Abstract
Inhibitor development is the major treatment complication in children with severe hemophilia A. It is not clear whether the risk of inhibitors is higher with recombinant factor VIII or with plasma-derived factor VIII. We used multivariate analysis to compare 2 cohorts of previously untreated patients (PUPs) with severe hemophilia A: 62 patients treated with the same brand of high-purity plasma-derived FVIII (pFVIII) containing von Willebrand factor (VWF) and 86 patients treated with full-length recombinant FVIII (rFVIII). In addition to the usual end points (all inhibitors, high inhibitors), we also examined a third end point (high inhibitors and/or immune tolerance induction). The risk of inhibitor development was higher in patients treated with rFVIII than in patients treated with pFVIII, regardless of other risk factors (F8 genotype; nonwhite origin; history of inhibitors in patients with a family history of hemophilia; age at first FVIII infusion). The adjusted relative risk (RRa) for inhibitor development with rFVIII versus pFVIII was 2.4 (all inhibitors), 2.6 (high inhibitors), and 3.2 (high inhibitors and/or immune tolerance induction), respectively, depending on the end point (above). The pathophysiology of this large effect must be understood in order to improve the characteristics of recombinant products and to reduce the incidence of inhibitors to FVIII.
Introduction
It has been recommended that the evaluation of the immunogenicity of new factor VIII products be carried out in previously treated patients (PTPs).1-3 However, inhibitors are more often observed in previously untreated patients (PUPs) with severe hemophilia A. Several risk factors for the development of inhibitors to factor VIII have been identified in patients with hemophilia A. The incidence of inhibitors depends on both genetic factors (severity of hemophilia, type of mutation,4 ethnicity,5 family history of inhibitors,6 the HLA genotype7,8 ) and nongenetic factors (age at first treatment,9,10 intensity of treatment,11 continuous infusion,12 and multiple product switches13 ). The influence of the type of FVIII concentrate in PUPs with severe hemophilia A is highly controversial.14,15 A low incidence of inhibitors (0%-11%) was observed in patients treated only with some plasma-derived FVIII concentrates in some studies,16-18 contrasting with an incidence of 24% to 33% in the first international prospective studies of recombinant factor VIII.19-21 To determine whether the incidence of inhibitors is indeed higher in PUPs with severe hemophilia A treated with recombinant FVIII, we reanalyzed individual data from 2 retrospective studies involving PUPs with severe hemophilia A who were treated with either a plasma-derived FVIII concentrate16 or a recombinant FVIII concentrate.22
Patients, materials, and methods
Study design
Two historic French cohorts of PUPs with severe hemophilia A were updated in late 2002 by sending questionnaires to the 24 French hemophilia centers involved in the two studies. The first cohort was treated with a French plasma-derived concentrate (FVIII-LFB)16 and the second with full-length recombinant factor VIII.22 The aim of this work was to compare the incidence of FVIII inhibitors in these two cohorts, independently of all other recorded risk factors. The study was approved by the institutional review board of the Medical University of Lille. The parents' informed consent was obtained in accordance with the Declaration of Helsinki.
Inclusion and exclusion criteria
Patients were eligible if they had previously untreated severe hemophilia A (FVIII:C < 1 IU/dL) and had been tested for the intron 22 inversion. They received the same brand of concentrate (FVIII LFB, Recombinate, or Kogenate) during the entire observation period. Patients were not eligible if they received a transfusion of red blood cell concentrate (RBC) or fresh frozen plasma (FFP) before the first infusion of FVIII concentrate.
Data collection
The following data were collected in each participating hemophilia treatment center: baseline factor VIII level, F8 genotype, white vs nonwhite origin, family history of hemophilia and family history of inhibitors, age at the first FVIII infusion, the FVIII brand, results of inhibitor assay with the Bethesda method,23 and cumulative exposure days (CEDs) to FVIII at each inhibitor assay. All these data were collected until inhibitor detection or the last visit when no inhibitor was detected. After inhibitor detection, we also recorded serial inhibitor titers and treatment (on-demand coagulation factor concentrate or immune tolerance induction [ITI]). ITI was defined as regular infusions of FVIII (daily, every other day, or 3 times a week) with the aim of inhibitor eradication. Only anonymous data were centralized for statistical analysis.
