The HTLV-1 transactivator protein Tax is essential for malignant transformation of CD4 T cells, ultimately leading to adult T-cell leukemia/lymphoma (ATL). Malignant transformation may involve development of apoptosis resistance. In this study we investigated the molecular mechanisms by which HTLV-1 Tax confers resistance toward CD95-mediated apoptosis. We show that Tax-expressing T-cell lines derived from HTLV-1–infected patients express elevated levels of c-FLIPL and c-FLIPS. The levels of c-FLIP correlated with resistance toward CD95-mediated apoptosis. Using an inducible system we demonstrated that both resistance toward CD95-mediated apoptosis and induction of c-FLIP are dependent on Tax. In addition, analysis of early cleavage of the BH3-only Bcl-2 family member Bid, a direct caspase-8 substrate, revealed that apoptosis is inhibited at a CD95 death receptor proximal level in Tax-expressing cells. Finally, using siRNA we directly showed that c-FLIP confers Tax-mediated resistance toward CD95-mediated apoptosis. In conclusion, our data suggest an important mechanism by which expression of HTLV-1 Tax may lead to immune escape of infected T cells and, thus, to persistent infection and transformation.
Introduction
Human T-cell leukemia virus type 1 (HTLV-1) has been implicated in the etiology of adult T-cell leukemia/lymphoma (ATL)1,2 and tropical spastic paraparesis/HTLV-1–associated myelopathy (TSP/HAM).3 Approximately 1% to 2% of HTLV-1–seropositive individuals have been reported to develop ATL, however, only after a latency period spanning decades of subclinical infection.4 The transactivator protein Tax of the causative virus HTLV-1 is essential for malignant transformation of CD4+ T lymphocytes,5-7 probably by increasing the expression of a set of cellular genes such as IL-2, CD25, c-fos, GM-CSF, c-myb, Lck, and p53.8 Tax does not bind directly to the DNA of its target genes but acts mainly by enhancing the activity of transcription factors like ATF/CREB, AP-1, and, most notably, NF-κB.9,10
Apoptosis is critical both to maintain tissue homeostasis and for a functional immune response.11,12 Thus, inhibition of apoptosis contributes to malignant transformation of cells and constitutes a mechanism of immune escape of virally infected cells.13 CD95 (APO-1/Fas) belongs to the subfamily of death receptors, which is part of the TNF/NGF receptor superfamily.14 The salient feature of death receptor signaling is the formation of a multimolecular complex of proteins triggered by receptor cross-linking with either agonistic antibodies15 or death ligands. The structure formed at the cell membrane is called the death-inducing signaling complex (DISC).16 The CD95 DISC consists of oligomerized, most probably trimerized, CD95; the adaptor FADD/Mort-1; 2 isoforms of caspase-8 (caspase-8/a and caspase-8/b)17 ; CAP3 (a molecule that contains the N-terminal death effector domains [DEDs] of caspase-8 and an as yet uncharacterized C-terminus); and caspase-10, the role of which remains largely elusive.18 At the DISC, pro–caspase-8 is cleaved and, thereby, activated. Active caspase-8 dissociates from the DISC to start the cascade of caspase activation that constitutes the execution phase of apoptosis. Our laboratory has identified 2 cell types, termed type I and type II, with respect to signaling pathways triggered by CD95.19 In type I cells, induction of apoptosis is accompanied by activation of large amounts of caspase-8 at the DISC, directly initiating a caspase cascade. In contrast, in type II cells DISC formation is markedly reduced and strong activation of caspases occurs after loss of the mitochondrial transmembrane potential (ΔψM).19 Activation of mitochondria upon CD95 triggering is mediated by cleavage of the proapoptotic BH3-only Bcl-2 family member Bid.20,21 Truncated Bid translocates to the mitochondria and induces loss of ΔψM, release of apoptogenic factors like cytochrome c and, subsequently, activation of caspase-9 and downstream caspases.22 Thus, in type II cells mitochondria are used as “amplifiers” to initiate the executionary apoptotic caspase cascade.
One major regulator of CD95-mediated apoptosis at the DISC level is c-FLIP (for review see Krueger et al23 ). Multiple splice variants of c-FLIP have been reported but so far only 3, designated c-FLIPS, c-FLIPR, and c-FLIPL, could be detected at the protein level.24,25 c-FLIPL contains tandem DEDs and a caspaselike domain. However, it lacks amino acid residues that are critical for caspase activity, most notably the cysteine of the catalytic center. c-FLIPS resembles its viral counterparts and has only 2 DEDs and a short C-terminal part that differs from c-FLIPL.26 The inhibition of apoptosis by c-FLIPL was shown to be through recruitment to and cleavage in the DISC, but because c-FLIPL is not an active protease, the cleavage is not reciprocated, resulting in an inactive caspase-8 molecule.27 Recruitment of c-FLIPS to the DISC prevents caspase-8 cleavage completely. Recently, it has been proposed that c-FLIPL, at low expression levels, can also act as an activator of caspase-8 by forming a heterodimer with pro–caspase-8 within the DISC.28 The expression of c-FLIP can be regulated via multiple pathways such as by MAP kinases,29 PI3K/Akt,30 or NF-κB.31,32 However, the relative contribution of these signaling pathways to the modulation of c-FLIP still remains elusive and might be cell-type dependent.
