responses against cancer or infectious microbes. Under a broad hypothesis that novel DC-stimulatory agents with clinical applicability can be discovered through large-scale screening of small chemicals, we sought to develop an unbiased functional screen. Here we report the development and validation of a DC-based biosensor system as a highly sensitive, high throughput (HTP)–compatible screening platform for the discovery of DC-targeted immunostimulants.
Materials and methods
Generation of the DC biosensor clone
The murine epidermal-derived DC line, XS106, was employed as an indicator. Phenotypic and functional features of this line are described elsewhere.19,20 We generated the interleukin-1β (IL-1β) promoter-driven yellow fluorescent protein (YFP) reporter construct by inserting a 4138-bp BamHI fragment of the murine IL-1β promoter21 into the BglII and BamHI sites of the pEYFP-1 vector containing an SV40 early promoter–driven neomycin resistance gene (BD Biosciences Clontech, Palo Alto, CA). The XS106 DC line was transduced with the construct by the gene gun, and stably transduced DC clones were then established by limiting dilution in the presence of G418 as described previously.19
Test samples and DC biosensor screening
A “diverse set” library of 1986 compounds selected based on the chemical structures and the availability in large quantities was obtained from the Developmental Therapeutics Program at the National Cancer Institute (NCI). In the first screening, these compounds (originally dissolved in dimethyl sulfoxide [DMSO] at 10 mM) were pooled (8 per group) and tested at 2 μM each (with the final DMSO concentration of 0.16%). A library of 880 Food and Drug Administration (FDA)–approved drugs was purchased from Prestwick (Washington, DC), and individual drugs were tested at 4 μg/mL in the first screening. The following agents purchased from Sigma (St Louis, MO) or Calbiochem (San Diego, CA) were tested at the indicated concentrations unless otherwise mentioned: lipopolysaccharide (LPS) from Escherichia coli (30 ng/mL), CpG ODNs 1668 (5′-TCCATGACGTTCCTGATGCT-3′) (1 μM), ATP (0.5 mM), ADP (0.5 mM), necrotic Pam 212 keratinocytes prepared by repeated freeze-and-thaw procedures (106 cells per milliliter), croton oil (CrO; 0.01% vol/vol), and phorbol myristate acetate (PMA; 50 ng/mL). None of the agents including the hit compounds (except for LPS) contained detectable amounts of endotoxin as tested by the QCL-1000 system (Cambrex, Walkersville, MD). The XS106-pIL1-YFP DC clone (2.5 × 105 cells per milliliter, 500 μL per tube) was cultured in complete RPMI 1640 supplemented with 0.5 ng/mL murine granulocyte-macrophage colony-stimulating factor (GM-CSF) and 5% NS fibroblast-conditioned medium in the continuous presence of test compounds and then examined for YFP expression and propidium iodide (PI) uptake by FACSCalibur (BD Biosciences Clontech). The FDA-approved drugs were screened in 96-well plates (2.5 × 105 cells per milliliter, 200 μL per well) from which indicator DCs were applied directly to the FACSCalibur in a fully automated fashion using an HTP sampler device (BD Biosciences Clontech). Liquid handling procedures were semiautomated with the Aquarius bench top multichannel pipetting system (Tecan, Durham, NC).
In vitro impacts of hit compounds on DCs
Following incubation with selected agents, XS106 DCs were examined for IL-1β mRNA expression using RiboQuant Multi-Probe RNase Protection Assay System (BD Biosciences Clontech). To test the impact on nuclear factor–κB (NF-κB) activation, XS106 DCs were incubated for 5 hours with selected agents, and nuclear extracts were then examined for NF-κB p65 and c-Rel using the enzyme-linked immunosorbent assay (ELISA)–based Mercury TransFactor kit (BD Biosciences Clontech), in which all NF-κB subunits are first captured by the NF-κB–binding DNA consensus sequence on the plate and individual subunits are then measured with subunit-specific antibodies. Bone marrow–derived DCs (BM-DCs) propagated from BALB/c mice were incubated for 24 hours with test agents and then examined for phenotypic and functional maturation as described previously.22 To test CC chemokine receptor-7 (CCR-7) expression, BM-DCs were first incubated with rat monoclonal antibody (mAb) against murine CCR-7 (eBioscience, San Diego, CA) or isotype-matched control rat immunoglobulin G (IgG) and then with fluorescein isothiocyanate (FITC)–conjugated goat anti–rat IgG. The samples were then stained with allophycocyanin (APC)–conjugated anti-CD11c mAb.
To test the impact on T-cell–stimulatory capacity, BM-DCs (derived from BALB/c mice) were incubated for 24 hours with 3 μg/mL colchicine (COL), washed extensively, and then examined for their potential to activate splenic T cells purified from C57BL/6 mice by 3H-thymidine uptake.22 In some experiments, OVA323-339 peptide (2 μg/mL) or phosphate-buffered saline (PBS) alone was added to the BM-DC cultures during the last 2 hours of incubation with COL. After extensive washing, these BM-DCs were cocultured with CD4 T cells purified from the DO11.10 transgenic mice as before.22,23 Following 24 hours of incubation with COL or vehicle alone, BM-DCs were incubated for 10 minutes with 5 μg/mL FITC-conjugated dextran (70 000 Da molecular weight; Sigma) at 4°C or 37°C, washed extensively, and then examined for FITC signals within the CD11c+ populations. Chemotaxis assays were performed using 6.5-mm Transwell units with a pore size of 5 μm (Costar, Cambridge, MA) as described previously.24 Briefly, BM-DCs (5 × 105 cells per well) were added to the upper chambers, whereas 100 ng/mL CCL-19 (R&D Systems, Minneapolis, MN) or PBS alone was added to the lower chambers. After 2 hours of incubation, migratory cells harvested from the lower chambers were examined for total cell numbers and the percentages of CD11c+ cells. The percentage DC migration was then calculated by dividing the output CD11c+ cells by the input CD11c+ cells.
