Abstract
Risk-adapted lymphoma treatment requires early and accurate assessment of prognosis. This investigation prospectively assessed the value of positron emission tomography with 2-[18F]fluoro-2-deoxy-D-glucose (FDG-PET) after two cycles of chemotherapy for prediction of progression-free survival (PFS) and overall survival (OS) in Hodgkin lymphoma (HL). Seventy-seven consecutive, newly diagnosed patients underwent FDG-PET at staging, after two and four cycles of chemotherapy, and after completion of chemotherapy. Median follow-up was 23 months. After two cycles of chemotherapy, 61 patients had negative FDG-PET scans and 16 patients had positive scans. Eleven of 16 FDG-PET–positive patients progressed and 2 died. Three of 61 FDG-PET–negative patients progressed; all were alive at latest follow-up. Survival analyses showed strong associations between early FDG-PET after two cycles and PFS (P < .001) and OS (P < .01). For prediction of PFS, interim FDG-PET was as accurate after two cycles as later during treatment and superior to computerized tomography (CT) at all times. In regression analyses, early interim FDG-PET was stronger than established prognostic factors. Other significant prognostic factors were stage and extranodal disease. Early interim FDG-PET is a strong and independent predictor of PFS in HL. A positive early interim FDG-PET is highly predictive of progression in patients with advanced-stage or extranodal disease.
Introduction
Modern combination chemotherapy and radiotherapy have raised the long-term survival from Hodgkin lymphoma (HL) to more than 80% over the past decades.1 However, the longer follow-up has shown serious long-term adverse effects of the treatment, including heart and lung disease, and secondary malignancies. HL patients have an excessive mortality directly related to these late treatment effects.2,3 At 15 years following treatment, the risk of death from HL is overtaken by the risk of death from other causes, and in early-stage HL, treatment-related illness accounts for more deaths than HL itself.4,5 In order to reduce the long-term effects of treatment, therapeutic strategies are becoming more tailored to the individual patient's prognosis. The aim is to achieve the highest cure rate with the least morbidity and mortality.6
Well-established pretreatment prognostic factors, such as clinical disease stage, number of involved regions, B symptoms, extranodal disease, bulky disease, patient age, blood counts, and biochemical parameters, have been shown to predict survival in large cohort studies.7-9 The treatment strategy is largely determined by these prognostic factors. Another important predictor of outcome is the response to treatment. Some patients fail to reach remission or relapse early after first-line therapy.10 These nonresponders generally have a much worse prognosis and need to be identified as early as possible to lower their risk of treatment failure, avoid unnecessary toxicity, and increase the chance of long-term survival. Conventional methods for treatment response monitoring are based on morphologic criteria, and a reduction in tumor size on computerized tomography (CT) is the most important determinant.11,12 However, this is not an accurate predictor of outcome, possibly because the malignant cells in HL make up only a small fraction of the tumor volume.13 Furthermore, the shrinkage of the tumor takes time and thus cannot form the basis for adjustment of therapy until late during treatment.
Functional imaging with positron emission tomography using 2-[18F]fluoro-2-deoxy-D-glucose (FDG-PET) enables evaluation of metabolic changes rather than the morphologic changes of the lymphoma during therapy. Several studies have shown the prognostic value of FDG-PET after a few cycles of chemotherapy in high-grade non-Hodgkin lymphoma (HG-NHL) patients.14-21 So far only a single large, retrospective study has evaluated the value of early FDG-PET treatment monitoring in HL.22
More individualized treatment regimens require improved early risk stratification for HL patients. Our hypothesis is that an early interim FDG-PET can play an important role in this assessment. The aim of the present study was to examine the prognostic value of interim FDG-PET after 2 cycles of chemotherapy in HL, in a prospective setting with systematic inclusion using standardized treatment protocols.