Several reports suggest an involvement of c-FLIP in the modulation of the immune response (for review see Krueger et al23 ). We recently demonstrated a potential physiologic role for c-FLIPS and found that it is upregulated upon short-term activation or restimulation of the T-cell receptor in primary human T cells. This finding correlates with resistance to CD95-mediated apoptosis and rescue of these cells from activation-induced cell death (AICD).33,34 In addition, we demonstrated upregulation of c-FLIPS after CD3/CD28 costimulation, which might contribute to protection toward AICD.35 It has been shown that high expression of FLIP promotes tumor growth and facilitates immune escape of tumors.36,37 Recent data suggest that overexpression of c-FLIP causes resistance of Hodgkin lymphoma cells to death receptor–mediated apoptosis.38,39
Tax has been reported to exert proapoptotic40,41 and antiapoptotic effects,42,43 the latter mainly through upregulation of Bcl-2 family members such as Bcl-2 and Bcl-xL.44-46 Our group has previously shown that T cells from ATL patients were susceptible to CD95-mediated apoptosis.47,48 However, these cells normally lack expression of viral gene products, suggesting that the onset of transformation occurs earlier than the manifestation of disease.49-51 T cells expressing Tax were reported to be resistant toward CD95-mediated apoptosis.52,53 In this study we investigated the mechanisms by which Tax confers resistance. We found that HTLV-1–infected and Tax–expressing cells express highly increased levels of c-FLIP. In addition, activation of Tax in an inducible system led to both resistance toward CD95-mediated apoptosis and increased expression of c-FLIP. Downmodulation of c-FLIP in this system using siRNA abolished apoptosis resistance, thus, directly demonstrating the functional relevance of Tax-mediated upregulation of c-FLIP.
Materials and methods
Antibodies and reagents
The monoclonal antibodies (mAbs) against FADD (clone 1; clone A66-2), Erk-1 (MK12), and Bcl-2 (6C8) and the polyclonal antibody against Bcl-xL were purchased from BD Biosciences (Heidelberg, Germany). The anti–α-tubulin mAb (clone B-5-1-2) was from Sigma (St Louis, MO). The anti–caspase-8 mAb C15, the anti–c-FLIP mAb NF6, the agonistic anti-CD95 mAb, anti–APO-1, and leucine zipper (LZ)–CD95L were described previously.15,24,54,55 The anti-Tax hybridomas (clone 168B17-46-34 and 168B17-46-50) were obtained through the AIDS Research and Reference Reagent Program (Division of AIDS, National Institute of Allergy and Infectious Diseases [NIAID], National Institutes of Health [NIH]) from Dr Beatrice Langton.56 Anti-CD3 antibodies (OKT3) were a kind gift from Dr Gerd Moldenhauer (DKFZ Heidelberg). The HRPO-conjugated goat anti–rabbit IgG and the polyclonal anti-CD95 antibody (C-20) were from Santa Cruz Biotechnology (Santa Cruz, CA). The HRPO-conjugated goat anti–mouse IgG1, IgG2a, and IgG2b were from Southern Biotechnology Associates (Birmingham, AL), and the polyclonal anti-Bid (p15) antibody (44-433) was from Biosource (Camarillo, CA). All other chemicals used were of analytical grade and purchased form Merck (Darmstadt, Germany) or Sigma.
Cell lines and cell culture
Jurkat cells stably transfected with an estrogen receptor–tax fusion protein (ERtax) or an estrogen receptor–truncated tax (lacking the first 12 amino acids) fusion protein (ERΔtax) were cultured in RPMI 1640 without phenol red (Invitrogen, Groningen, Netherlands) supplemented with 10% FCS (Invitrogen) and 2 mM Glutamax (Invitrogen).40 CEM and H9 as well as the HTLV-1–infected cell lines MT-2, HuT-102, SLB-1, ATL-3, CHAMP,57 and SP (obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH; SP from Dr Thomas Folks [Rowe et al58 ]) were cultured in RPMI 1640 (Invitrogen) supplemented with 10% FCS, 2 mM Glutamax, and 20 to 100 U/mL IL-2 for ATL-3, CHAMP, and SP cells.
siRNA
The vectors encoding siRNA against c-FLIP and control have been previously described.59 The specific target sequence for c-FLIP (accession no. U97074) was nucleotides 909-928 (5′-AAGGAGCAGGGACAAGTTAC-3′) and as control this sequence was mutated in 2 nucleotides, resulting in a nonfunctional siRNA (5′-AAGGTGCAGGTACAAGTTAC-3′).
Transient transfections
Transfection of Jurkat ERtax and ERΔtax cells was performed using the Amaxa system (Solution V, pulse S18) according to the manufacturer's instructions (Amaxa, Cologne, Germany).
Quantitative reverse transcriptase–polymerase chain reaction (RT-PCR)
RNA from cells was prepared using the Absolutely RNA Microprep kit (Stratagene, Amsterdam, The Netherlands) according to the manufacturer's instructions. Reverse transcription was performed using standard protocols. Quantitative PCR was done on a GeneAmp 5700 sequence detection system (PE Applied Biosciences, Foster City, CA) according to the manufacturer's instructions. TaqMan PCR Master Mix kits were from Eurogentec (Seraing, Belgium). Primers and probes used were as follows: 5′-c-FLIPL, GGC TCC CCC TGC ATC AC (50 nM); 3′-c-FLIPL, TTT GGC TTC CCT GCT AGA TAA GG (300 nM); probe c-FLIPL, CAG GAG GAT GTT CAT GGG AGA TTC ATG C (200 nM); 5′-c-FLIPS, ACC CTC ACC TTG TTT CGG ACT AT (300 nM); 3′-c-FLIPS, TGA GGA CAC ATC AGA TTT ATC CAA A (900 nM); probe c-FLIPS, AGA GTG CTG ATG GCA GAG ATT GGT GAG G (200 nM); 5′-β-actin, ACC CAC ACT GTG CCC ATC TAC GA (300 nM); 3′-β-actin, CAG CGG AAC CGC TCATTG CCAATG G (900 nM); probe β-actin, ATG CCC TCC CCC ATG CCATCC TGC GT (200 nM).
