Abstract
Late-onset erythropoietic protoporphyria (EPP) is a rare complication of myelodysplastic syndrome (MDS) but has not been described in association with a myeloproliferative disorder (MPD). EPP is normally an inherited disorder characterized by photosensitivity that starts in early childhood and results from overproduction of protoporphyrin secondary to ferrochelatase (FECH) deficiency. Severe liver disease occurs in 1% to 2% of patients. Here we report that severe photosensitivity and cholestatic liver disease in a patient with a myeloproliferative disorder was caused by excess protoporphyrin production from a clone of hematopoietic cells in which one FECH allele had been deleted. Our observations suggest that the usual explanation for the association of late-onset EPP with MPD and MDS is acquired somatic mutation of one FECH allele in bone marrow and show for the first time that the consequent overproduction of protoporphyrin may be severe enough to cause acute liver damage.
Introduction
Erythropoietic protoporphyria (EPP) is an uncommon inherited disorder of heme biosynthesis that results from partial deficiency of ferrochelatase (FECH). It is characterized clinically by childhood onset of lifelong photosensitivity and biochemically by overproduction of protoporphyrin in erythropoietic cells with accumulation of protoporphyrin in erythrocytes, plasma, skin, and liver.1 Clinical expression of EPP normally requires inheritance of an FECH mutation trans to a low-expression FECH allele,2-4 which is present in 13% of the United Kingdom population.4 In 1% to 2% of patients with EPP, deposition of protoporphyrin in the liver leads to progressive liver failure, usually after at least a decade of photosensitivity.1,5,6
Late onset of photosensitivity is rare in EPP.1 Only 10 patients have been reported in whom symptoms started after the age of 40 years,7-10 7 of whom also had refractory anemia with ring sideroblasts (RARS) or other myelodysplastic syndromes (MDSs). In one patient, EPP was recently shown to be caused by proliferation of a clone of hematopoietic cells in which one allele of the FECH gene had been deleted.9 Here we describe a patient with a myeloproliferative disorder (MPD) who developed severe photosensitivity and cholestatic liver disease. We postulated that these complications were caused by an acquired somatic mutation of the FECH gene in his hematopoietic cells.
Study design
A 62-year-old man was diagnosed with polycythemia vera (PV) and hyperuricemia (hemoglobin concentration, 17.5 g/L; hematocrit, 0.513 [51.3%]; leukocyte count, 11.1 × 109/L; platelet count, 191 × 109/L; red cell volume, 34.2 mL/kg [136% of predicted]; plasma volume, 34.4 mL/kg [92.6% of predicted]). Bone marrow examination was consistent with a myeloproliferative disorder (MPD), showing increased cellularity, no excess blasts, no ringed sideroblasts, and normal cytogenetics. He was treated with hydroxyurea, venesections, and allopurinol. Thirty-three months later, an increase in his white cell count (73 × 109/L; 79% neutrophils) was associated with splenomegaly and splenic infarction that required emergency splenectomy. Histology of the spleen showed extramedullary hematopoiesis; a liver biopsy also showed some extramedullary hematopoiesis but no porphyrin deposition or hepatocyte injury. Bone marrow examination showed little change (3% CD34+ cells, normal cytogenetics, grade 2 reticulin on trephine biopsy). He was managed with interferon-α 3Mu thrice weekly and intermittent hydroxyurea.
Fifteen months later, he was admitted with melaena and a 10-day history of deepening jaundice. He had been acutely photosensitive for 9 months, and this had worsened markedly over the previous 6 weeks. He had no previous or family history of photosensitivity or liver disease, consumed alcohol only occasionally, and was not receiving known hepatotoxic drugs. Examination showed jaundice, hepatomegaly, grade I encephalopathy, and a photoburn on his right foot without other skin lesions.
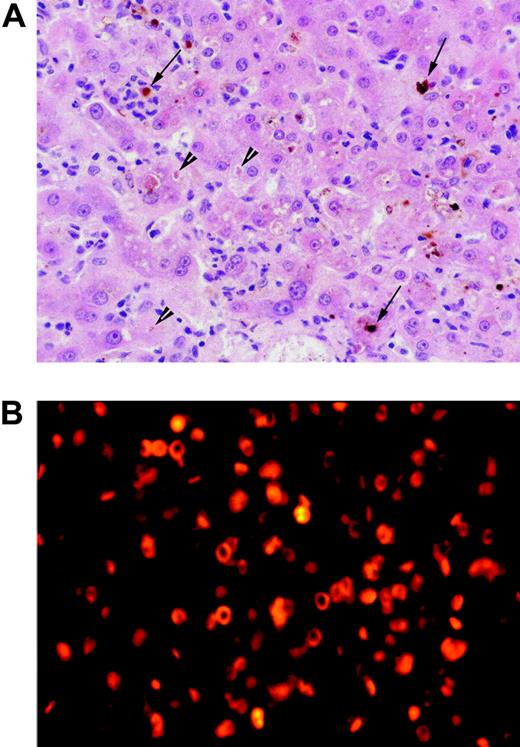
Hemoglobin concentration was 97 g/L (9.7 g/dL); mean cell volume, 89.3 fl; mean corpuscular hemoglobin, 25.8 pg; leukocyte count, 143.9 × 109/L (neutrophils, 74%; monocytes, 12%; basophils, 4%); and platelet count, 252 × 109/L; his prothrombin time was prolonged. Biochemical tests of liver function were abnormal (Figure 1A). His bone marrow was hypercellular; erythroid cells accounted for 32% of total nucleated cells and showed some dysplastic features; myeloid maturation was normal; megakaryocyte numbers were increased. The lesion on his right foot had the histologic appearances of an acute burn; the underlying dermis showed no intimal thickening of blood vessels or other changes of long-standing EPP. A liver biopsy (Figure 2A) showed canalicular cholestasis, bilirubin accumulation, focal eosinophilic single-cell necrosis, and protoporphyrin deposits. There was no fibrosis, cirrhosis, extrahepatic biliary obstruction, or extramedullary erythropoiesis.
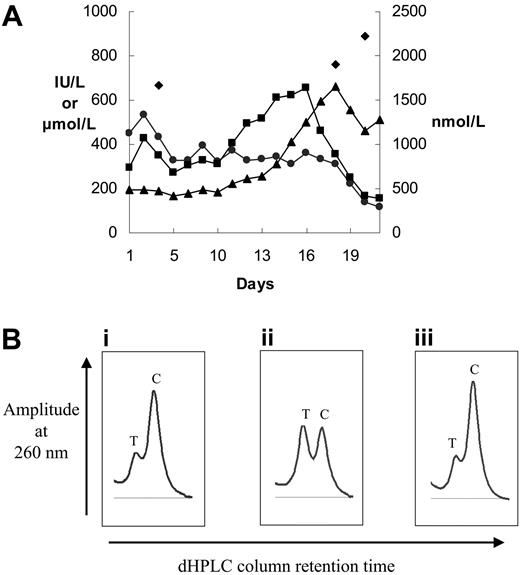
Liver disease in late-onset protoporphyria caused by deletion of an FECH gene in hematopoietic cells. (A) Biochemical tests of liver function and plasma protoporphyrin concentrations during the final phase of the patient's illness. Plasma protoporphyrin (nM) (♦), plasma bilirubin (μM) (▴), plasma alkaline phosphatase (IU/L) (•), and plasma aspartate aminotransferase (IU/L) (▪) measurements are shown. (B) Denaturing HPLC tracings (only homodimer peaks are shown) of intron 3 amplicons from (i) DNA from the patient's unfractionated bone marrow, (ii) germ-line DNA from an FECH IVS3-48C/T heterozygote, and (iii) a mixture of T and C amplicons containing 25% T amplicon.
Liver disease in late-onset protoporphyria caused by deletion of an FECH gene in hematopoietic cells. (A) Biochemical tests of liver function and plasma protoporphyrin concentrations during the final phase of the patient's illness. Plasma protoporphyrin (nM) (♦), plasma bilirubin (μM) (▴), plasma alkaline phosphatase (IU/L) (•), and plasma aspartate aminotransferase (IU/L) (▪) measurements are shown. (B) Denaturing HPLC tracings (only homodimer peaks are shown) of intron 3 amplicons from (i) DNA from the patient's unfractionated bone marrow, (ii) germ-line DNA from an FECH IVS3-48C/T heterozygote, and (iii) a mixture of T and C amplicons containing 25% T amplicon.
His liver disease progressed (Figure 1A) and he died 20 days after admission with a bleeding duodenal ulcer and a perforated abdominal viscus.
Informed consent was given according to the Declaration of Helsinki. Genomic DNA was extracted and the FECH gene sequenced4 ; for fluorescence gene dosage analysis,11 HMBS and PPOX exons were used as internal standards; the FECH IVS3-48 C/T allele ratio was determined by denaturing high-performance liquid chromatography (HPLC). Bone marrow cytogenetic analysis (GTL banding) and fluorescence in situ hybridization (FISH) were performed by standard methods, using a probe specific for BCL2 (LSI IGH/BCL2 Dual Fusion probe; Abbott Laboratories, Abbott Park, IL). Porphyrins were analyzed as described.12
Results and discussion
Porphyrin analyses showed a very high plasma protoporphyrin concentration (1657 nM; normal, < 10 nM) and other biochemical features characteristic of EPP (erythrocyte porphyrin, 112 μM erythrocytes [normal, 0.4-1.7 μM], > 90% protoporphyrin IX; fecal porphyrin, 2052 nmol/g dry weight [normal, 10-200 nmol/g] of which 96% was protoporphyrin). Fluorescence microscopy of bone marrow (Figure 2B) was consistent with EPP.13
His bone marrow karyotype showed abnormalities consistent with MPD in 7 of 9 metaphases, including partial deletion of one chromosome 18q with the breakpoint in the large 18q21 region in which the FECH gene lies at 18q21.31. Absence of the BCL2 gene at 18q21.33 suggested that the deletion was likely to include FECH. Gene dosage analysis of each FECH exon showed that one FECH allele was deleted in a subclone of MPD cells comprising about 30% of nucleated bone marrow cells; a similar degree of mosaicism of unfractionated bone marrow has been reported in the α-thalassemia–MDS syndrome caused by acquired ATRX mutations.14 Denaturing HPLC (Figure 1B) and comparison of DNA sequence chromatograms for germ-line and bone marrow DNA at a polymorphic site in intron 3 (FECH IVS3-48C/T) indicated that the FECH-deleted cells retained only the IVS3-48C allele. No point mutation was identified in the FECH gene in germ-line DNA from buccal cells or in DNA from bone marrow or peripheral blood cells. This indicates that the somatic mutation in our patient's hematopoietic cells was not trans to an inherited, previously clinically latent, FECH mutation.15
These molecular findings, together with the presence of porphyric erythroid cells in his bone marrow, are consistent with the hypothesis that our patient acquired EPP as the result of expansion of a clone of hematopoietic cells containing only one FECH allele. Furthermore, this was a low-expression allele, as indicated by the presence of a C nucleotide at FECH IVS3-48,2 which may explain the unusual severity of his EPP.