Patients, materials, and methods
Patients
The study was carried out in collaboration between the Danish lymphoma treatment centers at Copenhagen University Hospital, Rigshospitalet (RH), Herlev Hospital (HER), and Aarhus University Hospital (AUH) and the PET centers at RH and AUH. Ninety-nine consecutive patients with newly diagnosed HL were included from November 2001 until June 2004. Exclusion criteria were diabetes mellitus, pregnancy, and age younger than 18 years. The study was approved by the human investigations ethics committee of Copenhagen, Denmark, and was performed in accordance with the revised Helsinki Declaration. Written informed consent was obtained from all patients. Sixty-six patients were treated at RH; 16 patients, at HER; and 17 patients, at AUH. The following clinical data were obtained from all patients: sex, age, clinical stage (I-IV), number of involved nodal regions, extranodal involvement, presence of B symptoms, bulky disease (tumor > 10 cm and/or mediastinal bulk > 1/3 of thoracic diameter), histologic subtype according to the WHO classification,23 albumin, erythrocyte sedimentation rate (ESR), hemoglobin, leukocyte count, lymphocyte count, and International Prognostic Score (IPS).7 All patients underwent initial staging FDG-PET or FDG-PET/CT along with standard staging procedures, including CT. Seventy-seven patients had an early interim PET after 2 cycles of chemotherapy. Twenty-two patients were not PET scanned after 2 cycles of chemotherapy. This was due to several reasons: 5 patients received only radiotherapy and no chemotherapy, 1 patient died after just one cycle of chemotherapy, 7 patients missed the early interim scan due to lack of compliance (did not show up for the examination), 7 patients missed the scan due to problems on the hospital side (no referral, technical problems with scanner or cyclotron, etc), and finally, 2 patients were too ill at the time to undergo the procedure. The 77 patients who had an early interim PET show distributions of age, sex, and histologic subtype quite similar to the overall distributions, but in the group of 22 patients without an early interim PET there is a relative overrepresentation of patients with very limited disease (stage I) and of patients with a fatal outcome. The patient characteristics are given in Table 1.
Treatment
Treatment of early-stage disease was given according to the Nordic Lymphoma Group protocols.24 Patients with nodular lymphocyte predominance (NLP) HL and no risk factors (bulky disease, > 2 lymph node regions or 2 nonadjacent lymph node regions, ESR > 50 mm) were treated with involved field radiotherapy (IFRT) alone. Patients with NLP and risk factors were given 2 to 4 cycles of chemotherapy followed by IFRT. Patients with classic HL and no risk factors (bulky disease, > 2 lymph node regions, ESR > 50 mm) were given 2 cycles of anthracycline-containing chemotherapy followed by IFRT. Patients with CHL and one or more risk factors present were given 4 cycles of anthracycline-containing chemotherapy followed by IFRT. Patients with advanced-stage disease were treated with 6 to 8 cycles of anthracycline-containing chemotherapy. Advanced-stage patients were given either chemotherapy alone or a combination of chemotherapy and radiotherapy.
The majority of patients received ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine) in standard doses every 2 weeks (one cycle = 4 weeks) with dose modification, granulocyte stimulation, or delays depending on blood counts. IFRT was given with megavoltage energies to tumor doses of 30 to 36 Gy in 1.8-Gy daily fractions, 5 fractions per week. Radiotherapy plans were not influenced by the results of interim or postchemotherapy FDG-PET.
PET scans
A staging FDG-PET scan was performed before the start of treatment. Interim FDG-PET scans were performed within the last week before administration of the third (PET2) and fifth (PET4) chemotherapy cycles. Patients given more than 4 cycles of chemotherapy had an FDG-PET approximately 2 weeks after administration of the last dose. FDG was produced from onsite cyclotron and radiochemistry facilities. All FDG-PET scans were performed as half-body scans (midbrain to upper thigh) after a 6-hour fast. Emission data were acquired for 5 minutes per bed position starting 45 to 90 minutes after intravenous injection of approximately 400 MBq FDG. Fifty patients from RH were scanned in a GE LS Discovery PET/CT scanner (General Electric Medical Systems, Milwaukee, WI), 10 patients from HER and 2 patients from RH were scanned (at the RH PET center) in a GE Advance PET scanner (General Electric Medical Systems), and 15 patients from AUH were scanned using a Siemens/CTI ECAT Exact HR47PET scanner (Siemens/CTI, Knoxville, TN). Diazepam was given orally to some patients before FDG administration to avoid muscular uptake of the tracer. Examples of staging and early interim FDG-PET scans are shown in Figure 1.
Conventional restaging and treatment monitoring
Conventional restaging procedures included physical examination, CT, and laboratory screening. Restaging was performed after 4 cycles of chemotherapy according to the response criteria described by Lister et al, where a satisfactory response was defined as no new disease sites and a tumor reduction of minimum 50% in 2 dimensions on CT (CT4).8 Further restaging was performed after completion of first-line chemotherapy, after radiotherapy, then every 6 months, and after 2 years once yearly. The majority of patients also had a restaging CT already after 2 cycles of chemotherapy (CT2).