Western blot
Cells either unstimulated or treated with the indicated concentrations of LZ-CD95L for different periods of time were lysed in lysis buffer (20 mM Tris/HCl, pH 7.4; 1% Triton X-100; 10% glycerol; 150 mM NaCl; 1 mM PMSF; and protease inhibitors; Complete, Roche, Mannheim, Germany) for 15 minutes on ice and centrifuged (15 minutes, 14 000g). For Western blot analysis, postnuclear supernatant equivalents of 106 cells or 30 μg protein were used.
Surface staining
Cells (5 × 105) were fixed with 2% paraformaldehyde and incubated with 1 μg/mL anti–APO-1 for 15 minutes at 4°C, washed with PBS, incubated another 15 minutes with phycoerythrin-labeled goat antimouse antibodies (Dianova, Hamburg, Germany), and analyzed by FACScan (BD Biosciences).
Cytotoxicity assays
Cells (106) were stimulated with LZ-CD95L or left untreated for 18 hours at 37°C. Apoptosis was analyzed by FACScan according to the method of Nicoletti et al60 or by changes of the forward scatter/side scatter (FSC/SSC) pattern, reflected by loss of FSC intensity with concomitant stability or increase in SSC intensity as previously described.61,62 Both methods of analysis showed comparable results (Supplemental Figure S1-S3, available on the Blood website; click on the Supplemental Figures link at the top of the online article). Cells transfected with siRNA were stained with annexin V–Alexa594 (Molecular Probes, Eugene, OR) after apoptosis induction, and electronically gated eGFP+ cells were analyzed for annexin-V positivity. Specific apoptosis was calculated as follows: (% experimental apoptosis – % spontaneous apoptosis)/(100 – % spontaneous apoptosis) × 100.
Results
HTLV-1–infected T-cell lines are resistant to CD95-mediated apoptosis
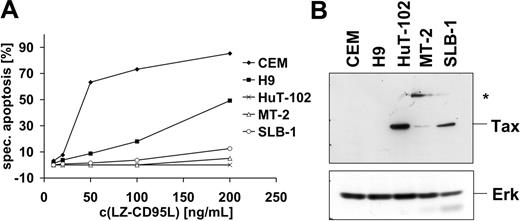
It has been reported previously that HTLV-1–infected cells are resistant to CD95-mediated apoptosis.52,53 In order to study the mechanism of apoptosis resistance, we analyzed 3 HTLV-1–infected T-cell lines, MT-2, SLB-1, and HuT-102, one of which, HuT-102, was derived from an HTLV-1–infected patient. In order to assess their sensitivity toward CD95-mediated apoptosis, cells were treated with different concentrations of recombinant LZ-CD95L for 16 hours and apoptosis was assessed by quantification of cells displaying subdiploid DNA amounts. All 3 cell lines were highly resistant to CD95-mediated apoptosis compared with the noninfected T-cell lines CEM and H9 (Figure 1A). All cell lines expressed Tax as assessed by Western blot (Figure 1B). MT-2 cells expressed relatively low amounts of Tax protein compared with SLB-1 and HuT-102 cells. In addition, MT-2 cells expressed an anti-Tax–reactive protein of 68 kDa that has been detected previously with different anti-Tax antibodies but remains yet uncharacterized (Figure 1B).63
HTLV-1–infected cells express increased amounts of c-FLIP
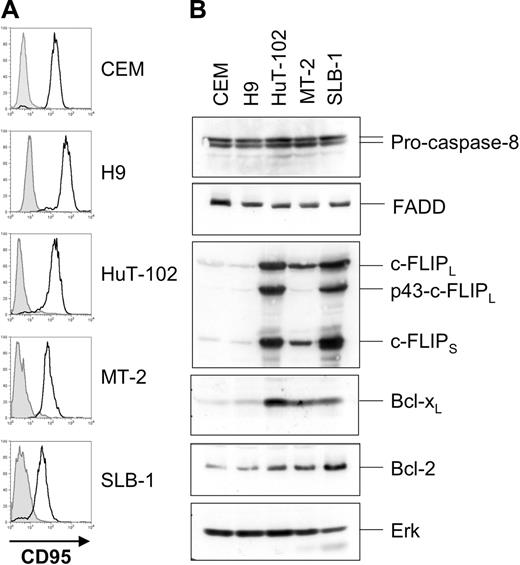
Next we analyzed expression levels of components of the CD95 pathway. MT-2, SLB-1, and HuT-102 cells expressed CD95 (Figure 2A), FADD, and pro–caspase-8 (Figure 2B) at levels comparable to those of the noninfected apoptosis-sensitive T-cell lines CEM and H9. Interestingly, the 3 HTLV-1–infected cell lines expressed highly elevated levels of both c-FLIPL and c-FLIPS compared with CEM and H9 cells. In accordance with previous reports we also found elevated expression of the antiapoptotic Bcl-2 family member Bcl-xL but only slightly elevated levels of Bcl-2.45
Apoptosis inhibition in HTLV-1–infected cells is a death-receptor–proximal event
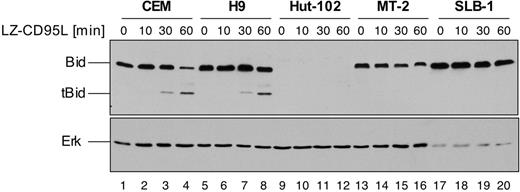
In CD95 type II cells, execution of CD95-mediated apoptosis depends on amplification of the apoptotic signal via activation of the mitochondrial pathway of apoptosis.19 Mitochondrial activation is mediated by caspase-8–triggered truncation of the BH3-only Bcl-2 family member Bid.20,21 In such type II cells, CD95-mediated apoptosis can be inhibited by antiapoptotic Bcl-2 family members. In order to address the functional role of elevated c-FLIP expression we therefore investigated cleavage of Bid as a death receptor proximal event, which is independent of mitochondria. HTLV-1–infected MT-2, SLB-1, and HuT-102 cells as well as noninfected CEM and H9 cells were incubated for up to 1 hour with LZ-CD95L, and cleavage of