Genotyping and mutations classification
Genetic analysis was performed in each hemophilia treatment center as part of the patients' standard care. All patients were initially tested for the presence of F8 intron 22 inversion and if negative for intron 1 inversion. The intron 22 inversion was detected using Southern blot technique24 or a long-fragment polymerase chain reaction (PCR) method.25 The F8 intron 1 inversion was detected by a multiplex PCR described by Bagnall et al.26 Mutation characterization was carried out on the patients without intron 1 and 22 inversions, by direct sequencing of the coding regions of the F8 gene. According to the experience of the German and British groups,27,28 the F8 gene mutations were classified in 2 groups: those considered as high-risk inhibitor mutations (ie, large insertion/deletion > 1 exon, nonsense mutations on the light chain, intron-22 or intron-1 inversion) and those considered as non–high-risk inhibitor mutations (ie, small insertion/deletion non–A run, nonsense mutations on the heavy chain, large insertion/deletion single exon, missense mutations on the light chain, small insertion/deletion A run, missense mutations on the heavy chain, splicing error).
Products
Plasma-derived FVIII. The plasma-derived FVIII concentrate (FVIII-LFB) was manufactured by LFB (Laboratoire Français du Fractionnement et des Biotechnologies, Les Ulis, France). It was prepared by ion-exchange chromatography and was solvent-detergent treated.29 FVIII:C-specific activity was more than 150 IU/mg protein. Von Willebrand factor (VWF) was present at a concentration of 200 to 400 IU VWF:Ag per 1000 IU FVIII:C, naturally stabilizing FVIII:C. There was no albumin in the formulation.
Recombinant FVIII. Two full-length recombinant FVIII preparations were used. Both were produced by biotechnology (Recombinate,30 Kogenate31 ), purified with monoclonal antibodies, and stabilized with human albumin. As the published cumulative inhibitor incidence rates for Recombinate and Kogenate were similar (ie, 30%),32 data from patients treated with Recombinate and Kogenate were combined for analysis (rFVIII group). We therefore compared plasma-derived FVIII (pFVIII) and recombinant FVIII (rFVIII).
End points
Two classic end points were used, namely “all inhibitors,” defined by an inhibitor titer of 0.6 or more Bethesda units (BU) and “high inhibitors” defined by a titer of more than 5 BU attained at any time.33 However the “all inhibitors” end point may exclude some low or transient inhibitor titers, while the “high inhibitors” end point may also be suboptimal as some patients were immediately placed on an ITI regimen when a low inhibitor titer was detected (≤ 5 BU), possibly modifying the course of the inhibitor titer and leading to an underestimation of the incidence of high inhibitors. We thus added a third end point—“high inhibitors and/or ITI”—combining a high inhibitor titer and/or a change in the treatment regimen after inhibitor detection. We also considered the concordance among these 3 end points, none of which was optimal.
Statistical analysis
Univariate and multivariate survival analyses were performed. The 3 end points were considered (all inhibitors, high inhibitors, and high inhibitors and/or ITI). The inhibitor-free survival time was defined as the number of CEDs until the discovery of the inhibitor or, for patients who had not developed an inhibitor, until the last inhibitor assay after infusion of clotting factor of origin. For univariate analysis, the survival curves were plotted with the Kaplan-Meier method for each end point and cofactor. In Cox multivariate analysis, the proportional hazards assumption was checked for each cofactor by plotting the curve ln(-ln(S(t)).34 Adjusted relative risk (RRa) and 95% confidence intervals were calculated, and the Wald test was applied to each cofactor. Cofactors mentioned in the literature and recorded in the 2 cohorts were included in the multivariate analysis, independently of their statistical significance in univariate analysis. All analyses and figures were done with Stata Statistical software (release 8.2; Stata, College Station, TX).
Results
One hundred forty-eight PUPs with severe hemophilia A, recruited in 24 French hemophilia centers, were included in the analysis, of whom 62 were treated with pFVIII and 86 with rFVIII. None of the patients was receiving primary prophylaxis. The characteristics of the 2 cohorts are listed in Table 1. Patients treated with pFVIII had longer follow-up than those treated with rFVIII, and also longer exposure to FVIII (median follow-up, 48.4 and 24.6 months; median CEDs, 108 and 38, respectively). The interval between 2 inhibitor tests was similar in the 2 cohorts. There was no significant difference (P values .073 to .602) between the 2 groups in terms of risk cofactors for inhibitor development.