In vivo testing of pharmacologic activities of COL
BALB/c mice (8- to 10-week-old females) received subcutaneous injections of ovalbumin (OVA; 400 μg per animal) together with COL (6 μg per animal) or vehicle alone at the base of the tail on days 0 and 7. On day 14, serum samples and inguinal LN-cell suspensions were examined for OVA-specific humoral responses by ELISA and proliferative responses to OVA by 3H-thymidine uptake, respectively.19 In an independent set of experiments, COL (1.2 μg per animal) or vehicle alone was subcutaneously injected to the right or left ear, respectively. Two days later, ear skin samples were harvested to examine the number of MHC II–positive Langerhans cells (LCs), expression levels of MHC II, and frequencies of CD86+ LCs in epidermal sheet preparations using an Olympus BX60 microscope (Olympus, Melville, NY) equipped with an Olympus DPIO digital camera and MetaMorph software (Universal Imaging, Downingtown, PA).25 Concurrently, cervical LN cells were examined by flow cytometry for total numbers of CD11c+ cells as well as for surface expression of MHC II and CD86 within the CD11c+ gated populations. All animal experiments were conducted according to the National Institutes of Health (NIH) guidelines and were approved by the Institutional Review Board.
Statistical analysis
Comparisons between 2 groups were performed using a 2-tailed Student t test, and more than 2 groups were compared by analysis of variance (ANOVA). Each experiment was repeated at least 3 times to assess reproducibility.
Results
Development of DC biosensor system
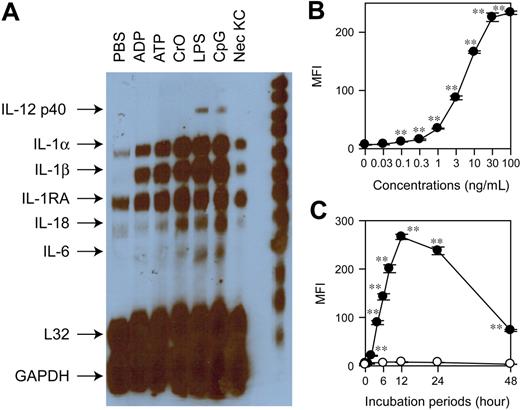
To assemble a DC-based biosensor system, we chose to employ a stable DC line XS106 established from the epidermis of newborn mice not only because this line maintains many features of skin-resident DCs19,20 but also because it responds to diverse stimuli by altering the phenotypes and functions.26 For example, we have observed recently that TLR ligands (LPS and CpG ODNs), purinergic type 2 receptor (P2R) ligands (extracellular ATP and ADP), necrotic keratinocytes, and a proinflammatory chemical, CrO, differentially regulate the expression of MHC II and CD86 as well as the production of tumor necrosis factor-α (TNF-α), IL-6, IL-12, vascular endothelial growth factor (VEGF), and macrophage inflammatory protein-1α (MIP-1α) by XS106 DCs.26 Interestingly, IL-1β mRNA, which was barely detectable by RNase protection assay in XS106 DCs after treatment with PBS alone, was commonly induced by all tested stimuli (Figure 1A). This implied that IL-1β mRNA expression might serve as a universal readout (or a surrogate marker) indicating the activation status of XS106 DCs. In an attempt to develop an HTP-compatible assay platform, we sought to engineer XS106 DCs to stably express the YFP gene under the control of the IL-1β promoter. Briefly, we introduced a 4.1-kb 5′-flanking fragment isolated from the murine IL-1β gene21 upstream of the YFP coding sequence without additional transcription control elements and then transduced the XS106 DC line with the resulting pIL1-YFP construct using the gene gun. After G418 selection, permanently transduced DC clones were established by 2 rounds of limiting dilution. A representative DC biosensor clone, termed the XS106-pIL1-YFP, expressed YFP in response to LPS stimulation in dose- and time-dependent fashions. LPS as low as 0.1 ng/mL was sufficient to induce significant YFP expression, indicating high sensitivity of our assay (Figure 1B). YFP expression became detectable within 120 minutes after LPS treatment, indicating rapid responsiveness (Figure 1C).
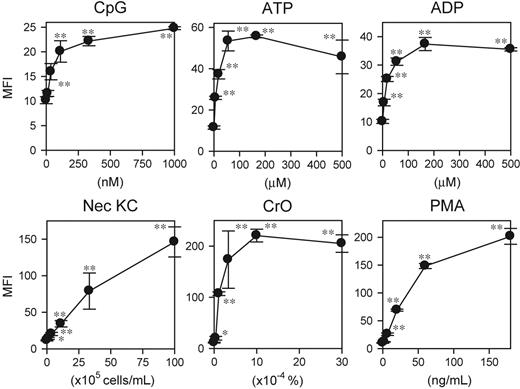
Development and characterization of DC biosensor system. (A) XS106 DCs were incubated for 6 hours with the indicated agents, including necrotic keratinocyte preparations (Nec KC), and then examined for cytokine mRNA profiles by RNase protection assay. (B) The XS106-pIL1-YFP DC biosensor clone was incubated for 16 hours with LPS at the indicated concentrations and then examined for the mean fluorescence intensity (MFI) for YFP signals (mean ± SD from triplicate samples). (C) The same clone was incubated with 30 ng/mL LPS (•) or PBS alone (○) for the indicated periods and then examined for YFP expression (mean ± SD; n = 3). Asterisks indicate statistically significant (**P < .01) differences compared with the nontreated control samples. Data shown in this figure are representative of at least 3 independent experiments producing similar results.
Development and characterization of DC biosensor system. (A) XS106 DCs were incubated for 6 hours with the indicated agents, including necrotic keratinocyte preparations (Nec KC), and then examined for cytokine mRNA profiles by RNase protection assay. (B) The XS106-pIL1-YFP DC biosensor clone was incubated for 16 hours with LPS at the indicated concentrations and then examined for the mean fluorescence intensity (MFI) for YFP signals (mean ± SD from triplicate samples). (C) The same clone was incubated with 30 ng/mL LPS (•) or PBS alone (○) for the indicated periods and then examined for YFP expression (mean ± SD; n = 3). Asterisks indicate statistically significant (**P < .01) differences compared with the nontreated control samples. Data shown in this figure are representative of at least 3 independent experiments producing similar results.
