DMT1 mediates the pH-dependent uptake of Fe2+ from the diet in duodenal enterocytes and in most other cells. It transfers iron from the endosomes to the cytosol following the uptake of the transferrintransferrin receptor complex. DMT1 mutations are responsible for severe hypochromic microcytic anemia in rodents and in 2 human patients described recently. We report a compound heterozygote for 2 new DMT1 mutations, associated with microcytic anemia from birth and progressive liver iron overload. The first mutation is a GTG deletion in exon 5, leading to the V114 in-frame deletion in transmembrane domain 2, and the second is a G → T substitution in exon 8 leading to the G212V replacement in transmembrane domain 5. Together with the 2 previously reported cases, this patient defines a new syndrome of congenital microcytic hypochromic anemia, poorly responsive to oral iron treatment, with liver iron overload associated paradoxically with normal to moderately elevated serum ferritin levels.
Introduction
Iron supply to the erythron requires efficient recycling of iron by macrophages following destruction of senescent red blood cells and absorption of iron from the diet by duodenal enterocytes, to compensate for daily losses. DMT1/NRAMP2/SLC11A2 (hereafter referred to as DMT1) is a divalent metal transporter with 12 transmembrane domains1 (TMs), which cotransport protons and Fe2+.2 It is expressed at the apical membrane of duodenal enterocytes3 and in recycling endosomes of peripheral tissues, especially in erythroid precursors, where it mediates transfer of iron internalized by transferrin from the endosomes to the cytoplasm.4 Several isoforms of the DMT1 mRNA are known,5,6 resulting from alternative splicing and/or the use of 2 alternative upstream promoter regions. The same mutation in DMT1 (G185R) is found both in the mk/mk mouse7 and in the Belgrade rat8 and causes a hypochromic, microcytic anemia due to a defect in intestinal absorption of iron and its use for erythropoiesis. In mice, selective inactivation of DMT1 confirmed the role of DMT1 in gut and bone marrow.9 The first mutation in human DMT1 was found in the homozygous state in a Czech patient with congenital severe hypochromic, microcytic anemia, and normal to slightly increased ferritinemia. Liver iron overload was diagnosed at the age of 19.10,11 The mutation, G1285C, affected the last nucleotide of exon 12, leading to an E399D replacement. This DMT1 mutant seems to have retained a full iron transport activity,12,13 the clinical phenotype of the patient resulting from preferential exon 12 skipping. Recently, Iolascon et al14 presented a compound heterozygote (c310-3_5del CTT and C1246T; R416C) with a microcytic, hypochromic anemia and liver iron overload. Here, we present a French patient carrying 2 new DMT1 mutations and showing a comparable phenotype.
Study design
The female proband was born in 1996 to healthy, nonconsanguineous French parents. Her young brother and sister also were healthy. The pregnancy (38 weeks and 3 days) had been marked by the discovery at 25 weeks gestational age of a left ventricular hypertrophy. Fetal karyotype was normal. At birth, the proband was hypotrophic (weight, 2.150 kg; size, 45.5 cm). There was no hepatosplenomegaly. Anemia was discovered: RBC, 4.36 × 1012/L; Hb, 83 g/L; MCV, 64.4 fL; MCHC, 296 g/L; and reticulocytes, 270 × 109/L (6.2%). Activities of G6PD and PK were normal. Packed red cells were transfused at day 0, the only transfusion ever administered. At day 12, Hb was 113 g/L, and the baby was discharged with iron supplementation. Thereafter, growth and development were considered normal. The family went abroad for 3 years, during which oral iron treatment was continued for 1 year. No strict medical follow-up was performed. The parents said that the child was pale, prone to tiredness, and avoided physical activity.