Characteristics of the cohorts
. | FVIII-LFB; n = 62 . | Recombinant FVIII*; n = 86 . |
|---|---|---|
| Follow-up | ||
| Period | 1988-1999 | 1991†-2002 |
| Median time to follow-up, mo (range) | 48.4 (1.0-127.5) | 24.6 (0.3-84.4) |
| Mean per patient of interval between 2 inhibitor tests | ||
| Median CEDs (25th-75th percentile) | 5.4 (4.0-7.4) | 5.2 (3.5-7.4) |
| Patients with average interval no higher than 10 CEDs (%) | 51 (82) | 72 (84) |
| Median, mo (25th-75th percentile) | 4.4 (3.3-6.3) | 3.2 (2.4-4.5) |
| Exposure‡ | ||
| Median CEDs (range) | 108 (1-544) | 38 (3-655) |
| Patients with at least 50 CEDs (%) | 40 (65) | 36 (42) |
| Patients with at least 100 CEDs (%) | 32 (52) | 25 (29) |
| Genetic cofactors, no. (%)§ | ||
| High-risk mutations | 36 (58) | 44 (51) |
| Other mutations | 23 (37) | 35 (41) |
| Nonwhite | 7 (11) | 17 (20) |
| Family history of hemophilia and inhibitor | 9 (15) | 6 (7) |
| Family history of hemophilia without inhibitor | 23 (37) | 23 (27) |
| Environmental cofactors, no. (%)§ | ||
| Age at first infusion∥ | ||
| Younger than 6 months | 17 (27) | 18 (21) |
| 6 to 11 months | 21 (34) | 29 (34) |
| End point (%) | ||
| Patients with inhibitors, 0.6 BU or more | 7 (11) | 27 (31) |
| No. of high inhibitors, more than 5 BU | 3 (5) | 13 (15) |
| No. of high inhibitors, more than 5 BU and/or ITI | 4 (6) | 19 (22) |
. | FVIII-LFB; n = 62 . | Recombinant FVIII*; n = 86 . |
|---|---|---|
| Follow-up | ||
| Period | 1988-1999 | 1991†-2002 |
| Median time to follow-up, mo (range) | 48.4 (1.0-127.5) | 24.6 (0.3-84.4) |
| Mean per patient of interval between 2 inhibitor tests | ||
| Median CEDs (25th-75th percentile) | 5.4 (4.0-7.4) | 5.2 (3.5-7.4) |
| Patients with average interval no higher than 10 CEDs (%) | 51 (82) | 72 (84) |
| Median, mo (25th-75th percentile) | 4.4 (3.3-6.3) | 3.2 (2.4-4.5) |
| Exposure‡ | ||
| Median CEDs (range) | 108 (1-544) | 38 (3-655) |
| Patients with at least 50 CEDs (%) | 40 (65) | 36 (42) |
| Patients with at least 100 CEDs (%) | 32 (52) | 25 (29) |
| Genetic cofactors, no. (%)§ | ||
| High-risk mutations | 36 (58) | 44 (51) |
| Other mutations | 23 (37) | 35 (41) |
| Nonwhite | 7 (11) | 17 (20) |
| Family history of hemophilia and inhibitor | 9 (15) | 6 (7) |
| Family history of hemophilia without inhibitor | 23 (37) | 23 (27) |
| Environmental cofactors, no. (%)§ | ||
| Age at first infusion∥ | ||
| Younger than 6 months | 17 (27) | 18 (21) |
| 6 to 11 months | 21 (34) | 29 (34) |
| End point (%) | ||
| Patients with inhibitors, 0.6 BU or more | 7 (11) | 27 (31) |
| No. of high inhibitors, more than 5 BU | 3 (5) | 13 (15) |
| No. of high inhibitors, more than 5 BU and/or ITI | 4 (6) | 19 (22) |
Recombinate (n = 62) or Kogenate (n = 24)
Only 3 patients included in the Recombinate PUPs clinical trial received their first treatment in 1991; the others were treated as from mid-1993, when Recombinate became available in France
The observation period was defined as the number of CEDs until the discovery of the inhibitor or, for patients having no inhibitor, until the last inhibitor test after infusion of clotting factor of origin
There is no significant difference for genetic and environmental cofactors between the 2 cohorts: F8 genotype (P = .601), ethnicity (P = .167), family history of inhibitors (P = .073), or age at first infusion (P = .602)
Mean age at the first infusion is 10.9 months in FVIII-LFB cohort and 12.6 in recombinant FVIII cohort (P = .273)