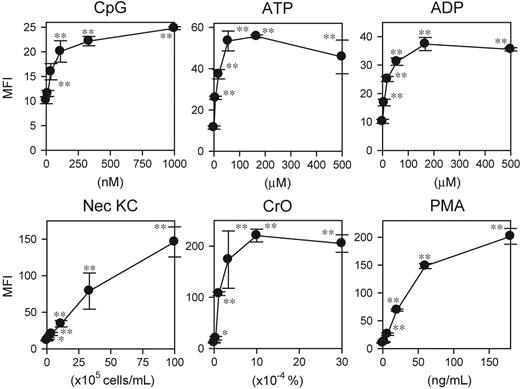
A key question concerned whether other DC-stimulatory signals would be also detectable with our DC biosensor system. Corroborating our RNase protection data, the XS106-pIL1-YFP DC clone expressed significant YFP signals in response to CpG ODNs, ATP, ADP, necrotic keratinocytes, and CrO (Figure 2). Moreover, the DC biosensor clone also responded to PMA as well as forskolin, an adenylate cyclase activating reagent (Figure 2 and data not shown). These observations document the broad reactivity of the DC biosensor clone to diverse pharmacologic and biologic stimuli.
Identification of DC-stimulatory potentials of topoisomerase I inhibitors
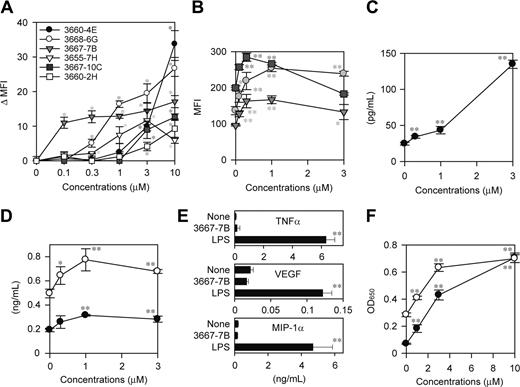
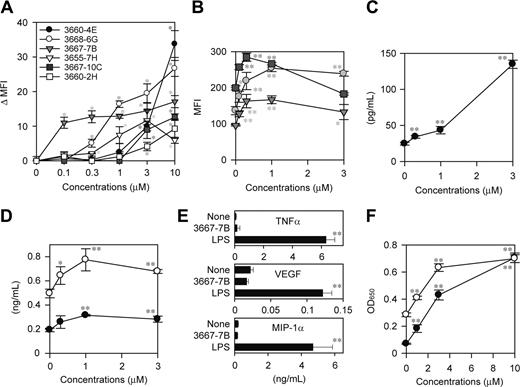
To assess the potential utility of the system for drug discovery, we screened a small chemical library from the NCI with the XS106-pIL1-YFP DC clone. In the first screen, test chemicals were grouped (8 compounds per group) and added to the cultures at 2 μM each. By testing 1986 chemicals in this manner, we selected 35 “positive” groups that induced YFP expression above the baseline level (mean + 3 SD observed in the absence of added chemicals) without affecting cell viability as measured by PI uptake. In the second screen, we tested individual chemicals in the positive groups at 2 μM and identified 16 compounds inducing significant (P < .05) YFP expression. From this panel, we finally selected 6 “hits” that induced significant (P < .01) YFP expression in a dose-dependent fashion in the range from 0.1 to 10 μM (Figure 3A).
To determine whether any of the hits identified with a genetically engineered DC clone would, in fact, alter the maturational state of bona fide DC populations, we examined the effect of each hit compound on surface expression of MHC II and the costimulatory molecules CD40 and CD80 by BM-DCs. Three of the hits from the NCI library, 3667-7B, 3667-10C, and 3668-6G, were found to significantly elevate the expression of all tested markers of DC maturation. For example, compound 3667-7B dose dependently up-regulated MHC II, CD40, and CD80 by BM-DCs in the range from 0.3 to 3 μM (Figure 3B). Moreover, compound 3667-7B also triggered significant production of IL-1β protein by BM-DCs in the same dose range (Figure 3C). This compound, however, induced only modest production of IL-6 and IL-12 p40 and no detectable production of TNF-α, VEGF, and MIP-1α by BM-DCs, although all these factors were released after LPS stimulation (Figure 3D-E). Likewise, the other hit compounds 3667-10C and 3668-6G identified in the NCI library caused phenotypic maturation of BM-DCs without triggering robust cytokine/chemokine production (data not shown).
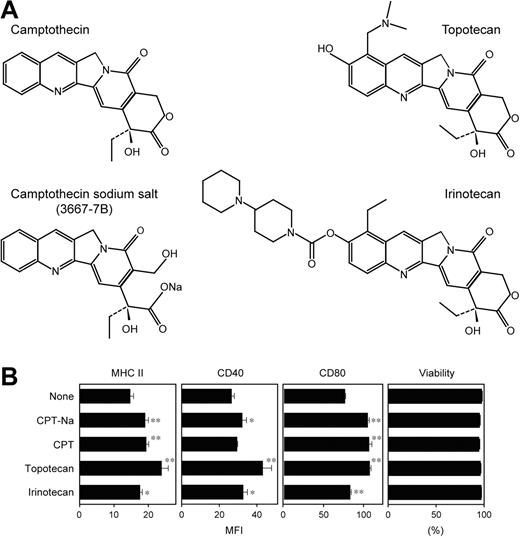
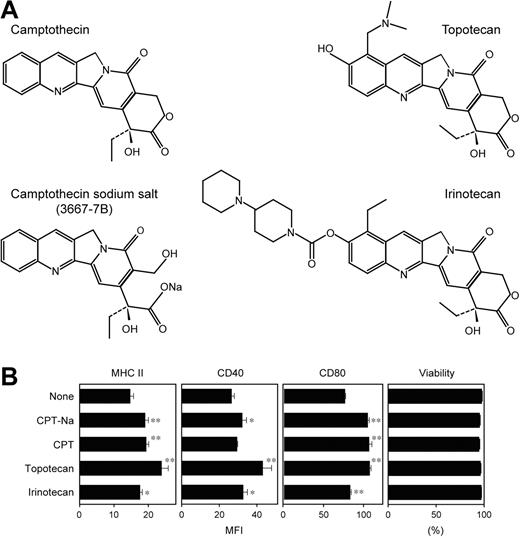
Compound 3667-7B was decoded as camptothecin sodium salt (CPT-Na), an anticancer drug within the family of topoisomerase I inhibitors. CPT and its derivatives induce reversible single-strand breaks and irreversible double-strand breaks, thereby inhibiting DNA replication.27 Interestingly, CPT derivatives have recently been reported to activate the NF-κB–dependent gene transactivation pathway, with the implication that the resulting shift in the balance between cell death and survival may lead to accelerated apoptosis of tumor cells.28-30 Because NF-κB activation has been observed in DCs after exposure to many external stimuli, such as TLR ligands, cytokines, and CD40 ligand,31-33 it was of particular interest to examine the potential impact of CPT-mediated DNA breaks on the NF-κB pathway in DCs. CPT-Na, indeed, induced significant activation of NF-κB p65 and c-Rel subunits in XS106 DCs in a dose-dependent manner (Figure 3F).
