Comment on Doughty et al, page 4458
B cells need to meet specific energy demands of their rapid exponential growth. Doughty and colleagues show that B-cell–receptor engagement increases glucose metabolism through PI-3K signaling.
Mature lymphocytes have specific energy requirements that allow them to be maintained in a quiescent state for prolonged periods of time, and to rapidly respond to mitogenic signals and undergo massive proliferation upon recruitment into the effector arm of the immune system. An intricate regulatory network connecting metabolism and functions has been unraveled for T cells in the past few years.1 Resting naive and memory T cells require energy to maintain housekeeping functions, and their ATP is mostly derived from oxidative phosphorylation in the mitochondrion. Engagement of the T-cell receptor by its cognate antigen triggers a functional switch to the effector phase, which requires a rapid and massive clonal expansion. The considerable corresponding energy requirements are met by ligation of costimulatory molecules, such as CD28, and/or the presence of growth factors, such as IL-2. These events have the dual effect of increasing glucose uptake and switching ATP synthesis from the TCA cycle to glucose catabolism.
In contrast, the regulation of the metabolic requirements of B cells is largely unknown. Doughty and colleagues show that ligation of the B-cell receptor (BCR) results in 2 fundamental events: increase in glucose uptake by increasing the expression of the facilitated Glut1 transporter, and a rapid and sustained increase in glucose metabolism, which occurs prior to cell size growth and proliferation. This increase in glucose utilization is an absolute requirement for B-cell growth. In addition to glycolysis, BCR ligation also triggers glucose catabolism through the pentose phosphate pathway. The significance of this new finding is currently unclear. Of interest, all the energy requirements for B-cell growth were met by the sole BCR engagement in the absence of costimulation or growth factors, which are required in T cells. It will be of great interest to determine the metabolic response of B cells to CD40 ligation, IL-4, or LPS exposure, all of which have significant impact on B-cell growth and signaling. Doughty and colleagues demonstrate that, similarly to T cells, the phosphatidylinositol 3-kinase (PI-3K) plays an essential role in increased glucose utilization in BCR-stimulated B cells. Conditional expression of activated protein kinase B/AKT, a downstream target of PI-3K, is sufficient to increase glucose utilization in the absence of BCR engagement. It is very satisfying that the authors were able to show that coengagement of BCR and FcγRIIb, a well-known inhibitory pathway upon ligation with IgG immune complexes,2 results in the opposite events in down-regulating glucose utilization through PI-3K. A somewhat surprising result from this study was the lack of involvement of the target of rapamycin (TOR) protein. Rapamycin is a potent inhibitor of energy metabolism and T-cell growth. It has beenFIG1 shown, however, that TOR participation is stimulus and/or cell-type specific.1 This may indicate that TOR is involved in B-cell metabolism in the presence of other stimulatory signals to be determined.
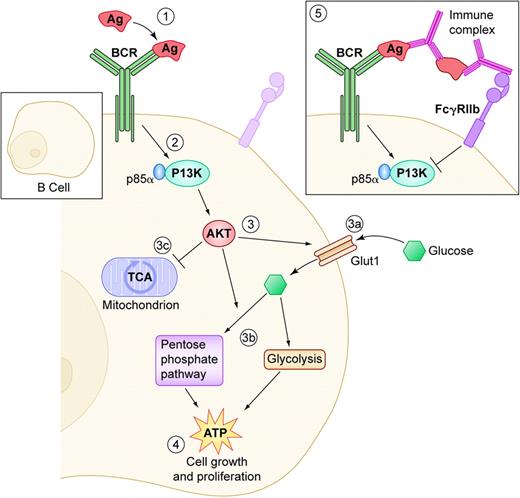
B-cell–receptor (BCR) engagement regulates glucose intake and utilization. (1) Antigen (Ag) binding triggers BCR signaling and (2) activation of phosphatidylinositol 3-kinase (PI-3K) and its p85α subunit. (3) This in turns activates AKT by recruiting it to the membrane. AKT has multiple effects on glucose transport and utilization: (3a) it increases Glut1 expression, which results in increased glucose intake and (3b) it increases glucose catabolism through glycolysis and the pentose phosphate pathway, (3c) to the expense of oxidative phosphorylation through the TCA cycle, which is the main pathway to generate ATP in resting lymphocytes. (4) All of these concerted events concur to greatly boost ATP production, which is necessary to fuel cell growth and proliferation. (5) Conversely, engagement of the negative regulator FcγRIIb by immune complexes results in the opposite PI-3K–mediated effects on glucose intake and utilization, starving B-cell proliferation of its energy supply. Illustration by Kenneth Probst.
B-cell–receptor (BCR) engagement regulates glucose intake and utilization. (1) Antigen (Ag) binding triggers BCR signaling and (2) activation of phosphatidylinositol 3-kinase (PI-3K) and its p85α subunit. (3) This in turns activates AKT by recruiting it to the membrane. AKT has multiple effects on glucose transport and utilization: (3a) it increases Glut1 expression, which results in increased glucose intake and (3b) it increases glucose catabolism through glycolysis and the pentose phosphate pathway, (3c) to the expense of oxidative phosphorylation through the TCA cycle, which is the main pathway to generate ATP in resting lymphocytes. (4) All of these concerted events concur to greatly boost ATP production, which is necessary to fuel cell growth and proliferation. (5) Conversely, engagement of the negative regulator FcγRIIb by immune complexes results in the opposite PI-3K–mediated effects on glucose intake and utilization, starving B-cell proliferation of its energy supply. Illustration by Kenneth Probst.
These new results should open the way to new studies on B-cell metabolism to determine how glucose utilization is altered not only by other stimulatory conditions, but also in the progression of B cells through peripheral development. The various B-cell subsets that have been described from transitional cells to plasma cells have very different functions in health and in disease and, one might speculate, different energy needs. A better understanding of these energy requirements and their regulation may allow targeting of specific B-cell subsets for amplification or reduction, according to therapeutic requirements. ▪