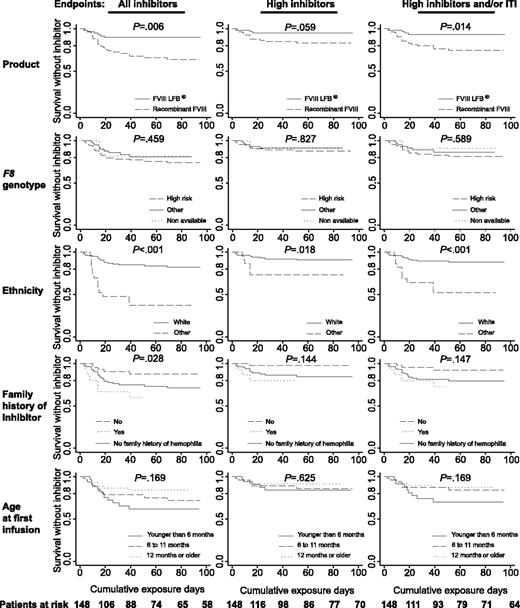
Kaplan-Meier analysis of survival without inhibitor period. The curves were plotted for each end point (from the left to the right: all inhibitors, high inhibitors, high inhibitors and/or ITI) and cofactors (from the top to the bottom: product, F8 genotype, ethnicity, family history of inhibitor, age at first infusion). The number of patients at risk for each cumulative exposure day is given at the bottom.
Kaplan-Meier analysis of survival without inhibitor period. The curves were plotted for each end point (from the left to the right: all inhibitors, high inhibitors, high inhibitors and/or ITI) and cofactors (from the top to the bottom: product, F8 genotype, ethnicity, family history of inhibitor, age at first infusion). The number of patients at risk for each cumulative exposure day is given at the bottom.
The F8 gene mutations are shown in Table 2. Ten patients remained as negative for the intron 22 inversion without further F8 gene analysis. Complete genotype results were thus available from 138 patients (93%). Eighty patients had high-risk inhibitor mutations (mostly intron 22 or intron 1 inversions) and 58 patients had other types of mutations. Both cohorts appeared to be homogeneous in terms of F8 genotype distribution.
F8 gene mutations distribution: number of patients in the cohorts and risk of inhibitor development associated to each mutation*
. | FVIII-LFB; n = 62 . | Recombinant FVIII;†n = 86 . |
|---|---|---|
| High risk (“null mutation”) | 36 | 44 |
| Large insertion/deletion 2 or more exons | 0 | 0 |
| Point mutation nonsense light chain | 2 | 4 |
| Inversion (intron 1 or intron 22) | 34 | 40 |
| Other mutations | 23 | 35 |
| Small insertion/deletion non-A run | 7 | 9 |
| Point mutation nonsense heavy chain | 3 | 11 |
| Large insertion/deletion single exon | 3 | 1 |
| Point mutation missense light chain | 2 | 4 |
| Small mutation insertion/deletion A run | 3 | 3 |
| Point mutation missense heavy chain | 4 | 6 |
| Splicing error | 1 | 1 |
| Nonavailable (intron 22–negative) | 3 | 7 |
. | FVIII-LFB; n = 62 . | Recombinant FVIII;†n = 86 . |
|---|---|---|
| High risk (“null mutation”) | 36 | 44 |
| Large insertion/deletion 2 or more exons | 0 | 0 |
| Point mutation nonsense light chain | 2 | 4 |
| Inversion (intron 1 or intron 22) | 34 | 40 |
| Other mutations | 23 | 35 |
| Small insertion/deletion non-A run | 7 | 9 |
| Point mutation nonsense heavy chain | 3 | 11 |
| Large insertion/deletion single exon | 3 | 1 |
| Point mutation missense light chain | 2 | 4 |
| Small mutation insertion/deletion A run | 3 | 3 |
| Point mutation missense heavy chain | 4 | 6 |
| Splicing error | 1 | 1 |
| Nonavailable (intron 22–negative) | 3 | 7 |
During the observation period, 34 (23%) of 148 patients developed an inhibitor: 7 (11%) of 62 treated with pFVIII and 27 (30%) of 86 with rFVIII (Table 1). High inhibitor titers were observed in 16 patients (3 treated with pFVIII and 13 with rFVIII). Regarding the third end point, 23 patients either developed a high titer inhibitor (n = 16) or started ITI early (n = 7) because of an inhibitor peak of 5 BU or less. The inhibitor was discovered before 10 CEDs in 9 patients, between 10 and 20 CEDs in 17 patients, between 21 and 50 CEDs in 5 patients, and after more than 50 CEDs in 3 patients, 1 of whom developed an inhibitor after surgery (after 286 pFVIII CEDs).