Two CPT analogs, topotecan (a water-soluble derivative containing a dimethylamino methyl side chain) and irinotecan (a prodrug requiring the cleavage of a dipiperidine side chain), are currently approved by the US FDA as chemotherapeutics in cancer patients (Figure 4A).27 As observed with CPT-Na, both topotecan and irinotecan also significantly elevated surface expression of MHC II, CD40, and CD80 by BM-DCs without affecting their viability (Figure 4B). However, CPT-Na, CPT, topotecan, and irinotecan all failed to significantly augment the capacity of BM-DCs to activate naive T cells in the primary allogeneic mixed leukocyte reactions—that is, a functional hallmark of DC maturation (data not shown). Thus, it appears that nuclear signals resulting from CPT-induced DNA breaks induce partial maturation of DCs.
Reactivity of DC biosensor clone to diverse stimuli. The XS106-pIL1-YFP DC clone was incubated for 16 hours with each test agent at the indicated concentrations and then examined for YFP expression (mean ± SD; n = 3). Asterisks indicate statistically significant YFP up-regulation compared with the nontreated samples (*P < .05, **P < .01). Data shown are representative of 3 independent experiments producing similar results.
Reactivity of DC biosensor clone to diverse stimuli. The XS106-pIL1-YFP DC clone was incubated for 16 hours with each test agent at the indicated concentrations and then examined for YFP expression (mean ± SD; n = 3). Asterisks indicate statistically significant YFP up-regulation compared with the nontreated samples (*P < .05, **P < .01). Data shown are representative of 3 independent experiments producing similar results.
Identification of DC-stimulatory compounds in the NCI library. (A) Six hits were identified by screening 1986 compounds in the NCI library with the DC biosensor system. The XS106-pIL1-YFP DC clone was incubated for 16 hours with individual hit compounds at the indicated concentrations and then examined for YFP expression. Data shown are the delta MFI values representing the YFP fluorescent signals above the baseline level detected in the absence of added stimuli (mean ± SD; n = 3). *P < .01 (compared with vehicle-treated samples). (B) BM-DCs were incubated for 24 hours with compound 3667-7B at the indicated concentrations and then examined for the surface expression of MHC II (circles), CD40 (triangles), and CD80 (squares) (mean ± SD; n = 3). *P < .05, **P < .01 (compared with vehicle-treated samples). (C-D) The supernatants of the same BM-DC cultures were examined for secretion of IL-1β (C), IL-6 (D, ○), and IL-12 p40 (D, •) (mean ± SD; n = 3). *P < .05, **P < .01 (compared with vehicle-treated samples). (E) BM-DCs were incubated for 24 hours with compound 3667-7B (1 μM), LPS (10 ng/mL), or vehicle alone and then examined for the production of indicated factor (mean ± SD; n = 3). **P < .01 (compared with vehicle-treated samples). (F) XS106 DCs were incubated for 5 hours with compound 3667-7B at the indicated concentrations and then examined for activation of NF-κB p65 (•) and c-Rel (○) (mean ± SD; n = 3). **P < .01 (compared with vehicle-treated samples).
Identification of DC-stimulatory compounds in the NCI library. (A) Six hits were identified by screening 1986 compounds in the NCI library with the DC biosensor system. The XS106-pIL1-YFP DC clone was incubated for 16 hours with individual hit compounds at the indicated concentrations and then examined for YFP expression. Data shown are the delta MFI values representing the YFP fluorescent signals above the baseline level detected in the absence of added stimuli (mean ± SD; n = 3). *P < .01 (compared with vehicle-treated samples). (B) BM-DCs were incubated for 24 hours with compound 3667-7B at the indicated concentrations and then examined for the surface expression of MHC II (circles), CD40 (triangles), and CD80 (squares) (mean ± SD; n = 3). *P < .05, **P < .01 (compared with vehicle-treated samples). (C-D) The supernatants of the same BM-DC cultures were examined for secretion of IL-1β (C), IL-6 (D, ○), and IL-12 p40 (D, •) (mean ± SD; n = 3). *P < .05, **P < .01 (compared with vehicle-treated samples). (E) BM-DCs were incubated for 24 hours with compound 3667-7B (1 μM), LPS (10 ng/mL), or vehicle alone and then examined for the production of indicated factor (mean ± SD; n = 3). **P < .01 (compared with vehicle-treated samples). (F) XS106 DCs were incubated for 5 hours with compound 3667-7B at the indicated concentrations and then examined for activation of NF-κB p65 (•) and c-Rel (○) (mean ± SD; n = 3). **P < .01 (compared with vehicle-treated samples).
Microtubule depolymerizing drugs trigger full maturation of DCs in vitro and in vivo
In an attempt to identify one or more compounds capable of driving full maturation of DCs, we next screened a second library composed of 880 biologically active and chemically diverse drugs that have been approved by the FDA for clinical use. To improve the HTP capability, we miniaturized the DC biosensor culture into a 96-well format and automated the process of flow cytometric measurement of YFP expression, a major bottleneck of the assay. By testing individual drugs at 4 μg/mL in the modified assay system, we identified a total of 40 drugs that dose dependently induced significant (P < .05) YFP expression. Of these hits, COL and podophyllotoxin (PDP), which induced YFP expression at 1 μg/mL and 0.03 μg/mL, respectively, were of particular interest because COL was reported in the 1970s to exhibit an adjuvant-like activity in experimental animals,34,35 although underlying mechanisms remain totally unknown.