Bid was assessed by Western blot analysis (Figure 3). Cleavage of Bid was readily detectable in noninfected CEM and H9 cells after stimulation of 30 to 60 minutes, as shown by the appearance of the 15-kDa truncated Bid (tBid) cleavage product (Figure 3 lanes 3, 4, 7, and 8). However, no cleavage could be detected in MT-2 and SLB-1 cells (Figure 3 lanes 13-20), indicating that CD95-mediated apoptosis is inhibited at a receptor proximal step upstream of Bid. Interestingly, we found that HuT-102 cells lack detectable expression of Bid. However, analysis of DISC formation revealed that HuT-102 cells form high amounts of DISC, comparable to prototypic CD95 type I cells (data not shown), indicating that Bid might not be required for CD95-mediated apoptosis in these cells. We also analyzed 3 additional HTLV-1–infected cell lines, ATL-3, CHAMP, and SP, derived from patients. All lines were resistant to CD95-mediated apoptosis and expressed Tax (Figure S2A-B). In addition, they showed similarly high expression levels of both c-FLIPL and c-FLIPS as HuT-102 cells, whereas, in contrast to HuT-102 cells, they also clearly expressed Bid (Figure S1C). These data suggest that elevated c-FLIP expression is a common feature of HTLV-1–infected cell lines derived from patients whereas lack of Bid expression is not.
HTLV-1–infected cells are resistant to CD95-mediated apoptosis. (A) HuT-102, MT-2, and SLB-1 cells were incubated with the indicated concentrations of LZ-CD95L for 16 hours. CD95-sensitive CEM and H9 cells were used as positive control. Apoptosis was determined by analyzing subdiploid DNA by fluorescence-activated cell sorter (FACS). Specific apoptosis was calculated as described in “Materials and methods.” The experiment shown is representative of 3 independent experiments. (B) Expression levels of Tax in HTLV-1–infected cells. Lysates of noninfected (CEM, H9) and HTLV-1–infected (HuT-102, MT-2, and SLB-1) cells were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis with antibodies against Tax. Analysis of Erk expression served as control for equal protein loading. An additional anti-Tax–reactive band specifically expressed in MT-2 cells is marked with an asterisk.
HTLV-1–infected cells are resistant to CD95-mediated apoptosis. (A) HuT-102, MT-2, and SLB-1 cells were incubated with the indicated concentrations of LZ-CD95L for 16 hours. CD95-sensitive CEM and H9 cells were used as positive control. Apoptosis was determined by analyzing subdiploid DNA by fluorescence-activated cell sorter (FACS). Specific apoptosis was calculated as described in “Materials and methods.” The experiment shown is representative of 3 independent experiments. (B) Expression levels of Tax in HTLV-1–infected cells. Lysates of noninfected (CEM, H9) and HTLV-1–infected (HuT-102, MT-2, and SLB-1) cells were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis with antibodies against Tax. Analysis of Erk expression served as control for equal protein loading. An additional anti-Tax–reactive band specifically expressed in MT-2 cells is marked with an asterisk.
Activation of HTLV-1 Tax leads to induction of c-FLIP and resistance to CD95-mediated apoptosis
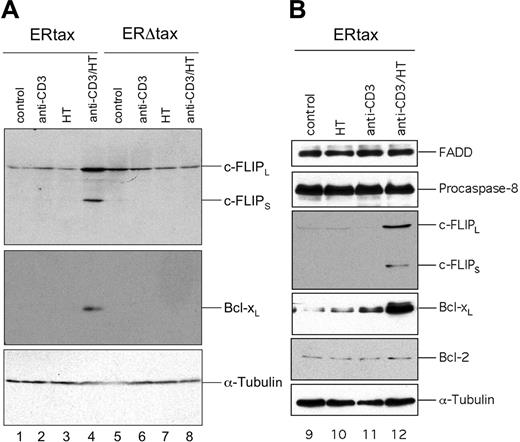
Next we addressed the question of whether the Tax protein itself or other HTLV-1 gene products are responsible for the elevated expression of c-FLIP. Therefore, we used an inducible system of Jurkat cells stably transfected with either ERtax or ERΔtax.40 ERtax can be activated by exogenous addition of 4-hydroxy-tamoxifen (HT). Jurkat ERtax and ERΔtax cells were stimulated with anti-CD3, HT, or both for 24 hours and assayed for the expression of proteins implicated in the CD95 pathway. ERtax cells stimulated with anti-CD3 plus HT simultaneously displayed strongly increased levels of c-FLIPL and c-FLIPS (Figure 4A lane 4). In addition, we observed an upregulation of Bcl-xL under these conditions (Figure 4A). In contrast, ERΔtax cells stimulated with anti-CD3 and HT and ERtax stimulated with anti-CD3 or HT alone did not upregulate any of these proteins (Figure 4A lanes 1-3 and 5-8). The expression of other components of the DISC, such as CD95 (data not shown), FADD, and pro–caspase-8 and the Bcl-2 family member Bcl-2, remained unaffected under any stimulation condition (Figure 4B).