In our patient, and others with EPP and MDS,9 inactivation of only one FECH allele appears sufficient for overproduction of protoporphyrin. This contrasts with inherited EPP where involvement of both alleles is required to decrease FECH activity by 70% or more.2 An unexpectedly severe phenotype has also been reported for acquired somatic mutations of the ATRX and α-globin genes in MDS.14
Photomicrographs of liver biopsy and bone marrow aspirate. (A) High-power photomicrograph of liver biopsy; the field shows a perivenular zone with canalicular cholestasis (arrowheads), vacuolar degeneration of hepatocytes, and prominent neutrophils and Kupffer cells, many of the latter containing protoporphyrin aggregates (arrows). Tissue was stained with hematoxylin and eosin and was examined through a Plan 40 ×/0.65 numerical objective lens, using an Olympus BX50 microscope fitted with an Olympus C-4040 digital camera (Olympus, Melville, NY). Images were acquired using Adobe Photoshop 5.0.2 (Adobe Systems, San Jose, CA). (B) Fluorescence microscopy of bone marrow aspirate showing intense red autofluorescence, indicating the presence of protoporphyrin in the cytoplasm of erythroid precursor cells and erythrocytes. Bone marrow smears were mounted in Vectashield (Alpha Laboratories, Eastleigh Hampshire, United Kingdom), and photomicrographs were taken with a Zeiss Axioplan microscope (Carl Zeiss, Welwyn Garden City, United Kingdom) using a tetramethylrhodamine isothiocyanate filter at 200 × total magnification using a 20 ×/0.50 objective lens.
Photomicrographs of liver biopsy and bone marrow aspirate. (A) High-power photomicrograph of liver biopsy; the field shows a perivenular zone with canalicular cholestasis (arrowheads), vacuolar degeneration of hepatocytes, and prominent neutrophils and Kupffer cells, many of the latter containing protoporphyrin aggregates (arrows). Tissue was stained with hematoxylin and eosin and was examined through a Plan 40 ×/0.65 numerical objective lens, using an Olympus BX50 microscope fitted with an Olympus C-4040 digital camera (Olympus, Melville, NY). Images were acquired using Adobe Photoshop 5.0.2 (Adobe Systems, San Jose, CA). (B) Fluorescence microscopy of bone marrow aspirate showing intense red autofluorescence, indicating the presence of protoporphyrin in the cytoplasm of erythroid precursor cells and erythrocytes. Bone marrow smears were mounted in Vectashield (Alpha Laboratories, Eastleigh Hampshire, United Kingdom), and photomicrographs were taken with a Zeiss Axioplan microscope (Carl Zeiss, Welwyn Garden City, United Kingdom) using a tetramethylrhodamine isothiocyanate filter at 200 × total magnification using a 20 ×/0.50 objective lens.
This is the first report of liver disease in late-onset EPP. In animals, protoporphyrin is acutely hepatotoxic and causes cholestasis by damaging the biliary excretory apparatus.6,16 It is likely that the very high plasma protoporphyrin levels in our patient had a similar hepatotoxic effect. The histology of his liver (Figure 2A) resembled that reported for allografts after orthotopic transplantation for EPP liver disease, where liver disease may recur within 9 months of exposure of a previously normal liver to excess protoporphyrin.17,18
Our findings, together with previous reports of gross abnormalities of chromosome 18 in bone marrow in some patients with RARS or MDS and EPP,7,9 show that an acquired somatic mutation of one FECH allele in hematopoietic cells is the usual explanation for the association of late-onset EPP with MPD and MDS, both conditions in which there is genetic instability.19,20 Furthermore, management of this uncommon complication of MDS and MPD should include measures to prevent fatal protoporphyric liver disease.5,6,17,18
Prepublished online as Blood First Edition Paper, September 8, 2005; DOI 10.1182/blood-2004-12-4939.
R.G.G., W.J.K., C.C.L., P.L., G.T.W., M.N.B., G.H.E., and A.K.B. contributed to the clinical care of the patient and interpretation of data; S.D.W. and M.M. carried out laboratory investigations and contributed to the analysis of data; G.H.E. designed the research; W.J.K., M.N.B., G.T.W., and G.H.E. wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms N. G. Mason and Ms J. R. Woolf, Department of Medical Biochemistry and Immunology, for skilled technical assistance, and Dr P. Thompson, Institute of Medical Genetics, for fluorescence microscopy.