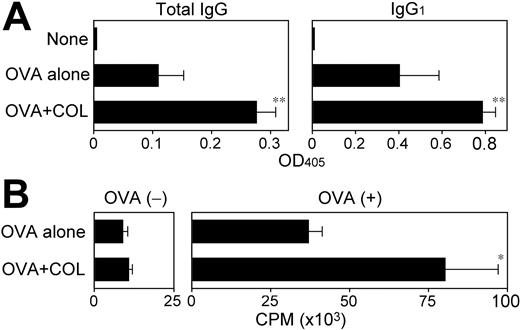
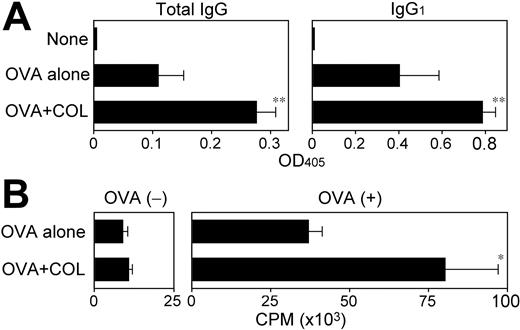
Corroborating those previous reports, coinjections of a relatively small amount (6 μg per animal) of COL with a foreign protein antigen OVA significantly boosted OVA-specific humoral responses (Figure 5A). Although OVA-reactive immunoglobulins of IgG1, IgG2a, and IgG2b isotypes were all readily detectable after immunization with OVA plus CpG ODNs, the mice immunized with OVA plus COL produced primarily the IgG1 isotype, with the implication that COL may preferentially augment Th2-directed immune responses. Immunization with OVA plus COL also triggered significant cellular immune responses as measured by OVA-specific proliferative responses and IL-2 production (Figure 5B and data not shown). These observations document the pharmacologic activity of COL to boost both humoral and cellular immune responses. We failed to confirm an adjuvant activity of PDP due to its substantial in vivo toxicity.
In vitro impacts of CPT derivatives on DCs. (A) Chemical structures of the 4 tested CPT derivatives are shown. Compound 3667-7B was decoded to be CPT-Na. (B) BM-DCs were incubated for 24 hours with each CPT derivative at 1 μM or vehicle alone and then examined for the surface expression of MHC II, CD40, and CD80 and for cell viability by PI uptake (mean ± SD; n = 3). Asterisks indicate statistically significant (*P < .05, **P < .01) increases compared with vehicle-treated controls.
In vitro impacts of CPT derivatives on DCs. (A) Chemical structures of the 4 tested CPT derivatives are shown. Compound 3667-7B was decoded to be CPT-Na. (B) BM-DCs were incubated for 24 hours with each CPT derivative at 1 μM or vehicle alone and then examined for the surface expression of MHC II, CD40, and CD80 and for cell viability by PI uptake (mean ± SD; n = 3). Asterisks indicate statistically significant (*P < .05, **P < .01) increases compared with vehicle-treated controls.
Adjuvant activities of COL to boost humoral and cellular immune responses. (A) BALB/c mice (n = 3 each) were left nonimmunized or immunized twice by subcutaneous injections of OVA (400 μg per animal) plus vehicle alone or COL (6 μg per animal). Serum samples collected 7 days after the second immunization were examined for OVA-specific immunoglobulins of the indicated isotypes (mean ± SD from 3 independent animals each). (B) The draining LN cells harvested from the same immunized mice were examined for proliferative responsiveness in the presence or absence of OVA (100 μg/mL) by 3H-thymidine uptake on day 4 (mean ± SD; n = 3 each). Statistically significant differences compared with the control panel immunized with OVA plus vehicle alone are indicated with asterisks (*P < .05, **P < .01).
Adjuvant activities of COL to boost humoral and cellular immune responses. (A) BALB/c mice (n = 3 each) were left nonimmunized or immunized twice by subcutaneous injections of OVA (400 μg per animal) plus vehicle alone or COL (6 μg per animal). Serum samples collected 7 days after the second immunization were examined for OVA-specific immunoglobulins of the indicated isotypes (mean ± SD from 3 independent animals each). (B) The draining LN cells harvested from the same immunized mice were examined for proliferative responsiveness in the presence or absence of OVA (100 μg/mL) by 3H-thymidine uptake on day 4 (mean ± SD; n = 3 each). Statistically significant differences compared with the control panel immunized with OVA plus vehicle alone are indicated with asterisks (*P < .05, **P < .01).
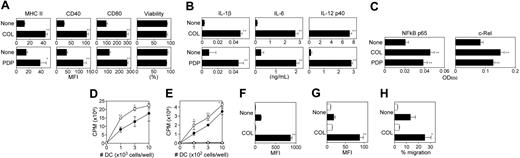
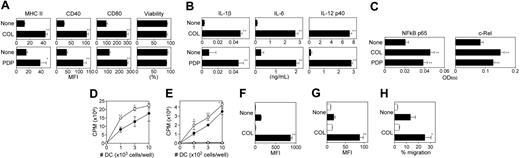
A key question concerned whether the inhibitors of microtubule polymerization would, indeed, provoke DC maturation as suggested in the DC biosensor screen. COL and PDP both elevated surface expression of MHC II, CD40, and CD80 by BM-DCs without affecting the cell viability (Figure 6A). Moreover, both triggered robust production of IL-1β, IL-6, and IL-12 p40 by BM-DCs (Figure 6B). With regard to pharmacologic mechanisms, both COL and PDP induced significant activation of NF-κB p65 and c-Rel subunits in XS106 DCs (Figure 6C). Importantly, pretreatment of BM-DCs with COL significantly augmented their capacity to activate allogeneic T cells in the primary mixed leukocyte reactions (Figure 6D) as well as their ability to present the OVA323-339 peptide to native CD4 T cells purified from the DO11.10 transgenic mice (Figure 6E). COL pretreatment also improved the ability of BM-DCs to uptake FITC-conjugated dextran (Figure 6F). This observation, which may first appear rather contradictory to the notion that DC maturation is accompanied by a diminished endocytic capacity,36 is in complete agreement with the most recent report that BM-DCs exhibit enhanced uptake of FITC-dextran following stimulation with TLR ligands.37 Interaction between CCR-7 (expressed by mature DCs) and CCL-19 (produced by lymphatic endothelial cells) has been shown to mediate LN-directed DC homing.38,39 Interestingly, COL treatment up-regulated the surface expression of CCR-7 by BM-DCs (Figure 6G) and also augmented their chemotactic migration to CCL-19 (Figure 6H). In summary, unlike CPT derivatives (which induced only phenotypic maturation of DCs), COL was found to trigger full maturation of DCs characterized by elevated expression of MHC II, costimulatory molecules, and CCR-7, production of multiple cytokines, boosted T-cell–stimulatory potentials, and improved migration toward CCL-19.