HTLV-1–infected cells express increased amounts of c-FLIP. (A) Surface expression of CD95 on HuT-102, MT-2, and SLB-1 cells. CEM and H9 cells served as positive controls. Filled histograms represent the isotype control; open histograms represent CD95 surface expression. (B) Expression of components of the CD95 signaling pathway in HTLV-1–infected cells. Lysates of CEM, H9, HuT-102, MT-2, and SLB-1 cells were subjected to SDS-PAGE and Western blot analysis with antibodies against c-FLIP, FADD, caspase-8, Bcl-xL, and Bcl-2. Analysis of Erk expression served as control for equal protein loading.
HTLV-1–infected cells express increased amounts of c-FLIP. (A) Surface expression of CD95 on HuT-102, MT-2, and SLB-1 cells. CEM and H9 cells served as positive controls. Filled histograms represent the isotype control; open histograms represent CD95 surface expression. (B) Expression of components of the CD95 signaling pathway in HTLV-1–infected cells. Lysates of CEM, H9, HuT-102, MT-2, and SLB-1 cells were subjected to SDS-PAGE and Western blot analysis with antibodies against c-FLIP, FADD, caspase-8, Bcl-xL, and Bcl-2. Analysis of Erk expression served as control for equal protein loading.
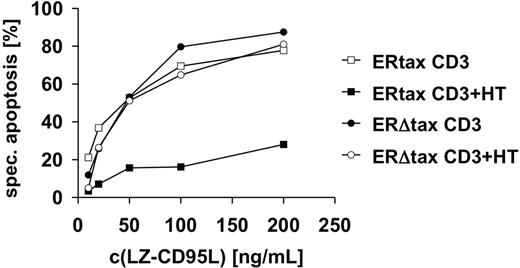
We then assessed whether the induction of antiapoptotic proteins had functional consequences. Jurkat ERtax and ERΔtax cells were stimulated with anti-CD3 or anti-CD3 plus HT and subsequently incubated with different concentrations of LZ-CD95L. Cotreatment of anti-CD3 and HT blocked CD95-mediated apoptosis of ERtax cells, whereas ERΔtax cells remained unaffected (Figure 5). These results indicate that induction of the antiapoptotic proteins c-FLIPL, c-FLIPS, and Bcl-xL by Tax confers resistance to CD95-mediated apoptosis.
Activation of HTLV-1 Tax reduces CD95-dependent cleavage of Bid
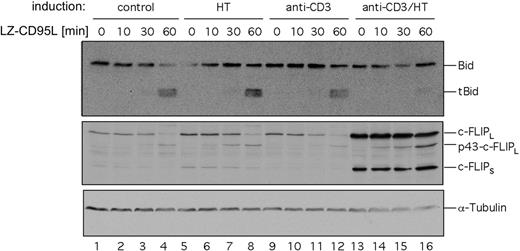
Jurkat cells have been characterized as CD95 type II cells.19 Therefore, in these cells CD95-mediated apoptosis can be blocked not only by interfering with DISC activation through c-FLIP but also by inhibition of the mitochondrial apoptosis pathway through Bcl-2 family members. To dissect the contributions of c-FLIP and Bcl-xL to the inhibition of CD95-mediated apoptosis we investigated cleavage of Bid upon CD95 triggering. Jurkat ERtax cells were stimulated with anti-CD3, HT, or both for 24 hours and subsequently incubated with LZ-CD95L for up to 60 minutes. Cleaved Bid was readily detectable in unstimulated cells or cells stimulated with anti-CD3 or HT alone (Figure 6 lanes 1-12), whereas almost no Bid cleavage was detectable by Western blot in cells stimulated with anti-CD3 plus HT (Figure 6 lanes 13-16). We conclude from these results that the induction of c-FLIP by Tax leads to inhibition of Bid cleavage and, consequently, contributes to resistance to CD95-mediated apoptosis.
Lack of CD95-mediated cleavage of Bid in HTLV-1–infected MT-2 and SLB-1 cells. CEM, H9, HuT-102, MT-2, and SLB-1 cells were treated with 200 ng/mL LZ-CD95L for the indicated period of time and lysed. Lysates were subjected to SDS-PAGE and Western blot analysis with antibodies against Bid and Erk. The 22-kDa Bid and 15-kDa truncated Bid (tBid) are indicated. Analysis of Erk expression served as control for equal protein loading.
Lack of CD95-mediated cleavage of Bid in HTLV-1–infected MT-2 and SLB-1 cells. CEM, H9, HuT-102, MT-2, and SLB-1 cells were treated with 200 ng/mL LZ-CD95L for the indicated period of time and lysed. Lysates were subjected to SDS-PAGE and Western blot analysis with antibodies against Bid and Erk. The 22-kDa Bid and 15-kDa truncated Bid (tBid) are indicated. Analysis of Erk expression served as control for equal protein loading.