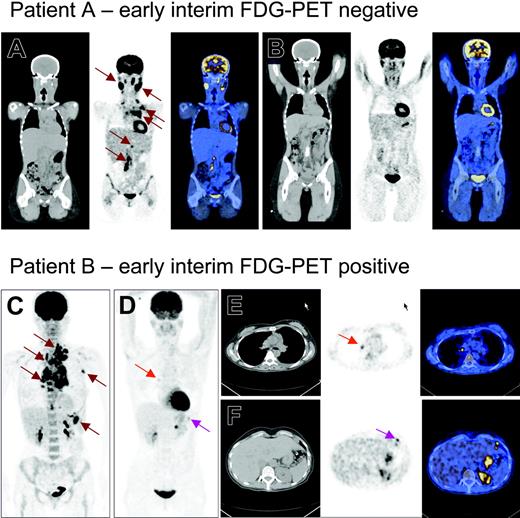
PET and PET/CT images of 2 patients with stage III Hodgkin lymphoma. Panels A-B and E-F are PET/CT images with CT, PET, and fusion images from left to right. Panels C-D are PET images. Patient A initially had involvement of cervical regions, left axilla, mediastinum, and the para-aortic glands in retroperitoneum (A, brown arrows). After 2 cycles of chemotherapy, CT still showed cervical and mediastinal swelling, while PET showed only physiologic uptake. Patient A is now in the fourth year of complete remission. Patient B initially had involvement of the left base of the neck, left axilla, mediastinum, and the spleen (C, brown arrows). After 2 cycles of chemotherapy (D-F), there was still some pathologic FDG uptake in the mediastinum (red arrows) and upper abdomen (pink arrows). The patient was considered in remission after treatment ended but later relapsed in the mediastinum.
PET and PET/CT images of 2 patients with stage III Hodgkin lymphoma. Panels A-B and E-F are PET/CT images with CT, PET, and fusion images from left to right. Panels C-D are PET images. Patient A initially had involvement of cervical regions, left axilla, mediastinum, and the para-aortic glands in retroperitoneum (A, brown arrows). After 2 cycles of chemotherapy, CT still showed cervical and mediastinal swelling, while PET showed only physiologic uptake. Patient A is now in the fourth year of complete remission. Patient B initially had involvement of the left base of the neck, left axilla, mediastinum, and the spleen (C, brown arrows). After 2 cycles of chemotherapy (D-F), there was still some pathologic FDG uptake in the mediastinum (red arrows) and upper abdomen (pink arrows). The patient was considered in remission after treatment ended but later relapsed in the mediastinum.
Data analysis
PET images were displayed as whole-body projections and as transaxial, coronal, and sagittal tomographic sections. High resolution images were produced with ordered subset expectation maximation iterative reconstruction (OSEM), using transmission scans for correction, or CT data, when available. Two experienced nuclear medicine physicians read all scans, and the results were decided by consensus. The nuclear medicine physicians were blinded from all other clinical information than the diagnosis, and the clinicians were blinded from the results of PET in order to prevent impact on the given treatment.
Standardized uptake value (SUV) was calculated for the 50 patients examined in the RH PET/CT scanner.25 Regions of interest (ROIs) were drawn representing each lymph node region (regions listed in the Cotswolds classification) and organ (lungs, spleen, and liver) on all transaxial and coronal slices. ROIs were normalized for injection dose and body weight, and the maximum voxel value was recorded in each region or organ. Maximum values were used in order to minimize partial volume effects and to enhance the reproducibility of the measurements. The highest SUV measured on the early interim scan, in any region or organ where the staging scan showed increased uptake, was used for prognostic stratification (SUVmax).
Statistical analysis
For the study of the prognostic value of interim FDG-PET, progression-free survival (PFS) and overall survival (OS) were chosen as end points. PFS was defined as the time from diagnosis to first evidence of progression or relapse, or to disease-related death. Data were censored if the patients were alive and free of progression/relapse at last follow-up. OS was defined as the time from diagnosis to death from any cause. Data were censored if the patients were alive at last follow-up. There were no deaths unrelated to HL. Survival according to FDG-PET and CT results were depicted using Kaplan-Meier plots.26 Differences between groups were analyzed using log-rank test.27 Proportional survival at certain times was determined using Kaplan-Meier statistics. Univariate regression analyses were used to assess the value of all the prognostic factors for the prediction of PFS. Multivariate proportional hazards (Cox) regression analyses were applied to test the PET2's independency of established prognostic factors for the prediction of PFS.28 Schoenfeld and Martingale residuals plots were used to check for assumptions of proportional hazards and linearity. The plots were evaluated visually with the help of locally weighted regression fits (lowess curves).29 Receiver operating characteristics (ROC) curves were used to optimize the cut-off points for SUVmax. Differences in SUV between groups were analyzed with Student t tests, assuming inequality of variances. All tests were 2-sided with 5% as the level of significance. All data analyses were performed using the statistical software package SPSS 13.0 (SPSS, Chicago, IL).29,30
Results
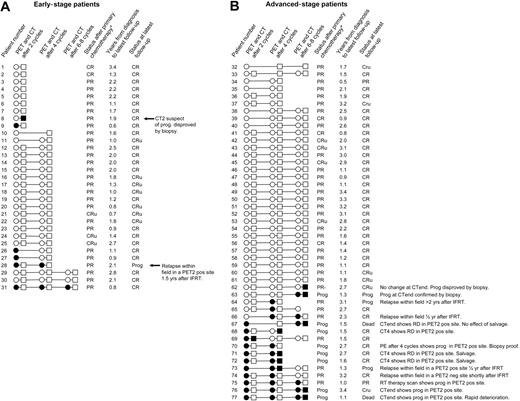
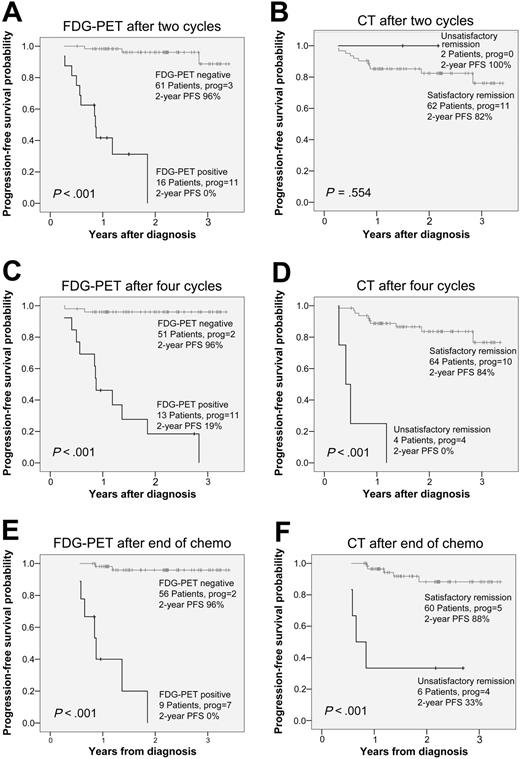
All 77 patients had abnormal FDG uptake on staging FDG-PET. An overview of all patients' FDG-PET and CT results during and after therapy as well as clinical follow-up information is given in Figure 2. Conventional restaging procedures combined with biopsy results showed primary refractory disease in 8 patients. Four patients progressed before completion of first-line chemotherapy (nos. 68, 70, 71, and 72). Four patients showed an unsatisfactory response after completion of first-line chemotherapy (nos. 63, 67, 76, and 77). Six patients relapsed after having reached a satisfactory response with first-line chemotherapy. Five of the 6 relapsing patients had received radiotherapy, and they all relapsed inside the irradiated fields. The 2-year PFS of all 77 patients was 80.8% (95% CI, 71.0%-90.6%). PFS according to the results of PET2, PET4, and PETend as well as CT2, CT4, and CTend is displayed in Figure 3.
Overview of all patients' FDG-PET and CT results during and after therapy as well as clinical follow-up information. ○ and • represent negative and positive FDG-PET scans, respectively. □ and ▪ represent satisfactory and unsatisfactory remission on CT, respectively. Early-stage patients are sorted according to numbers of chemotherapy cycles given and subsequently FDG-PET results after 2 and 4 cycles. Advanced-stage patients are sorted according to PET results. PE indicates physical examination (patient no. 70); RD, refractory disease; IFRT, involved field radiotherapy; Prog, progression; CR, complete remission; CRu, complete remission uncertain; PR, partial remission; and PR-, unsatisfactory partial remission. *Restaging after completion of chemotherapy using conventional restaging methods.