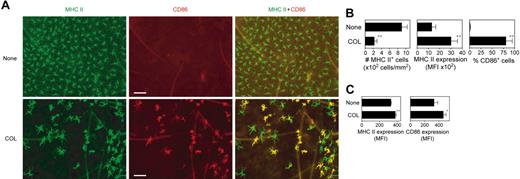
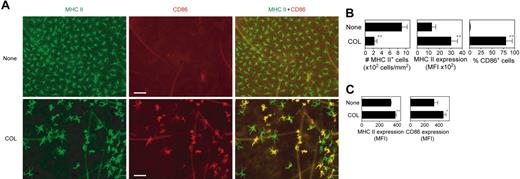
We next injected a small amount of COL subcutaneously into right ears of BALB/c mice to test its in vivo impact on epidermal LCs, which represent prototypic immature DCs that reside in peripheral epithelial tissues. Compared with the control skin sites injected with vehicle alone (ie, left ears of the same animals), COL injection induced striking (more than 75%) reduction in surface densities of MHC II–positive LCs (Figure 7A-B). The MHC II–positive LCs that remained in the epidermal compartment after COL injection showed an increased cell size and irregular elongation of dendritic processes, 2 characteristic morphologic changes of in vivo maturing LCs40 COL injection also elevated MHC II expression on epidermal LCs and induced the expression of CD86 in about 75% of LCs. Moreover, COL injection significantly up-regulated surface expression of MHC II and CD86 by CD11c+ DCs in the draining LNs (Figure 7C). Consistent with our in vitro findings that COL augmented CCR-7 expression and CCL-19–directed chemotaxis by BM-DCs, significantly (P < .05) larger numbers of CD11c+ DCs were recovered from the cervical LNs draining the COL injection sites (46.0 × 103 ± 13.5 × 103 cells per mouse; n = 3) than from those draining the vehicle injection sites (17.7 × 103 ± 2.6 × 103 cells per mouse; n = 3). Notably, the ear thickness remained unchanged (P > .5) after COL injection (199 ± 4.3 μm; n = 6) compared with the thickness before injection (195 ± 5.4 μm). Likewise, no inflammatory changes were detected in COL-injected sites by histologic analysis (data not shown). Thus, we interpreted these results to unveil a previously unrecognized pharmacologic activity of COL to trigger in situ maturation and migration of tissue-resident DCs.
In vitro impacts of microtubule depolymerizing drugs on DC maturation. (A) BM-DCs were incubated for 24 hours with COL (3 μg/mL), PDP (1 μg/mL), or vehicle alone and then examined for the surface expression of MHC II, CD40, and CD80 and for cell viability by PI uptake (mean ± SD; n = 3). (B) The supernatants of the same BM-DC cultures were examined for the secretion of the indicated cytokines (mean ± SD; n = 3). (C) XS106 DCs were incubated for 5 hours with COL (3 μg/mL), PDP (1 μg/mL), or vehicle alone and then examined for NF-κB activation (mean ± SD; n = 3). (D) BM-DCs generated from BALB/c mice were incubated for 24 hours with 3 μg/mL COL (○) or vehicle alone (•), washed extensively, and then cocultured at the indicated numbers with allogeneic T cells (5 × 104 cells per well) purified from C57BL/6 mice. Data shown are 3H-thymidine uptake on day 4 (mean ± SD; n = 3). (E) BM-DCs were incubated for 24 hours with 3 μg/mL COL (open symbols) or vehicle alone (closed symbols) in the presence (circles) or absence (triangles) of 2 μg/mL OVA323-339 peptide during the last 2 hours of the incubation period. After extensive washing, DCs were cocultured at the indicated numbers with CD4 T cells (5 × 104 cells per well) purified from the DO11.10 transgenic mice. Data shown are 3H-thymidine uptake on day 3 (mean ± SD; n = 3). (F) Following 24 hours of incubation with 3 μg/mL COL or vehicle alone, BM-DCs were examined for their capacity to uptake FITC-dextran at 4°C (open bars) or 37°C (closed bars). Samples were then examined for FITC fluorescent signals (mean ± SD; n = 3) within the CD11c+ populations. (G) Following 24 hours of incubation with 3 μg/mL COL or vehicle alone, BM-DCs were stained with anti–CCR-7 mAb (closed bars) or isotype-matched control IgG (open bars). Data shown are fluorescent signals (mean ± SD; n = 3) within the CD11c+ populations. (H) Following 24 hours of incubation with 3 μg/mL COL or vehicle alone, BM-DCs were examined for their migratory capacity toward CCL-19 (closed bars) or PBS alone (open bars). Data shown are the percentage DC migration (mean ± SD; n = 3). In all panels, statistically significant changes as compared with controls treated with vehicle alone are indicated with asterisks (*P < .05, **P < .01).