Activation of HTLV-1 Tax leads to induction of c-FLIP and Bcl-xL. Jurkat ERtax and ERΔtax cells were stimulated with 30 μg/mL anti-CD3, 2 μM HT, or both for 24 hours. (A) Lysates were subjected to Western blot analysis with antibodies against c-FLIP and Bcl-xL. Expression of tubulin was analyzed as loading control. (B) No modulation of the DISC components FADD and pro–caspase-8 and of Bcl-2. ERtax cells were stimulated with 30 μg/mL anti-CD3, 2 μM HT, or both for 24 hours. Lysates were subjected to Western blot analysis with antibodies against FADD, pro–caspase-8, and Bcl-2. Expression of tubulin was analyzed as loading control. Induction of c-FLIP and Bcl-xL was used as positive control for stimulation.
Activation of HTLV-1 Tax leads to induction of c-FLIP and Bcl-xL. Jurkat ERtax and ERΔtax cells were stimulated with 30 μg/mL anti-CD3, 2 μM HT, or both for 24 hours. (A) Lysates were subjected to Western blot analysis with antibodies against c-FLIP and Bcl-xL. Expression of tubulin was analyzed as loading control. (B) No modulation of the DISC components FADD and pro–caspase-8 and of Bcl-2. ERtax cells were stimulated with 30 μg/mL anti-CD3, 2 μM HT, or both for 24 hours. Lysates were subjected to Western blot analysis with antibodies against FADD, pro–caspase-8, and Bcl-2. Expression of tubulin was analyzed as loading control. Induction of c-FLIP and Bcl-xL was used as positive control for stimulation.
Knockdown of c-FLIP restores sensitivity to CD95-mediated apoptosis in cells expressing active Tax
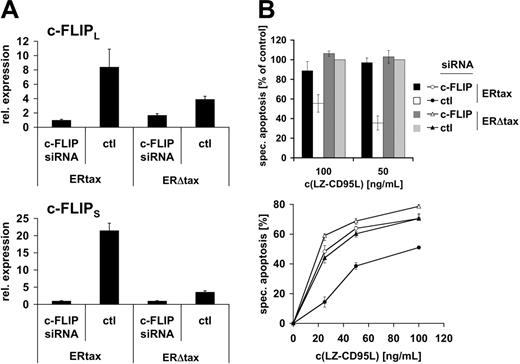
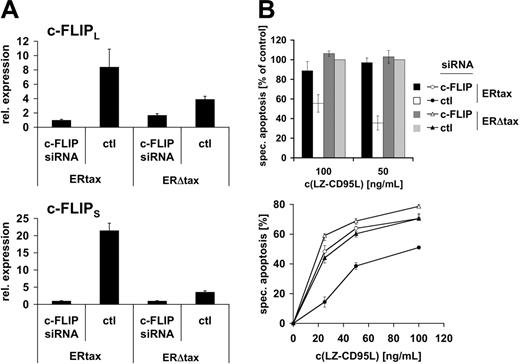
We have shown in short-term kinetics that active Tax prevents CD95-mediated Bid cleavage both in HTLV-1–infected cells and in the inducible system, suggesting that apoptosis resistance is mediated by a receptor proximal event. However, these experiments do not formally exclude that another, yet unknown, component rather than c-FLIP might confer apoptosis resistance. Alternatively, minute amounts of cleaved Bid might trigger the mitochondrial amplification loop. To directly test the role of c-FLIP in Tax-mediated resistance to CD95-triggered apoptosis we employed a knockdown approach using siRNA. Jurkat ERtax and ERΔtax cells were stimulated via CD3 for 16 hours. Cells were transfected with vectors encoding siRNA against c-FLIP or a nonspecific control and eGFP to identify transfected cells. Subsequently, cells were treated with HT for 24 hours and viable cells were recovered by Ficoll gradient centrifugation. Quantitative RT-PCR was performed to monitor the suppression effect of c-FLIP siRNA (Figure 7A). Both c-FLIPL and c-FLIPS mRNA levels were increased in control-transfected–stimulated ERtax cells compared with stimulated ERΔtax cells. Notably, the difference in c-FLIPS expression was much higher than in c-FLIPL mRNA expression, which is consistent with the protein expression data (Figure 4). Transfection of ERtax and ERΔtax cells with siRNA against c-FLIP led to suppression of c-FLIPS and c-FLIPL mRNA levels in ERtax cells by maximally 22-fold and 9-fold, respectively (Figure 7A). The efficiency of c-FLIP downmodulation was robust and amounted generally to 80% to 98% (3 independent experiments). Interestingly, as shown in Figure 7A, siRNA-mediated knockdown resulted in similarly low expression levels of c-FLIP in both ERtax and ERΔtax cells, despite strong induction in ERtax cells after stimulation. This indicates a high efficiency of the siRNA approach over a broad range of c-FLIP expression. In addition, these data suggest that there may be a minimal level of c-FLIP expression leading to elimination of cells with lower c-FLIP expression, possibly due to spontaneous or CD3-mediated apoptosis. Apoptosis sensitivity of differently stimulated and transfected ERtax and ERΔtax cells was analyzed after 16 hours of stimulation with different concentrations of LZ-CD95L. In agreement with previous results (Figure 5), control siRNA-transfected ERtax cells stimulated with anti-CD3 plus HT displayed reduced apoptosis when compared with ERΔtax cells (Figure 7B; Figure S3). Importantly, c-FLIP siRNA-transfected ERtax cells were clearly less resistant toward CD95-mediated apoptosis when compared with control siRNA-transfected cells (Figure 7B; Figure S3). These results clearly indicate that the upregulation of c-FLIP directly contributes to Tax-induced resistance toward CD95-mediated apoptosis.