Figure 1 shows Kaplan-Meier curves for the 3 end points and the risk cofactors. The curves for the 3 end points were similar for a given cofactor. For example, the curve for the cumulative inhibitor incidence during pFVIII therapy was always above the corresponding curve during rFVIII therapy. P values are shown on the graphs. The cumulative incidence of inhibitors after 50 CEDs is shown in Table 3 for the 3 end points. Table 3 shows also the RRa in multivariate analysis for each risk cofactor for the 3 end points. A significant difference was found between pFVIII and rFVIII for the end points “all inhibitors” and “high inhibitors and/or ITI,” with respective adjusted relative risk (RRa) values of 2.4 and 3.2 for rFVIII compared with pFVIII. The RRa for the “high inhibitors” end point was similar (2.6) but not statistically significant. For the 3 end points, nonwhite patients were at a higher risk of inhibitor development than whites (RRa between 3.5 and 6.7). Patients with a family history of hemophilia and inhibitors were also at a higher risk than patients with a history of hemophilia without inhibitors for the end points “all inhibitors” (RRa = 6.3) and “high inhibitors and/or ITI” (RRa = 5.8). Late age at first infusion (≥ 12 months vs < 6 months) is associated with a lower risk of inhibitor for the same 2 end points (RRa = 0.3). Finally, although high-risk mutations tendered to be associated with a higher risk of inhibitors (RRa between 1.6 and 2.5), the difference appeared to be nonsignificant. It must be noted, however, that the CI for the “all inhibitors” end point is included in the 1.1 to 5.6 range.
Multivariate analysis (Cox model) of inhibitor incidence according to risk factors
. | All inhibitors . | . | . | . | High inhibitors . | . | . | . | High inhibitors and/or ITI . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | CII (%) . | RRa . | 95% CI . | P . | CII*(%) . | RRa . | 95% CI . | P . | CII (%) . | RRa . | 95% CI . | P . | |||||||||
| Product | .049 | .157 | .045 | ||||||||||||||||||
| FVIII-LFB | 10.3 | 1.0 | – | 5.2 | 1.0 | – | 6.9 | 1.0 | – | ||||||||||||
| Recombinant FVIII* | 32.3 | 2.4 | 1.0-5.8 | 15.0 | 2.6 | 0.7-9.6 | 23.7 | 3.2 | 1.0-9.7 | ||||||||||||
| F8 genotype | .085 | .710 | .251 | ||||||||||||||||||
| Other mutations | 20.9 | 1.0 | – | 9.6 | 1.0 | – | 14.8 | 1.0 | – | ||||||||||||
| High-risk mutations | 25.1 | 2.5 | 1.1-5.6 | 11.8 | 1.6 | 0.5-5.0 | 18.7 | 2.2 | 0.9-5.9 | ||||||||||||
| Nonavailable (intron 22 neg.) | 20.0 | 2.1 | 0.4-10.3 | 10.0 | 1.4 | 0.1-13.1 | 10.0 | 1.5 | 0.2-13.8 | ||||||||||||
| Ethnicity | <.001 | .026 | <.001 | ||||||||||||||||||
| White | 15.0 | 1.0 | – | 7.8 | 1.0 | – | 10.5 | 1.0 | – | ||||||||||||