In vitro impacts of microtubule depolymerizing drugs on DC maturation. (A) BM-DCs were incubated for 24 hours with COL (3 μg/mL), PDP (1 μg/mL), or vehicle alone and then examined for the surface expression of MHC II, CD40, and CD80 and for cell viability by PI uptake (mean ± SD; n = 3). (B) The supernatants of the same BM-DC cultures were examined for the secretion of the indicated cytokines (mean ± SD; n = 3). (C) XS106 DCs were incubated for 5 hours with COL (3 μg/mL), PDP (1 μg/mL), or vehicle alone and then examined for NF-κB activation (mean ± SD; n = 3). (D) BM-DCs generated from BALB/c mice were incubated for 24 hours with 3 μg/mL COL (○) or vehicle alone (•), washed extensively, and then cocultured at the indicated numbers with allogeneic T cells (5 × 104 cells per well) purified from C57BL/6 mice. Data shown are 3H-thymidine uptake on day 4 (mean ± SD; n = 3). (E) BM-DCs were incubated for 24 hours with 3 μg/mL COL (open symbols) or vehicle alone (closed symbols) in the presence (circles) or absence (triangles) of 2 μg/mL OVA323-339 peptide during the last 2 hours of the incubation period. After extensive washing, DCs were cocultured at the indicated numbers with CD4 T cells (5 × 104 cells per well) purified from the DO11.10 transgenic mice. Data shown are 3H-thymidine uptake on day 3 (mean ± SD; n = 3). (F) Following 24 hours of incubation with 3 μg/mL COL or vehicle alone, BM-DCs were examined for their capacity to uptake FITC-dextran at 4°C (open bars) or 37°C (closed bars). Samples were then examined for FITC fluorescent signals (mean ± SD; n = 3) within the CD11c+ populations. (G) Following 24 hours of incubation with 3 μg/mL COL or vehicle alone, BM-DCs were stained with anti–CCR-7 mAb (closed bars) or isotype-matched control IgG (open bars). Data shown are fluorescent signals (mean ± SD; n = 3) within the CD11c+ populations. (H) Following 24 hours of incubation with 3 μg/mL COL or vehicle alone, BM-DCs were examined for their migratory capacity toward CCL-19 (closed bars) or PBS alone (open bars). Data shown are the percentage DC migration (mean ± SD; n = 3). In all panels, statistically significant changes as compared with controls treated with vehicle alone are indicated with asterisks (*P < .05, **P < .01).
In situ maturation of LCs induced by local injection of COL. (A) COL (1.2 μg per animal) or vehicle alone was subcutaneously injected into the right or left ears of BALB/c mice, respectively. Epidermal sheets prepared from the ear skin samples 48 hours after injection were stained for MHC II (green) and CD86 (red). Scale bar, 50 μm (original magnification 200×;20×/0.5 NA objective). (B) The same epidermal sheet preparations were examined for the number of MHC II–positive LCs, surface expression levels (MFI) of MHC II on LCs, and the frequency of CD86+ cells within MHC II+ LC populations using MetaMorph software. Data represent the mean ± SD (n = 3) measured by counting more than 10 independent fields in each specimen. (C) Cervical LNs were harvested 48 hours after subcutaneous injection of COL or vehicle alone to examine surface expression of MHC II and CD86 on CD11c+ DC populations by flow cytometry. Data represent the mean ± SD (n = 3) of MFI values. *P < .05, **P < .01 (compared with the controls treated with vehicle alone).
In situ maturation of LCs induced by local injection of COL. (A) COL (1.2 μg per animal) or vehicle alone was subcutaneously injected into the right or left ears of BALB/c mice, respectively. Epidermal sheets prepared from the ear skin samples 48 hours after injection were stained for MHC II (green) and CD86 (red). Scale bar, 50 μm (original magnification 200×;20×/0.5 NA objective). (B) The same epidermal sheet preparations were examined for the number of MHC II–positive LCs, surface expression levels (MFI) of MHC II on LCs, and the frequency of CD86+ cells within MHC II+ LC populations using MetaMorph software. Data represent the mean ± SD (n = 3) measured by counting more than 10 independent fields in each specimen. (C) Cervical LNs were harvested 48 hours after subcutaneous injection of COL or vehicle alone to examine surface expression of MHC II and CD86 on CD11c+ DC populations by flow cytometry. Data represent the mean ± SD (n = 3) of MFI values. *P < .05, **P < .01 (compared with the controls treated with vehicle alone).
Discussion
We have established here the DC-based biosensor system as a novel platform for time- and cost-efficient discovery of DC-stimulatory drugs. Despite the growing interests in academia and industry in the clinical use of immunostimulants as therapeutics for cancer and infectious diseases and also as vaccine adjuvants,41 no systematic effort has been reported in the literature toward discovery of newer immunostimulants. Most of the currently available immunostimulants (including CpG ODNs and imiquimod) were originally identified by testing synthetic compounds or natural products for their in vivo potentials to augment adaptive immune responses. By testing the in vitro impact of rationally selected compounds on DC phenotype and function, many agents have been shown to deliver DC-stimulatory signals. Our DC biosensor screen has several major advantages over such conventional approaches. First, the XS106-pIL1-YFP DC clone expressed significant YFP signals upon exposure to LPS at 0.1 ng/mL, the concentration 100- to 300-fold lower than ordinary detection limits with phenotypic or functional assays. Secondly, the DC biosensor clone responded to a wide variety of biologic and pharmacologic agents. Third, we experienced minimal variations in terms of assay sensitivity and specificity. Finally, we have been able to improve a single-handed screening capacity up to more than 1000 samples per day by combining a few standard semiautomated assay devices. These features (ie, high sensitivity, broad reactivity, high reproducibility, and HTP compatibility) may allow large-scale, unbiased functional screening for the discovery of a new class of immunostimulants that are designed to boost immune responses by inducing full maturation and mobilization of tissue-resident DCs.