Overview of all patients' FDG-PET and CT results during and after therapy as well as clinical follow-up information. ○ and • represent negative and positive FDG-PET scans, respectively. □ and ▪ represent satisfactory and unsatisfactory remission on CT, respectively. Early-stage patients are sorted according to numbers of chemotherapy cycles given and subsequently FDG-PET results after 2 and 4 cycles. Advanced-stage patients are sorted according to PET results. PE indicates physical examination (patient no. 70); RD, refractory disease; IFRT, involved field radiotherapy; Prog, progression; CR, complete remission; CRu, complete remission uncertain; PR, partial remission; and PR-, unsatisfactory partial remission. *Restaging after completion of chemotherapy using conventional restaging methods.
FDG-PET results
PET2 was read as positive in 16 cases. There was interpretation disagreement on the PET2 status in 2 cases that were reviewed; one was finally assessed as positive and one as negative. Both patients are in CR. A positive PET2 predicted primary refractory disease in 7 of the 8 cases (nos. 63, 68, 70-72, 67, and 76-77). Four PET2-positive patients relapsed after having reached a satisfactory response with first-line chemotherapy (nos. 28 and 73-75), while the remaining 5 PET2-positive patients were in remission at the end of the follow-up period (nos. 9, 26-27, 31, and 69).
In 10 of the 11 PET2-positive patients who progressed, the site of progression showed abnormal uptake on PET2. Only one patient relapsed in a PET2-negative site. Five PET2-positive patients had not progressed at the time of the analysis. They were all in good PR after chemotherapy. Three of them (nos. 9, 26-27) had a negative FDG-PET after radiotherapy and by the time of analysis 4, 6, and 11 months later they were in CR. Patient no. 69 was FDG-PET negative after 4 and 8 cycles and was in CR 12 months after completion of chemotherapy. Patient no. 31 was FDG-PET positive throughout the chemotherapy course. At the time of the analysis, he had completed radiotherapy only 2 months previously and had not yet had a new FDG-PET scan.
Sixty-one scans were reported as negative, including 3 scans with areas of low-grade, low-volume FDG uptake not considered to represent malignant disease. Sixty of 61 PET2-negative patients reached good remission after first-line therapy. Three of them later relapsed. Nine of 16 PET2-positive and 60 of 61 PET2-negative patients had a satisfactory remission after first-line chemotherapy. This difference is highly significant (χ-square, P < .001).
Of the 14 patients with progressive disease, one patient had a very rapid clinical progression and was too frail for second-line therapy to be instituted (no. 77). The remaining 13 patients went on to receive second-line chemotherapy and stem-cell transplantation. Two of 14 patients who experienced progressive disease within the follow-up period died. Both patients who died had positive early interim FDG-PET scans. With 2 deaths among 16 FDG-PET–positive patients and no deaths among 61 FDG-PET–negative patients, there was a significant difference in OS between the FDG-PET–negative and the FDG-PET–positive groups (log rank, P < .01). However, given the excellent overall short-term survival and the very few fatal events, we shall not further consider OS as an end point in this paper.
PET4 was positive in 13 of 64 patients. Eleven of them had either primary refractory disease or relapsed at a later stage. Patient no. 68 had growing tumor masses on CT4 and recurrence of B symptoms and is thus clearly regarded as having had primary refractory disease although PET4 was negative. FDG-PET after completion of first-line chemotherapy (PETend) was positive in 9 of 65 patients. Two of them had satisfactory response with conventional restaging methods and are in CR after very short follow-up periods (nos. 9 and 31). Of the remaining 7 PETend-positive patients, 4 patients had unsatisfactory CTend results and were treated as refractory disease (nos. 63, 67, and 76-77), while 3 patients had good PR on CTend but later relapsed (nos. 28, 66, and 75).
Kaplan-Meier survival curves depicting the progression-free survival of HL patients according to FDG-PET and CT results after 2 and 4 cycles of chemotherapy, and after completion of chemotherapy. Total number of patients, number of patients with progression, and progression-free survival rate after 2 years are given for all groups.
Kaplan-Meier survival curves depicting the progression-free survival of HL patients according to FDG-PET and CT results after 2 and 4 cycles of chemotherapy, and after completion of chemotherapy. Total number of patients, number of patients with progression, and progression-free survival rate after 2 years are given for all groups.
SUV analyses
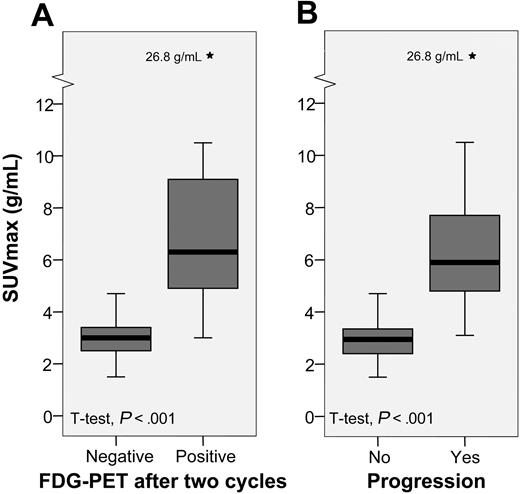
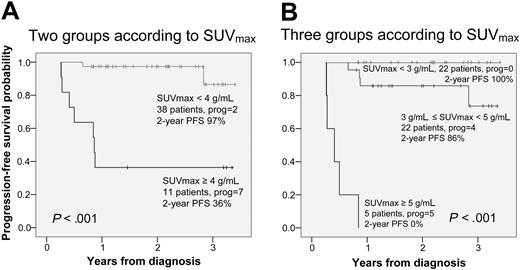
An overview of the SUVmax data is presented in Table 2 and Figure 4. SUVmax was significantly higher among patients who progressed compared with patients who entered and stayed in remission. Since 20% to 25% of all patients are expected to experience short-term treatment failure, it is reasonable to choose a cut-off point around the 75th to 80th percentiles for prediction of PFS. The 75th percentile SUVmax value was 3.8 g/mL and the 80th percentile SUVmax value was 4.3 g/mL. Additional ROC curves confirmed a cut-off point of 4 g/mL as the optimal balancing point between sensitivity and specificity for the prediction of progression. Furthermore, the ROC tables showed 100% specificity for SUVmax more than 5 g/mL and 100% sensitivity for SUVmax less than 3 g/mL (data not shown). In Figure 5, we present the PFS with SUVmax values stratified into two groups by a cut-off point of 4 g/mL (panel A), and three groups by cut-off points of 3 g/mL and 5 g/mL (panel B). The upper half of Table 2 shows that one patient with SUVmax less than 4 g/mL was regarded as PET2 positive and 4 patients with SUVmax more than 4 g/mL were regarded as PET2 negative. None of those 5 patients have experienced progression.
Box plots showing the distributions of SUVmax. Black bars represent the median value, gray boxes represent the interquartile range (IQR), and whiskers represent the range. *Extreme outliers, defined as values more than 3 × IQR away from the box.
Box plots showing the distributions of SUVmax. Black bars represent the median value, gray boxes represent the interquartile range (IQR), and whiskers represent the range. *Extreme outliers, defined as values more than 3 × IQR away from the box.
Univariate analyses
Univariate survival analyses showed significant predictive value of PET2, SUVmax, clinical stage, number of involved regions, extranodal involvement, B symptoms, leukocyte count, and the IPS. They failed to show a predictive value of age, sex, bulky disease, histologic subtype, sedimentation rate, hemoglobin, and lymphocyte count (Table 3). PET2 and SUVmax were tested in bivariate analyses against all of the other prognostic factors listed in Table 3. These analyses showed that PET2 as well as SUVmax were independent of and stronger than all the other factors, when assessed one on one (data not shown).
Multivariate analyses
Due to the high PFS in HL, a proper multivariate analysis of PFS including all known prognostic factors is made impossible by the relatively low number of events. However, trivariate analyses were possible. Apart from the PET variables, the 2 strongest predictors from the univariate analyses, clinical stage and extranodal involvement, were chosen. These analyses are displayed in Table 4, showing very strong independent values of PET2 and SUVmax for the prediction of PFS. The presence of extranodal disease also has an independent, although less strong, predictive value. Tested against the PET variables and extranodal disease, clinical stage failed to show independent prognostic properties.
Progression-free survival according to SUVmax. (A) One SUVmax cut-off value at 4 g/mL. (B) Two SUVmax cut-off values at 3 g/mL and 5 g/mL.
Progression-free survival according to SUVmax. (A) One SUVmax cut-off value at 4 g/mL. (B) Two SUVmax cut-off values at 3 g/mL and 5 g/mL.
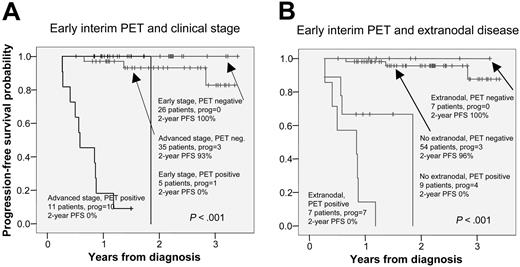
The prognostic value of PET2 combined with extranodal disease and Ann Arbor stage are presented in Table 5 and Figure 6. A positive PET2 in addition to either advanced disease or extranodal disease clearly predicted the patients to be at high risk of progression (10/11 and 7/7). No patient with early-stage disease and a negative PET2 progressed (0/26), and only 3 of 54 PET2-negative patients with the absence of extranodal disease progressed.
Discussion
Modern treatment regimens for early-stage HL show very high cure rates. As cure rates have improved over the years, late adverse treatment effects have become a matter of increasing concern. In both early and advanced HL, further risk-adapted therapy is being introduced to achieve high cure rates with minimal long-term morbidity and mortality. In the case of advanced-stage HL, where the prognosis is less favorable, efforts have also concentrated on intensifying chemotherapy to improve the chances of cure.31 Primary refractory disease, in particular, has the worst prognosis with conventional chemotherapy. Early relapse also has a worse prognosis than late relapse. Salvage high-dose chemotherapy with hematopoietic stem cell transplantation improves the outcome of both groups.32-34
Risk-adapted therapy depends on a reliable prognostic stratification as early as possible during treatment. While the prognosis can be estimated using well-established and validated pretreatment prognostic indices,7 response to treatment is probably the most important single prognostic factor for the individual patient. Radiologic studies have significant limitations in assessing response to treatment. This has led to a focus on nuclear medicine procedures in treatment monitoring, and several studies assessed the value of gallium-67 scintigraphy for early prediction of treatment outcome in HL.35-37 Three studies of the predictive value of an early interim FDG-PET included very small subgroups of HL patients, who have been analyzed as parts of larger mixed lymphoma populations.17-19 In 2004, Friedberg et al published a study where 22 de novo HL patients were FDG-PET scanned after 3 cycles of chemotherapy. After a median follow-up of 24 months, 4 of 5 interim FDG-PET–positive patients had progressed and 15 of 17 FDG-PET–negative patients were in sustained remission.38 In a retrospective study of 85 patients, Hutchings et al recently published the first large study of the prognostic value of early interim FDG-PET in HL. With a median follow-up of more than 3 years, this study showed that FDG-PET after 2 to 3 cycles of chemotherapy had a strong negative predictive value in the early stages and a strong positive predictive value in the advanced stages of the disease, independent of other known prognostic factors.22 Although mostly retrospective and subject to significant bias, these studies suggest that an early FDG-PET is predictive of complete response and superior to FDG-PET after completion of treatment for prediction of disease progression.
Progression-free survival according to early interim FDG-PET and measures of disease progression. Panel A shows clinical stage; panel B, extranodal disease.
Progression-free survival according to early interim FDG-PET and measures of disease progression. Panel A shows clinical stage; panel B, extranodal disease.
In the present study, the majority of patients showed a good response on PET2 (61 negative, 16 positive), reflecting the chemosensitivity of the disease. Early response on FDG-PET was predictive of both primary treatment response and survival, with a 2-year PFS for PET2-negative patients of 96.0% compared with 0% for PET-positive patients. Early interim FDG-PET, assessed qualitatively (PET2) as well as semiquantitatively (SUVmax), was stronger than all pretreatment prognostic factors when evaluated independently in univariate regression analyses, with clinical stage and extranodal disease also showing considerable prognostic strength. In multivariate regression analyses, PET2 was shown to be independently a stronger predictor of PFS than clinical stage and extranodal disease.
PET2, PET4, and PETend have a high prognostic value and are more accurate for the prediction of PFS than CT at the corresponding times (Figure 3). The optimal timing of an interim FDG-PET in lymphoma patients is a field of discussion. Our data show no obvious difference between the prognostic value of FDG-PET after 2 and 4 cycles. Since treatment modifications, if indicated, should take place as early as possible after the response assessment, we find PET2 preferable to PET4 or PETend as platform for decisions on modifications of treatment strategy. However, more definitive answers to this issue will be obtained only through randomized studies.
The negative predictive value of early interim FDG-PET is extremely high in early-stage patients. This is not particularly surprising, since early-stage HL generally has an excellent prognosis. We confirm the findings from Hutchings et al that the positive predictive value is very high in advanced-stage patients.22 In the present study, all patients but one with advanced stage and a positive PET2 progressed within 18 months. We also confirm earlier findings of a high positive predictive value of FDG-PET after completion of first-line therapy for HL.39,40
Romer et al demonstrated that a reduction in SUV and metabolic rate of FDG was predictive of progression in NHL.41 More recently, Torizuka et al investigated a mixed lymphoma population of 20 patients (3 with HL) and found that the absolute maximal SUV on the early monitoring scan was predictive of outcome.19 Our results confirm that this is likely to be true for HL as well as for NHL. SUVmax, a simple and easily obtainable semiquantitative measure of residual metabolic tumor activity, is shown to have independent prognostic value. On the other hand, it is important to acknowledge that the predictive value of SUVmax is based on cut-off points determined by the distribution of SUVmax values in our own data. The observed prognostic properties of SUVmax are not necessarily reproducible in a different patient group, but they do generate the hypothesis that using SUVmax might add to the prognostic information of early interim FDG-PET.
Along with the important prognostic properties of treatment response assessed with early interim FDG-PET, the regression analyses pointed toward clinical stage and extranodal disease as the most important additional prognostic factors. Table 5 and Figure 6 display the value of early interim FDG-PET in combination with clinical stage and extranodal disease. The combination of early interim FDG-PET and one of the 2 measures of dissemination identifies a large group with an excellent prognosis and a smaller group with a very high risk of disease progression. This is probably due to the fact that disease dissemination and initial response to treatment are the most important determinants of outcome in HL. If disease dissemination is seen as an indicator of tumor aggressiveness, one can speculate that the predictive value of interim FDG-PET is higher in the more aggressive forms of the disease.
Since the median follow-up is 23 months and approximately one third of treatment failures in HL occur more than 2 years after diagnosis, a number of new relapses can still be expected. Five PET2-positive patients who had not progressed at the time of analysis were all followed for fewer than 12 months after completing treatment. One can speculate that those patients are at increased risk of relapse in the future. However, there is also the possibility of relapses in the large PET2-negative group, which would reduce the negative predictive value of early interim FDG-PET. Thus, the actual difference in long-term PFS between PET2-positive and PET2-negative patients might be lower than our data presently suggest.
In conclusion, an early interim FDG-PET scan after 2 cycles of chemotherapy may help risk-adapted treatment strategies by selecting good-prognosis HL patients for less intensive and less morbid treatment. Likewise, if added to the information of important pretreatment prognostic factors, early interim FDG-PET identifies a group, where a vast majority is destined to short-term progression or relapse. This could be important in the selection of patients for early treatment intensification. To our knowledge, the present study is the first to show in a prospective and systematic setting the important prognostic value of FDG-PET early during induction treatment in HL. The method is highly reliable for early prediction of remission and progression-free survival in advanced-stage disease.
Prepublished online as Blood First Edition Paper, September 8, 2005; DOI 10.1182/blood-2005-06-2252.
M. Hutchings designed and performed research, analyzed PET images, analyzed data, and wrote the paper. A.L. designed and performed research, and produced and analyzed PET images. M. Hansen designed and performed research and enrolled patients. L.M.P. was responsible for the design, performed research, enrolled patients, and recorded clinical data. T.B. produced and analyzed PET images. J.J. enrolled patients and recorded clinical data. S.B. produced and analyzed PET images. S.K. designed and performed research, and produced and analyzed PET images. F.D. designed and performed research, enrolled patients, and recorded clinical data. A.-M.B. designed research, enrolled patients, and recorded clinical data. A.K.B. analyzed CT images. L.S. designed and performed research, and supervised data analysis and writing of the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to acknowledge Dr Liselotte Højgaard for her strong support throughout this study. The authors have no financial interests in products studied in this work.