| Other | 62.8 | 6.7 | 2.9-15.3 | 26.8 | 3.5 | 1.2-10.3 | 47.7 | 5.6 | 2.2-13.9 | ||||||||||||
| Family history of inhibitor | .006 | .121 | .042 | ||||||||||||||||||
| No | 12.4 | 1.0 | – | 2.5 | 1.0 | – | 7.8 | 1.0 | – | ||||||||||||
| Yes | 40.0 | 6.3 | 1.9-20.8 | 20.0 | 10.2 | 1.1-99.4 | 27.3 | 5.8 | 1.3-27.1 | ||||||||||||
| No family history of hemophilia | 25.1 | 4.6 | 1.6-13.3 | 13.6 | 7.4 | 0.9-58.4 | 18.8 | 5.0 | 1.3-18.4 | ||||||||||||
| Age at first infusion | .031 | .546 | .043 | ||||||||||||||||||
| Younger than 6 months | 38.1 | 1.0 | – | 16.0 | 1.0 | – | 30.0 | 1.0 | – | ||||||||||||
| 6 to 11 months | 21.3 | 0.5 | 0.2-1.3 | 10.6 | 0.8 | 0.2-2.8 | 12.7 | 0.4 | 0.1-1.0 | ||||||||||||
| 12 months or older | 15.7 | 0.3 | 0.1-0.7 | 8.3 | 0.5 | 0.1-1.8 | 12.3 | 0.3 | 0.1-0.8 | ||||||||||||
. | All inhibitors . | . | . | . | High inhibitors . | . | . | . | High inhibitors and/or ITI . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | CII (%) . | RRa . | 95% CI . | P . | CII*(%) . | RRa . | 95% CI . | P . | CII (%) . | RRa . | 95% CI . | P . | |||||||||
| Product | .049 | .157 | .045 | ||||||||||||||||||
| FVIII-LFB | 10.3 | 1.0 | – | 5.2 | 1.0 | – | 6.9 | 1.0 | – | ||||||||||||
| Recombinant FVIII* | 32.3 | 2.4 | 1.0-5.8 | 15.0 | 2.6 | 0.7-9.6 | 23.7 | 3.2 | 1.0-9.7 | ||||||||||||
| F8 genotype | .085 | .710 | .251 | ||||||||||||||||||
| Other mutations | 20.9 | 1.0 | – | 9.6 | 1.0 | – | 14.8 | 1.0 | – | ||||||||||||
| High-risk mutations | 25.1 | 2.5 | 1.1-5.6 | 11.8 | 1.6 | 0.5-5.0 | 18.7 | 2.2 | 0.9-5.9 | ||||||||||||
| Nonavailable (intron 22 neg.) | 20.0 | 2.1 | 0.4-10.3 | 10.0 | 1.4 | 0.1-13.1 | 10.0 | 1.5 | 0.2-13.8 | ||||||||||||
| Ethnicity | <.001 | .026 | <.001 | ||||||||||||||||||
| White | 15.0 | 1.0 | – | 7.8 | 1.0 | – | 10.5 | 1.0 | – | ||||||||||||
| Other | 62.8 | 6.7 | 2.9-15.3 | 26.8 | 3.5 | 1.2-10.3 | 47.7 | 5.6 | 2.2-13.9 | ||||||||||||
| Family history of inhibitor | .006 | .121 | .042 | ||||||||||||||||||
| No | 12.4 | 1.0 | – | 2.5 | 1.0 | – | 7.8 | 1.0 | – | ||||||||||||
| Yes | 40.0 | 6.3 | 1.9-20.8 | 20.0 | 10.2 | 1.1-99.4 | 27.3 | 5.8 | 1.3-27.1 | ||||||||||||
| No family history of hemophilia | 25.1 | 4.6 | 1.6-13.3 | 13.6 | 7.4 | 0.9-58.4 | 18.8 | 5.0 | 1.3-18.4 | ||||||||||||
| Age at first infusion | .031 | .546 | .043 | ||||||||||||||||||
| Younger than 6 months | 38.1 | 1.0 | – | 16.0 | 1.0 | – | 30.0 | 1.0 | – | ||||||||||||
| 6 to 11 months | 21.3 | 0.5 | 0.2-1.3 | 10.6 | 0.8 | 0.2-2.8 | 12.7 | 0.4 | 0.1-1.0 | ||||||||||||
| 12 months or older | 15.7 | 0.3 | 0.1-0.7 | 8.3 | 0.5 | 0.1-1.8 | 12.3 | 0.3 | 0.1-0.8 | ||||||||||||
CII indicates cumulative incidence of inhibitor at 50 CEDs; –, not applicable.