By screening a limited number of small compounds, we have identified several unexpected DC-stimulatory drugs. CPT-Na was one of the hits selected from the NCI library. Phenotypic maturation of BM-DCs was induced by all tested CPT derivatives, including topotecan and irinotecan, which are clinically used as chemotherapeutics. CPT was originally discovered in 1966 as a plant-derived alkaloid with a potent antitumor activity in the NCI drug discovery program42 and was subsequently found to induce single-strand and double-strand DNA breaks by inhibiting topoisomerase I, a key enzyme catalyzing the relaxation of supercoiled chromosomal DNA during replication.43 To the best of our knowledge, this is the first report documenting the pharmacologic activity of any CPT derivative or topoisomerase I inhibitor to trigger DC maturation. Very recently, we have identified several additional chemotherapeutics with potent DC-stimulatory potentials by screening more than 50 anticancer drugs on the market (H.T., N.M., H.M., and A.T., manuscript submitted). Thus, it is tempting to speculate that selected chemotherapeutics may exert their pharmacologic activities not only by directly blocking the growth of tumor cells but also by augmenting naturally occurring protective immunity against tumor-associated antigens via DC activation. Studies are in progress in our laboratory to test this concept.
By screening a total of 880 FDA-approved drugs, we identified DC-stimulatory potentials of 2 microtubule depolymerizing drugs, COL and PDP, which have been used for more than 1000 years in folk medicine. Currently, COL is widely used in the treatment of gout, Behçet disease, and familial Mediterranean fever, while PDP is applied topically to patients with anogenital warts. We now demonstrate that COL is capable of triggering in situ maturation and migration of tissue-resident DCs and augmenting both humoral and cellular immune responses. Although COL was reported in the 1970s to exert an adjuvant-like activity,34,35 this is, once again to the best of our knowledge, the first report showing DC-stimulatory potentials of any microtubule depolymerizing drug. Local subcutaneous administration of relatively small amounts of COL was found to induce in situ maturation of epidermal LCs and to augment adaptive immune responses to an antigen that was coinjected to the same skin sites. This observation may first appear somewhat contradictory to the clinical use of COL as an antiinflammatory drug. In fact, contact hypersensitivity reactions to a topically applied antigen have been suppressed experimentally by intraperitoneal administration of larger amounts of COL during the elicitation, but not sensitization, phase.44 Microtubules play crucial roles not only in cellular mitosis but also in cell adhesion, migration, and phagocytosis, and COL has been reported to inhibit all these cellular activities at relatively high concentrations.45-48 For example, in vitro migration of macrophages and other leukocytes was inhibited by COL at 100 μg/mL or even much higher doses.49,50 By contrast, we observed that COL at 3 μg/mL elevated CCR-7 expression by BM-DCs and augmented their in vitro migratory potential toward CCL-19, consistent with the report documenting augmented macrophage migration after treatment with 0.5 to 1 μg/mL COL.51 Moreover, local injection of a small amount (1.2 μg per animal) of COL resulted in reduced surface densities of epidermal LCs and concomitant increases in CD11c+ DCs in the draining LNs, suggesting COL-triggered LC mobilization. Thus, COL is likely to produce opposing immunologic outcomes depending upon the dose, timing, and route of administration. In this regard, our finding may provide a conceptual basis to optimize the efficacy and safety of COL treatment by altering administration protocols and even to employ COL as a DC-targeted vaccine adjuvant. At the same time, our observations with COL further validate the utility of a DC biosensor screen to predict potential impacts of test compounds on DCs in living animals.
CPT-Na and COL both activated NF-κB p65 and c-Rel subunits in DCs. These observations may not be totally unexpected from a biochemical viewpoint because CPT derivatives and microtubule depolymerizing reagents have been reported to trigger NF-κB activation in non-DC populations.28-30,52,53 DCs have been shown to activate the NF-κB pathway upon sensing external stimuli, such as microbial products via TLRs, proinflammatory signals via cytokine receptors, and T-cell–derived activation signals via CD40.31-33 Blockade of the NF-κB pathway in DCs by NF-κB decoy ODNs (containing an NF-κB cis-element), a proteasome inhibitor (known to block IκB degradation), or overexpression of the IκBα gene (achieved by viral transfection) has severely impaired their antigenpresenting capacity, indicating the functional significance of NF-κB activation during DC maturation.54,55 In this regard, our observations imply that the NF-κB pathway can be activated in DCs not only by external stimuli but also by pharmacologic disruption of intracellular events, such as DNA replication and microtubule polymerization.
The DC biosensor prototype developed in this study may require some modifications for its broader applications. First, several test compounds were found to produce false negative signals due to the autofluorescence. To overcome this technical problem, we had to test all hit compounds with the parental XS106 DC line (nontransduced with the YFP gene). Secondly, we assessed the state of DC maturation by measuring IL-1β promoter activities. Because IL-1β mRNA expression is inducible in DCs as well as other cell types, one may choose an alternative marker that is more selective to DCs. Finally, we employed a single DC line derived from murine epidermis as indicator DCs. Considering the recent identification of several DC subsets with functional diversity,56 one may predict that not all the hits identified with this particular DC line will uniformly induce the maturation of other DC populations. In fact, 3 of the 6 hit compounds identified in the NCI library failed to produce significant maturational changes in BM-DCs. Use of a human-derived DC line would be a most desirable modification, especially for the purpose of drug discovery. Despite these and other potential shortcomings, we believe that the DC biosensor prototype represents a major breakthrough in the fields of DC immunobiology and pharmacology.
In conclusion, we have established a highly sensitive, HTP-compatible, unbiased functional screen to detect diverse stimuli causing DC activation. The DC-based biosensor system may have broad applications in biomedical research, including the discovery of DC-targeted immunostimulants (and immunosuppressants) and the real-time detection of microbial pathogens in environmental and clinical samples. Moreover, our findings with topoisomerase I inhibitors and microtubule depolymerizing agents now form the basis for a new concept that DC maturation is inducible by a broad spectrum of pharmacologic agents causing “intracellular” danger signals.
Prepublished online as Blood First Edition Paper, July 7, 2005; DOI 10.1182/blood-2005-03-1161.
Supported by National Institutes of Health (NIH) grants (RO1-AI46755, RO1-AR35068, RO1-AR43777, and RO1-AI43232 [A.T.]), the Dermatology Foundation Career Development Award (N.M.), and the American Cancer Society Junior Investigator Award (N.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Pat Adcock for secretarial assistance and Brant Ward for helpful comments. We also thank Clifford Bellone (St Louis University School of Medicine) for providing the murine IL-1β promoter.