It was not until 2001 to 2002 that thorough investigations were initiated. A hypochromic, microcytic anemia was confirmed (Table 1). The bone marrow was rich, 30% of nucleated cells were erythroid precursors, the more mature cells keeping a basophilic cytoplasm. Acidophilic erythroblasts were rare. There were no sideroblasts and no extracellular iron deposits. Haptoglobin and bilirubin levels were normal, as well as Hb electrophoresis and analysis of red cell membrane proteins. Porphyrin assessment showed a discrete elevation of protoporphyrins in feces compatible with iron-deficient anemia.
After several months of oral iron supplementation, a clinical survey performed in July 2002 showed a persistent microcytic anemia with normal serum iron, a high transferrin saturation, and surprisingly low serum and erythrocyte ferritin levels (Table 1). Continuous oral iron supplementation resulted in a rise of 10 to 20 g/L in Hb concentration with a striking improvement of quality of life. Discrepancy between high transferrin saturation and low ferritin levels led us to evaluate liver iron stores by magnetic resonance imagining (MRI).15 It showed a severe liver iron overload with 250 ± 50 μmoles iron/g liver (N < 36), leading to interruption of the iron treatment. Cardiac MRI was normal.
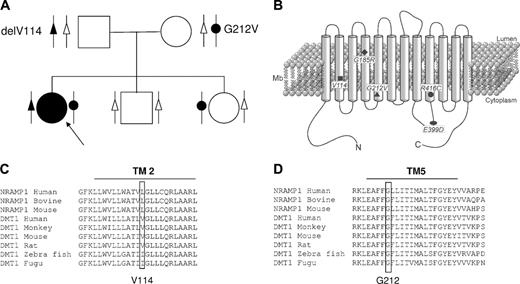
Family tree of the patient with new DMT1 mutations, position and conservation of these and previous mutations. (A) Pedigree of the family with 2 new DMT1 mutations. The black symbols denote a mutated allele, and the white symbol a normal allele. Arrow indicates the proband. (B) Schematic representation of the DMT1 molecule and position of the amino acid changes described in the mk mice (G185R) and in human patients. delV114 and G212V are reported here. R416C is a heterozygous mutation associated with c310-3_5delCTT described by Ioalescon et al.14 E399D is a homozygous mutation described by Mims et al.10 Multiple sequence alignments of DMT1 orthologs are shown for residues forming the second predicted transmembrane domain (C) and the fifth transmembrane domain (D).
Family tree of the patient with new DMT1 mutations, position and conservation of these and previous mutations. (A) Pedigree of the family with 2 new DMT1 mutations. The black symbols denote a mutated allele, and the white symbol a normal allele. Arrow indicates the proband. (B) Schematic representation of the DMT1 molecule and position of the amino acid changes described in the mk mice (G185R) and in human patients. delV114 and G212V are reported here. R416C is a heterozygous mutation associated with c310-3_5delCTT described by Ioalescon et al.14 E399D is a homozygous mutation described by Mims et al.10 Multiple sequence alignments of DMT1 orthologs are shown for residues forming the second predicted transmembrane domain (C) and the fifth transmembrane domain (D).
After informed consent from the parents, blood was obtained for genetic analysis and for establishment of a lymphoblastoid cell line. DNA also was obtained from 55 healthy white patients. To screen for mutations in TfR1 and ferritin subunits cDNA, RNA was extracted from the lymphoblastoid cell line using the RNAplus reagent (QBiogen, Illkirch, France), reversed transcribed, and sequenced using the Big Dye terminator kit (Applied Biosystems, Courtaboeuf, France). To sequence DMT1 gene, genomic DNA was extracted from peripheral blood collected on EDTA (ethylenediaminetetraacetic acid). Each exon of the DMT1 gene was amplified by polymerase chain reaction (PCR) with a set of primers complementary to flanking intron sequences (Table S1, available on the Blood website; see the Supplemental Table link at the top of the online article). The PCR fragments were sequenced using the BigDye terminator kit. Mutations were confirmed by sequencing on the reverse strand.