Recombinate (n = 62) or Kogenate (n = 24)
Discussion
The comparison of these 2 cohorts of previously untreated patients based on multivariate analysis shows that rFVIII (Recombinate or Kogenate) carries about a 2.5- to 3-fold higher risk than pFVIII (FVIII-LFB) for inhibitor development. The patients were managed in a number of French hemophilia treatment centers during approximately the same period. There was no significant difference between the 2 treatment groups in terms of risk cofactors for inhibitor development, although this does not guarantee comparability for known and unknown cofactors in a nonrandomized study. To overcome this difficulty, we performed multivariate analyses with 3 different end points and all recorded risk cofactors. Most previous studies of inhibitor development in previously untreated patients used only 2 end points (all inhibitors and high inhibitors). Our third end point (“high inhibitors and/or ITI”) reflects current medical practice, ITI started immediately on inhibitor detection, which might mask some high inhibitor titers. The adjusted relative risks for the 3 end points were concordant for each risk cofactor, validating this third end point.
Multivariate analysis identified a strong independent risk cofactor common to the 3 end points, namely the ethnic origin. Nonwhites were at a 3.5- to 6.7-fold higher risk than whites, regardless of other risk cofactors. An increased risk of inhibitors in African-American hemophiliacs has previously been reported.5,19,20 Three additional independent risk cofactors were identified for the 2 end points “all inhibitors” and “high inhibitors and/or ITI”: the family history of inhibitors; young age at first infusion; and the type of product (recombinant or plasma derived). Patients with family history of inhibitors were at a 5.8- and 6.3-fold higher risk than patients with a family history of hemophilia without inhibitors, a link that has also previously been reported.35 Young age at first FVIII treatment was recently reported to be a potential risk cofactor for inhibitor development.9,10 This was confirmed by our multivariate analysis, which showed 0.3-fold fewer inhibitors in children treated for the first time after 12 months of age compared with those treated before 6 months of age. Regarding the F8 genotype, the mutations profile in our patients was typical of what would be expected in a group of severe hemophilia A patients (63.5% had severe molecular defects: 50% inversions, 13.5% nonsense).36-38 It is well established that the F8 genotype is a critical risk factor for the development of inhibitors.4,27,28 With the exception of large insertion/deletion of multiple exons (a very rare mutation) that are associated with the highest risk of inhibitor (68%-88%), the other high-risk mutations (inversions, nonsense light chain) are at 30% to 40% risk of inhibitor—about twice more than other mutations such as small insertion/deletion non–A run or nonsense heavy chain, and large deletion/insertion single exon, which are at 15% to 20% risk.27,28 We found in multivariate analysis for these high-risk mutations an RRa ranging from 1.6 to 2.5, which confirms the influence of the F8 genotype on the risk of inhibitor as previously reported. Larger cohorts probably would have reached a significant level, especially for the “all inhibitors” end point.