Activation of HTLV-1 Tax induces resistance toward CD95-mediated apoptosis. Jurkat ERtax and ERΔtax cells were incubated with 30 μg/mL anti-CD3, 2μM HT, or both for 24 hours and subsequently stimulated with the indicated concentrations of LZ-CD95L for an additional 16 hours. Apoptosis was determined by FSC/SSC analysis. Specific apoptosis was calculated as described in “Materials and methods.” The experiment shown is representative of 5 independent experiments.
Activation of HTLV-1 Tax induces resistance toward CD95-mediated apoptosis. Jurkat ERtax and ERΔtax cells were incubated with 30 μg/mL anti-CD3, 2μM HT, or both for 24 hours and subsequently stimulated with the indicated concentrations of LZ-CD95L for an additional 16 hours. Apoptosis was determined by FSC/SSC analysis. Specific apoptosis was calculated as described in “Materials and methods.” The experiment shown is representative of 5 independent experiments.
Activation of HTLV-1 Tax reduces CD95-dependent cleavage of Bid. Jurkat ERtax cells were stimulated with 30 μg/mL anti-CD3, 2 μM HT, or both for 24 hours. Surviving cells were isolated by Ficoll gradient centrifugation and stimulated with 200 ng/mL LZ-CD95L for the indicated periods of time. Cell lysates were subjected to Western blot analysis with antibodies against Bid. Expression of tubulin was analyzed as loading control. Induction of c-FLIP was analyzed as positive control for stimulation.
Activation of HTLV-1 Tax reduces CD95-dependent cleavage of Bid. Jurkat ERtax cells were stimulated with 30 μg/mL anti-CD3, 2 μM HT, or both for 24 hours. Surviving cells were isolated by Ficoll gradient centrifugation and stimulated with 200 ng/mL LZ-CD95L for the indicated periods of time. Cell lysates were subjected to Western blot analysis with antibodies against Bid. Expression of tubulin was analyzed as loading control. Induction of c-FLIP was analyzed as positive control for stimulation.
Knockdown of c-FLIP restores sensitivity toward CD95-mediated apoptosis upon Tax expression. (A) Jurkat ERtax and ERΔtax cells were stimulated with 20 μg/mL anti-CD3 for 16 hours and subsequently transfected with vectors encoding c-FLIP or control (ctl) siRNA. Cells were then treated with 2 μMHT for 24 hours and viable cells were recovered by gradient centrifugation. mRNA expression levels of c-FLIPL (top panel) and c-FLIPS (bottom panel) were assessed by quantitative PCR and normalized to β-actin mRNA. (B) Cells were treated and transfected as described in panel A. Apoptosis was triggered with the indicated concentrations of LZ-CD95L for 16 hours and analyzed by annexin V staining of electronically gated eGFP+ cells. (Top panel) Data of 3 independent experiments are shown normalized with respect to apoptosis induction of ERΔtax cells transfected with control siRNA (nonnormalized data for all experiments are provided in Figure S3). Error bars represent SEM. (Bottom panel) Individual experiment showing nonnormalized data in triplicate ± SEM. c-FLIP siRNA indicates cells transfected with siRNA against c-FLIP; and ctl, cells transfected with control siRNA. Specific apoptosis was calculated as described in “Materials and methods.”
Knockdown of c-FLIP restores sensitivity toward CD95-mediated apoptosis upon Tax expression. (A) Jurkat ERtax and ERΔtax cells were stimulated with 20 μg/mL anti-CD3 for 16 hours and subsequently transfected with vectors encoding c-FLIP or control (ctl) siRNA. Cells were then treated with 2 μMHT for 24 hours and viable cells were recovered by gradient centrifugation. mRNA expression levels of c-FLIPL (top panel) and c-FLIPS (bottom panel) were assessed by quantitative PCR and normalized to β-actin mRNA. (B) Cells were treated and transfected as described in panel A. Apoptosis was triggered with the indicated concentrations of LZ-CD95L for 16 hours and analyzed by annexin V staining of electronically gated eGFP+ cells. (Top panel) Data of 3 independent experiments are shown normalized with respect to apoptosis induction of ERΔtax cells transfected with control siRNA (nonnormalized data for all experiments are provided in Figure S3). Error bars represent SEM. (Bottom panel) Individual experiment showing nonnormalized data in triplicate ± SEM. c-FLIP siRNA indicates cells transfected with siRNA against c-FLIP; and ctl, cells transfected with control siRNA. Specific apoptosis was calculated as described in “Materials and methods.”
Discussion
The HTLV-1 transactivator protein Tax is essential for malignant transformation of CD4 T cells, ultimately leading to ATL.5-7 A key feature of malignant transformation may be the induction of apoptosis resistance.12 In this study we investigated the molecular mechanisms by which HTLV-1 Tax confers resistance toward CD95-mediated apoptosis. We show that Tax-expressing T-cell lines, some of which are derived from HTLV-1–infected patients, contained strongly elevated amounts of 2 splice variants of c-FLIP, c-FLIPS and c-FLIPL, which correlated with the resistance toward CD95-mediated apoptosis. Moreover, by analysis of early cleavage of Bid, a direct caspase-8 substrate, into tBid we show that the apoptosis-signaling cascade in Tax-expressing cells is blocked at the death receptor proximal level. This suggests that elevated expression of c-FLIP proteins, which exert their function at the DISC, are critical for apoptosis resistance induced by HTLV-1. In addition, using a Tax-inducing system in Jurkat T cells,40 we demonstrate that activation of Tax strongly induces c-FLIPS and c-FLIPL expression and, consequently, leads to resistance to CD95-mediated apoptosis after CD3 stimulation. Consistent with previous reports,44,45,53 we also observe an increase in Bcl-xL expression upon Tax activation. Furthermore, using a c-FLIP siRNA knockdown technique, we demonstrate a direct role for c-FLIP in Tax-mediated resistance to CD95-mediated apoptosis. Thus, our studies provide evidence that up-regulation of c-FLIP by Tax may constitute a mechanism for immune evasion of HTLV-1–infected cells.
Interestingly, in the Tax-inducible system used in this study, protection from apoptosis was only observed upon stimulation via the T-cell receptor (TCR)/CD3. Previously, we have shown that costimulation through the T-cell accessory molecule CD28 led to upregulation of c-FLIPS and Bcl-xL and to concomitant reduction of AICD.35 Thus, the protective effect of Tax on activated T cells is strongly reminiscent of that induced by CD28 costimulation. Ligation of CD28 amplifies TCR-mediated T-cell activation at several levels that involve PI3K, PKB/Akt, VAV, ITK, and GSK3, ultimately leading to enhanced activation of TCR-inducible transcription factors, most prominently NF-κB.64 In a similar manner, HTLV-1 Tax is known to exert its function by enhancing the activity of NF-κB.65 The PKB/Akt pathway has been implicated in the regulation of c-FLIP expression levels,30 and c-FLIP has been reported to be an NF-κB target gene.31,32 This suggests that Tax-mediated induction of c-FLIP is executed by NF-κB.
Not only antiapoptotic effects, as described in this study and elsewhere,42,43 but also proapoptotic effects have been ascribed to HTLV-1 Tax.40,41,66 It has been reported that Tax enhances AICD of T cells within 72 hours and leads to apoptosis without an additional trigger within 7 days, probably due to an enhanced pro-oxidative state of the cells.40,66 Nicot and Harrod41 reported that the proapoptotic effect of Tax is dependent on its interaction with CBP/p300, but in this study apoptosis was assayed over a prolonged period of time. So far, no convincing results have been provided to clarify the apparently contradictory findings of the role of Tax in regulating apoptosis. One possible explanation could be that Tax exerts its function in a kinetically biphasic manner, first by inducing antiapoptotic proteins such as Bcl-xL and c-FLIP and then by changing the oxidative state of the cell.66 Thus, Tax may exert a proapoptotic effect at a later stage. This hypothesis might in part explain why the expression of Tax is only observed during a short period of time after infection.
It has been shown that some HTLV-1–transformed T-cell lines as well as leukemia cells from ATL patients are sensitive to CD95-mediated apoptosis.47,48 However, we and others could show that HTLV-1–transformed cell lines that retained expression of Tax are resistant to CD95-mediated apoptosis.52 Moreover, exogenous Tax could induce apoptosis resistance in primary human T cells.52 These divergent observations, together with the finding that Tax is only expressed at the early stages of infection67 and that ATL cells normally lack expression of viral gene products,49-51 point toward an early role of Tax during the course of infection and transformation. Early expression of Tax has been reported to elicit a strong response of Tax-specific cytotoxic T lymphocytes (CTLs).67 The 2 major pathways of CTL-mediated cytotoxicity comprise the CD95 system and the perforin-granzyme system.68 Thus, induction of c-FLIP by HTLV-1 Tax is likely to provide an immune escape mechanism during both early infection and transformation events. This hypothesis is supported by the finding that both viral FLIP (v-FLIP) and c-FLIP mediate the immune escape of tumors.36,37 Tumors with high expression levels of c-FLIP were shown to escape from T-cell–mediated immunity in vivo, although the perforin-granzyme pathway was not impaired. In addition, it was demonstrated that in vivo tumor cells were selected for elevated c-FLIP levels36 and v-FLIP, which structurally resembles c-FLIPS, promoted tumor establishment and progression in vivo by preventing death receptor–mediated cytotoxicity.37
In conclusion, as shown here, Tax-mediated induction of c-FLIP proteins provides a possible mechanism by which expression of HTLV-1 Tax can lead to immune escape of infected T cells and, thus, to persistent infection and transformation.
Prepublished online as Blood First Edition Paper, January 10, 2006; DOI 10.1182/blood-2005-06-2567.
Supported by grants from the Deutsche Forschungsgemeinschaft (DFG; SFB 405), the Deutsche Krebshilfe, the Deutsches Krebsforschungszentrum/Israeli Ministry of Science (DKFZ/MOS) German-Israeli Projectcooperation, and the Sander Stiftung.
A.K. and S.C.F. contributed equally to this work.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Drs Ralph Grassmann (University of Erlangen, Germany), Hiroaki Mitsuya (Kumamoto University School of Medicine, Kumamoto, Japan), Mordechai Aboud (Ben Gurion University of the Negev, Beer Sheva, Israel), and Gerd Moldenhauer (DKFZ, Heidelberg, Germany) for providing cell lines and antibodies. We thank Kathrin Kappes for expert technical assistance and we are grateful to Nina Oberle for helpful discussions.