Results and discussion
In this paper, we report the third case of congenital microcytic hypochromic anemia due to DMT1 mutations. Previous sequencing had found no mutation in the transferrin receptor 1 and in both H and L ferritin cDNAs.
The patient is a compound heterozygote for 2 new mutations, consisting in a deletion of a GTG codon in exon 5 (position 428_430 on the cDNA sequence from GenBank NM 000617), leading to the in-frame deletion of V114 in TM2 and a G → T substitution in exon 8 (position 723 on the cDNA sequence), leading to a G212V replacement in TM5. The father is heterozygous for the delV114 mutation, and both the mother and the sister are heterozygous for the G212V mutation (Figure 1A,B). They all are asymptomatic. These mutations were found neither in single nucleotide polymorphisms (SNPs) databases (http://www.ncbi.nlm.nih.gov/SNP/) nor detected by sequencing the corresponding exons in the DNA from 55 healthy white individuals. V114, although not entirely conserved throughout the Nramp family, is located in TM2, and its deletion is likely to be deleterious (Figure 1C). G212 is conserved throughout species as well as in Nramp1, another divalent metal transporter of the same family (Figure 1D), and this substitution was predicted to be “damaging” by the multicriteria software polyphen (http://www.bork.embl-heidelberg.de/PolyPhen/). On the other hand, the G → V substitution is rather conservative, and the mutated DMT1 molecule might have retained some iron transport function, explaining why the anemia is less severe than in the 2 previously reported cases, who were dependent on transfusions and/or required Epo treatment.11,14
Taken together, these 3 cases clearly highlight the role of DMT1 in erythropoiesis and demonstrate that the erythroid precursors in the bone marrow rely mostly on the endosomal transferrintransferrin receptor pathway for iron uptake. This has already been proposed, based on the observation that hpx mice, deficient in transferrin,16 and patients with atransferrinemia17 have a severe microcytic hypochromic anemia, although they have high serum iron values, and rapidly develop liver iron overload. In these cases, non-transferrin–bound iron (NTBI) is not used by erythroid cells but is taken up by hepatocytes and induces liver iron overload. It is conceivable that patients with DMT1 mutations who have high serum transferrin saturation also have serum NTBI, contributing to hepatocyte iron loading. However, in the patients with DMT1 mutations, and in contrast to what is observed in patients with atransferrinemia and high NTBI, serum ferritin levels were not as increased as expected from the degree of iron overload. In the present patient, serum ferritin levels remained low even in the face of an 8-fold increase in liver iron concentration, and in the other 2 patients reported previously, serum ferritin levels were normal or only moderately elevated.11,14 An alternative hypothesis to the constitution of liver iron overload is that a defective DMT1 in hepatocyte endosomal compartments impairs iron efflux toward the cytosol, leading to intracellular accumulation of iron in a compartment that does not trigger ferritin synthesis and/or secretion. Finally, the liver iron overload that develops in these patients raises the intriguing possibility that intestinal iron absorption does not rely entirely on DMT1. Human intestinal absorption probably compensates for deficiency in ferrous iron uptake by absorbing heme iron.11 The recent identification of an intestinal heme transporter18 further strengthens this hypothesis. Clearly, the hallmark of this new recessive syndrome defined by these 3 patients with DMT1 mutations is a congenital hypochromic microcytic anemia, poorly responsive to iron treatment and associated with progressive accumulation of iron in hepatocytes. It is noteworthy that in this condition, serum ferritin levels are not predictive of the liver iron overload, so that the evaluation of the iron status of these patients should not rely solely on serum iron indices. Other methods such as MRI should be used to evaluate tissue iron stores.
C.B. designed research and wrote the paper; J.D. contributed to the child's follow-up and wrote the paper; G.H. performed sequencing; B.G. analyzed data; M.d.M. contributed to the child's follow-up; and G.T. contributed to the child's follow-up, designed research, and wrote the paper.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, January 26, 2006; DOI 10.1182/blood-2005-10-4269.