Independently of each of these cofactors, the multivariate analysis showed that patients treated with rFVIII (Recombinate or Kogenate) were at a 2.4- to 3.2-fold higher risk of inhibitor development than those treated with pFVIII (FVIII-LFB). The P value was significant for the end points “all inhibitors” and “high inhibitors and/or ITI,” but not for “high inhibitors,” probably owing to the limited number of events (n = 16). The role of recombinant FVIII as a risk factor for inhibitor development has been a subject of intense debate for more than 10 years, some physicians favoring rFVIII15 and others pFVIII.14 Only a randomized double-blind study comparing rFVIII with pFVIII in previously untreated patients could provide a firm answer to this question. However, this would be difficult to perform because of the large number of patients required, the duration of such a study relative to a given product's lifecycle, and the fact that many medical teams and parents would be reluctant to use pFVIII in PUPs. Our multivariate analysis taking into account most known risk cofactors for inhibitor development offers new arguments in this discussion, suggesting that the nature of the product indeed plays a role in inhibitor development. Two possible explanations for a lower risk of inhibitor development with the pFVIII used in this study have been reported in the literature, namely the presence of immunomodulatory activity(ies) copurified along with FVIII and the presence of von Willebrand factor (VWF). Immunomodulatory activity associated with transforming growth factor β (TGFβ) has been observed with some, but not all, plasma-derived products.39 TGFβ is present in relatively large amounts in FVIII LFB39 but is not the only immunomodulating cytokine present in plasma-derived concentrates.40 Regarding von Willebrand factor, this factor indeed has a protective action against inhibitor development, as demonstrated in an animal model.41 It binds to the C2 domain of FVIII, which is frequently the target of anti-FVIII antibodies42 and contains the binding site for phospholipids (PLs). The thrombin cleavage within the C2 domain leads to the release of FVIIIa from its complex and increase in FVIIIa affinity for PLs.43 Difference in PL binding to FVIII has been demonstrated between different types of FVIII concentrates; the highest PL affinity was associated with recombinant products and the lowest with intermediate purity plasma-derived concentrates.44 The increase in PL affinity has been correlated with a higher frequency of inhibitor development.45 Moreover, some recombinant FVIII molecules are unable to bind VWF,46 possibly enhancing immunogenicity relative to plasma FVIII.
The 3 products used in this study are no longer on the market, owing to their short lifecycle. The question of whether to maintain plasma-derived FVIII on the market is regularly debated in the hemophilia community. Testing the VWF hypothesis could have important implications for the development of future generations of rFVIII. A double-blind randomized clinical trial comparing the same brand of rFVIII with and without recombinant VWF in PUPs with severe hemophilia A should be acceptable to the medical community and families. However, the new generation of rFVIII products may be orientated more toward rFVIII molecules with prolonged half-lives or rFVIII molecules that are active by the oral route.
Appendix
Hemophilia treatment centers (HTCs) participating in the 2 studies (in order of the number of patients followed): HTC Bicêtre (T. Lambert, A. Rafowicz, R. d'Oiron); HTC Necker-Enfants Malades (C. Rothschild, M. F. Torchet, F. Legrand); HTC Lille (J. Goudemand, B. Wibaut); HTC Marseille (H. Chambost); HTC Caen (A. Borel-Derlon); HTC Toulouse (S. Claeyssens); HTC Nantes (E. Fressinaud, M. Trossaert, M. Fiks-Sigaud); HTC Brest (B. Pan Petesch); HTC Tours (C. Guérois, B. Fimbel); HTC Le Mans (P. Moreau); HTC Angers (P. Beurrier); HTC Grenoble (G. Pernod); HTC Reims (P. Pouzol, P. Nguyen); HTC Dijon (F. Dutrillaux, F. Volot); HTC Rennes (A. M. Berthier, B. Coatmelec); HTC Montpellier (J. F. Schved); HTC Strasbourg (A. Faradji); HTC Limoges (S. Gaillard); HTC Montmorency (A. Hassoun); HTC Nancy (M.-E. Briquel); HTC Lyon (A. Durin); HTC Bordeaux (V. Guérin); HTC Besançon (M. A. Bertrand); and HTC Le Chesnay (J. Peynet).
Prepublished online as Blood First Edition Paper, September 15, 2005; DOI.
J.G., C.R., Y.L., T.C., and V.D. designed the research; J.G., C.R., C.V., T.L., H.C., A.B.-D., S.C., and members of the study group performed the research; V.D. and T.C. analyzed the data; J.G., C.R., Y.L., T.C., and C.V. wrote the paper; and J.G. and C.R. served as cochairpersons of the working group and contributed equally to the design and execution of this work as well as to the preparation of the paper.
A complete list of the members of the FVIII-LFB and Recombinant FVIII study groups appears in the “Appendix.”
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Prof Harold Roberts for critical review of the article and helpful discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal