Comment on Goudemand et al, page 46
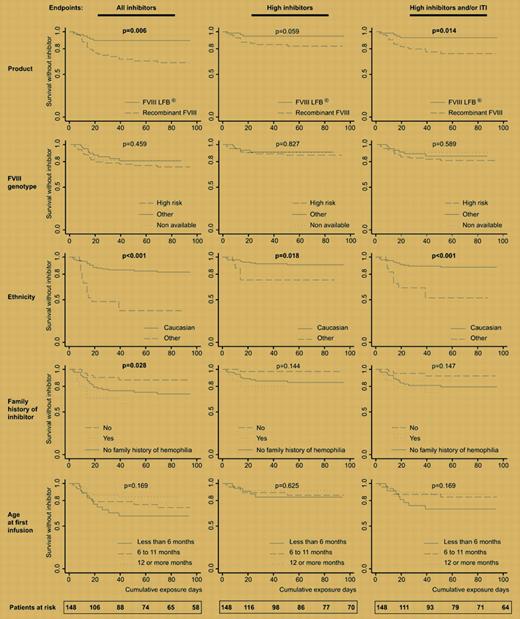
For the first time, Goudemand and colleagues show unequivocally that a plasma-derived partially purified concentrate of factor VIII was about 3-fold less likely to give rise to inhibitors than either of 2 highly purified full-length recombinant products.
Inhibitors to factor VIII are now the main and indeed the sole serious complication of replacement therapy for hemophilia A. Their etiology, means for prevention, and means for treating them are the subject of much research and debate.1 Goudemand and colleagues compared the results of 2 large retrospective treatment trials in patients with haemophilia A, seeking to detect an effect of concentrate type on incidence of inhibitors. The primary data were from 2 separately published trials, 1 using an ion exchange-purified plasma-derived concentrate (FVIII-LFB), the other using recombinant full-length factor VIII (r.FVIII), both in previously untreated patients (PUPs) with severe hemophilia A.2,3 The trials were updated to include data up to 2002. Additional information as to mutation genotype and ethnicity, and other putative risk factors were incorporated into the analysis.
The Kaplan-Meier plots of survival without inhibitor versus cumulative exposure days tell the story well. Ethnicity was known previously to be a risk factor for inhibitors, with African Americans being particularly prone to the complication.4 The very large effect of nonwhite ethnicity seen in these and other trials demands explanation, but none is presently available.
The influence of mutation type has also been extensively studied, with severe gene lesions being most prone to be associated with inhibitor development. It is somewhat surprising that in the present study the effect of genotype was not more pronounced, but earlier data on this relationship have come from published reports5 that may have selection bias or a large single-center study of historical whole-life inhibitor status,6 so they cannot be directly compared to a PUP study of limited duration.
Allowing for these and other factors, which were fairly well balanced between the FVIII-LFB and the recombinant FVIII-treated groups, it still remained the case that the partially purified concentrate from human plasma was much less likely to provoke an antibody response than was highly purified synthetic protein. After regression the relative risk ratio for rfVIII versus FVIII-LFB was 3.2 [confidence interval, 1.0-9.7] for development of a high-titered inhibitor (> 5 Bethesda units) and/or being given immune tolerance induction.
How is one to explain this superiority of a relatively low-tech product made from pooled plasma over a high-tech genetically engineered protein? The first obvious difference between products is that FVIII-LFB contains the natural carrier protein von Willebrand factor. Some animal experimental data support a role for VWF in suppressing antibodies to at least the C2 domain of FVIII to which VWF binds. Several other potentially immune modulatory proteins are also present in plasma-derived concentrates such as transforming growth factor β, which is (or was—the concentrate is no longer available) present in large amounts in FVIII-LFB.
To settle these questions, as the authors point out, a large randomized study comparing rFVIII with or without rVWF will be necessary, likewise for the immune modulatory proteins. Such a large effect of concentrate type on the incidence of inhibitors certainly ought to be taken seriously in the search for better therapy. But it seems unlikely that any such trials will in fact be undertaken, since the life-time of products in the marketplace is so short and other developments like modifications to prolong half-life are more likely to attract investment and effort. Meanwhile, clinicians may wonder whether there is a case for treating PUPs with lower purity plasma-derived factor VIII after all. ▪
Kaplan-Meier analysis of survival without inhibitor period. See the complete figure in the article beginning on page 46.
Kaplan-Meier analysis of survival without inhibitor period. See the complete figure in the article beginning on page 46.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal