Abstract
Human T cell leukemia virus type 1 (HTLV-1), the cause of adult T cell leukemia (ATL), induces clonal expansion of infected T-cells in nonleukemic individuals and immortalizes T cells in vitro. The resistance against apoptotic stimuli of these cells hints at a viral survival function in addition to a proliferation-stimulating activity. Here we describe the up-regulation of the antiapoptotic HIAP-1/CIAP-2 gene as a consistent phenotype of HTLV-1–transformed and ATL-derived cultures and its stimulation by the viral oncoprotein Tax. Cotransfections revealed a 60-fold increase of HIAP-1 promoter activity mediated by Tax mainly via nuclear factor-κB (NF-κB) activation. To address the relevance of virally increased HIAP-1 levels for the survival of HTLV-1–transformed cells, its expression was RNA interference (RNAi) suppressed using a lentiviral transduction system. This resulted in a dramatic reduction of cell growth, a strong induction of apoptosis rates, and increased caspases 3/7 activity, which is known to be suppressed by HIAP-1. Thus, the Tax-mediated HIAP-1 overexpression is required to suppress endogenous apoptosis and, therefore, is essential for the survival of HTLV-1–transformed lymphocytes. Moreover, this points to HIAP-1 as an important target of the HTLV-1–mediated NF-κB activation.
Introduction
The human T cell leukemia virus type 1 (HTLV-1) infection is causally linked with the development of a severe and fatal lymphoproliferative disorder of CD4+ T cells, the adult T cell leukemia (ATL), and of the neurodegenerative disease HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP).1,4 Both diseases develop only after prolonged viral persistence. The HTLV-1 infection seems to stimulate growth and survival of the T-lymphocytes in carriers, since they expand to detectable clones, which can persist over many years even in nonleukemic individuals.5,6 This notion is corroborated by the virus's capacity to stimulate permanent T-lymphocyte growth in vitro, resulting in T-cell immortalization. Hence, it is highly probable that viral gene functions are not only involved in immortalization of T-cell cultures, but also cause the clonal expansion of patient lymphocytes. Such growth-stimulating functions provide means to replicate the (pro-) viral genome without producing virus particles.
Being a prototypic delta-retrovirus, HTLV-1 encodes the regulatory nonstructural proteins Tax and Rex, which are essential for viral replication,7 and the accessory proteins p12, p30, and p13. While Rex acts at a posttranscriptional level8,9 to control the expression of the structural proteins, Tax strongly enhances viral gene expression by transactivating the HTLV-1 long-terminal-repeat promoter.10 Although the accessory proteins are important for viral infectivity and replication by influencing cellular signaling and gene expression,11,16 p12, p13, and p30 are dispensable for lymphocyte immortalization.17,18
Biochemically, Tax can stimulate transcription by affecting various pathways. Nuclear factor-κB (NF-κB) is activated by binding and stimulating IKKγ, a component of the inhibitor of kappa B kinase.19 Transactivation of various cellular promoters is mediated by binding to the transcriptional activators CREB and SRF, and to the coactivators p300/CBP.10,20 Tax also induces activator protein-1 (AP-1), a transcription factor complex composed of members of the Fos/Jun family,21,22 and stimulates transcription via nuclear factor of activated T-cell (NF-AT) elements.23,25
Tax confers the transforming properties on the virus. It is capable of immortalizing T cells26,27 and is leukemogenic in transgenic mice.28 It interferes with normal cell-cycle control by dysregulating control checkpoints.29 In particular, Tax is capable of stimulating the G1 phase by binding to and activating cyclin-dependent kinase holoenzymes; it also inhibits DNA repair and induces aneuploidy.30,35 Importantly, it interferes with tumor suppressor functions; for instance, it inactivates p53.36,37 Moreover, Tax can stimulate or repress the expression of cellular proteins involved in cell survival and proliferation. Among those are proto-oncogenes (c-FOS, EGR1), cytokines, and cytokine receptors,20,25,38 as well as cell-cycle regulators (p21WAF1/CIP1). Tax-mediated modulation of cellular gene expression may also explain the resistance of HTLV-1–positive cells to various proapoptotic stimuli.39,40 For example, it renders HTLV-1–transformed cells resistant to tumor necrosis factor α (TNFα)–induced apoptosis41 and reduces the sensitivity of Tax-expressing cells and T cells of Tax-transgenic mice to Fas-mediated apoptosis.10,42,43 Reduced sensitivity to apoptosis inducers is also a common feature of leukemic and HTLV-1–transformed T cells.44,45
Systematic comparison of the transcriptomes46,50 of HTLV-1–infected and uninfected cells hinted at genes of potential relevance for the survival of HTLV-1–transformed cells, among them HIAP-1 (human inhibitor of apoptosis 1)/CIAP-2 (cellular inhibitor of apoptosis 2). HIAP-1 represses apoptosis since it binds and inhibits caspases 3, 7, and 9.51,52 Because HIAP-1 was also identified as a possible oncogene53 involved in tumor progression, we analyzed its role in HTLV-1 transformation. Here we show that strong up-regulation of HIAP-1 expression by Tax is a consistent feature of HTLV-1–transformed cell lines. Overexpression is caused by efficient transactivation of the HIAP-1 promoter, mainly via NF-κB. Suppression of HIAP-1 expression by RNAi results in strongly reduced cellular proliferation, apoptosis, and increased caspases 3/7 activity in HTLV-1–transformed cells. Thus, Tax induces HIAP-1 overexpression, which is essential for the survival of HTLV-1–transformed lymphocytes.
Materials and methods
Cell culture
The HTLV-negative acute lymphoblastic leukemia (ALL) T-cell lines Jurkat, Molt-4, and CCRF-CEM (CCL-119; American Type Culture Collection, Manassas, VA), the HTLV-negative Sezary syndrome T-cell line HuT-78, the HTLV-1–transformed T-cell lines (C91-PL and MT-2), and ATL-derived cultures (JuanaW, Champ, PaBe, StEd and ATL1) were propagated as described.25,49,54 Eva cells are peripheral blood lymphocytes from a patient with HAM/TSP, which had been in permanent culture for 8 weeks since isolation from a blood sample. The culture was established and kept as described.49 All ATL-derived cell cultures and Eva cells require exogenous interleukin-2 (IL-2) for growth. Peripheral blood lymphocytes (PBLs) were prepared from 3 healthy donors using ficoll gradient centrifugation as described earlier.49 Tesi cells are primary human T cells, immortalized by a conditional expression cassette for the HTLV-1 Tax protein, which was transduced with a rhadinoviral vector.27 Tesi cells were propagated as described.49 Tesi cells were harvested after 10 days of cultivation in the absence and presence of 1 μg/mL tetracycline (Tet), which represses Tax expression.
RNA quantification
Total RNA was prepared from cell cultures (Macherey-Nagel, Düren, Germany). Reverse transcriptase (RT) and polymerase chain reaction (PCR) reactions were performed as described.25 The cDNA preparations were controlled by amplification of β-actin cDNA. (Primers: β-actin, GGGAAATCGTGCGTGACAT and GAACTTTGGGGGATGCTCGC; HIAP-1, CCAA CAGATCTGGCACGAGC and CACTTGCAAGCTGCTCAGGA; and blasticidin S deaminase, CTCAAGAAGAATCCACCCTC and TCACTGTCCTTCACTATCGC). For quantification of HIAP-1 and Tax RNA expression, real-time RT-PCR was applied. Primer and probes for HIAP-1 were purchased (Applied Biosystems, Foster City, CA). Sequences for Tax and for β-actin real-time RT-PCR reagents were taken from the literature.55,56 The PCR was performed on an ABI PRISM 7700 (Applied Biosystems) using 500-ng template cDNA and Absolute quantitative (Q) PCR Mixes (Abgene, Surrey, United Kingdom). Cycling conditions: 50°C for 2 minutes, 95°C for 15 minutes, 95°C for 15 seconds, and 60°C for 1 minute, 40 cycles. Each experiment was performed in duplicate or triplicate. For quantification, calibration curves were established using dilutions of plasmid-cloned cDNA of HIAP-1, Tax, and β-actin. The human housekeeping gene β-actin was used for internal calibration. The relative copy number of HIAP-1 mRNA was calculated by the following formula: HIAP-1 mRNA copy number = (value of HIAP-1 copies)/(value of β-actin copies). The relative copy number of Tax mRNA was determined in analogy. For estimation of copy numbers per cell, β-actin was quantitated from Jurkat cells. Briefly, the RNA from 1 × 106 Jurkat cells resulted in 90 μg cDNA, containing about 1.304 × 106 (± 0.269 × 106) copies β-actin/μg cDNA. Accordingly, the copy number of β-actin transcripts per cell was calculated to be 117 ± 24. The correlation between Tax and HIAP-1 mRNA loads was analyzed by Pearson correlational analysis (correlation coefficient, r = .992; significance, P = .008).
Promoter analysis
Based on the published sequence57 (GenBank accession no. AF233684), the HIAP-1 promoter was cloned from genomic DNA using Pwo polymerase and restriction sites-containing primers (GATCGCTAGCGAGATATGT GACAGAGAGAT, GACTAAGCTTCTGGCCTTTTCCGTGCCGGA). The purified PCR product was inserted into the pGL3-Basic vector (Promega, Mannheim, Germany) to obtain pGL3-HIAP1P. The NF-κB deletion mutants (HIAP1PD-147/136, HIAP1PD-196/187, HIAP1PD-209/200, and HIAP1PD-147/136D-196/187D-209/200) were generated via PCR using chimeric primers (HIAP1PD-147/136: TGTGTGGTTATTACCGCTAAGTCCTAAAAGGAAAG and CTTTCCTTTTAGGAC TTAGCGGTAATAACCACACAC; HIAP1PD-196/187: GTCATGGAAATCCCCGAGAGGCCACTGATTAAGAGG and CCTCTTAATCAGTGGCCTCTCGGGGATTTCCATGAC; and HIAP1PD-209/200: TCATGCTTTTGGGTCATGGAGTGGGTTTGCCAGGCC and GCCTGGCAAACCCACTCCATGACCCAAAAGCATGA). Cycling conditions: 94°C for 2 minutes, 35 cycles at 94°C for 15 seconds, 60°C for 30 seconds, and 72°C for 90 seconds. All promoter variants were sequenced to validate their identity. To test the HIAP-1 promoter stimulation by Tax, luciferase assays were performed in Jurkat T cells as described.25 The Tax expression vectors pcTax (HTLV-1 TaxWT) and M7, M22, and M47 (Tax mutants) were used.58 Luciferase activity was normalized to protein concentration and relative luciferase activity was calculated as multiple of the control (HIAP1P + pcDNA3.1).
Immunoblots
For the HIAP-1 protein detection, immunoblots were performed as described.25,32,49 The blots were incubated for 12 hours at 4°C with a 1:1000 dilution of anti–HIAP-1/BIRC3 rabbit monoclonal antibodies (Epitomics, Burlingame, CA). As internal standard β-actin was detected on each immunoblot after removal of the HIAP-1–specific antibodies (anti–β-actin mouse monoclonal antibodies 1:2500; Sigma, Taufkirchen, Germany). Specific bands were visualized using secondary antibodies and the enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotechnologies, Freiburg, Germany).
Apoptosis assays
Propidium iodide (PI) staining was performed as described.59 Cells were washed and resuspended in 25 μg/mL PI. To extract the nuclei, the cells were shaken for 30 minutes with 2% Triton and 0.5 g/L sodium citrate. The staining was measured in the flow cytometer (FACSCalibur; Becton Dickinson, San Jose, CA). As an apoptosis control, uninfected cells were treated with 1 μg/mL etoposide for 24 hours.
Because HIAP-1 specifically binds and inhibits caspases 3/7, the activity of these caspases was determined. For 1 assay 5000 cells were lysed and incubated with the caspases 3/7–specific substrate (Z-DEVD-aminoluciferin) according to the manufacturer's instructions (Caspase-Glo 3/7 Assay; Promega, Mannheim, Germany). Released aminoluciferin was detected in a luciferase reaction. The luminescence signal is proportional to the amount of active caspases 3/7. The relative caspases 3/7 activity in cells was calculated as multiple of the empty vector control.
Lentiviral transduction of shRNA
Double-stranded oligonucleotides encoding short hairpin RNAs (shRNAs) specific for HIAP-1 and a nonsense shRNA60 were ligated into the pSUPER vector61 (oligonucleotides sinonsense: GATCCCCGCGCGCTTTGTAGGATTCGTT CAAGAGACGAATCCTACAAAGCGCGCTTTTTGGAAA and AGCTTTTCCAAAAAGCGCGCTTTGTAGGATTCGTCTCTTGAACGAATCCTCAAAGCCGGG; oligonucleotides siHIAP1 (target sequence 143-161 bp of the open reading frame [ORF]): GATCCCCGGAGTCTTGCTCGTGCTGGTTCAAGAGACCAGCACGAGCAAGCTCCTTTTTGGAAA, AGCTTTTCCAAAAAGGAGTCTTGCTCGTGCTGGTCTCTTGAACCAGCACGAGCAAGACTCCGGG). The pSUPER-derived plasmids were ClaI/XbaI-digested and the shRNA expression cassettes, containing the shRNA sequences and the H1 promoter, were cloned into the lentiviral vector pNL-SIN-CMV-BLR,62 which encodes blasticidin S deaminase as selection marker. Vesicular stomatitis virus–glycoprotein (VSV-G)–pseudotyped lentiviruses were produced in 293T cells. For 1 tissue-culture plate of 10-cm diameter, 9 μg lentiviral vector (pNL-SIN-CMV-BLR, pNL-siHIAP1 or pNL-sinonsense), 0.5 μg Rev, 0.5 μg Tat, and 0.25 μg VSV-G expression plasmids62 were transfected using lipofectamine (Invitrogen, Karlsruhe, Germany). After 48 and 72 hours, the lentivirus-containing supernatant was collected and concentrated (53 000g, 2 hours, 4°C). For infection, 1.5 × 106 lymphocytes and 1 mL viral concentrate were centrifuged for 1 hour at 1200g in the presence of 8 μg/mL polybrene (Sigma). A part of the cells was selected with 8 μg/mL blasticidin S hydrochlorid (Sigma). Lentivirally transduced lymphocytes were harvested after 4 to 6 days for apoptosis assays, and RNA/protein analysis. To establish growth curves, the cell concentration was adjusted to 1.5 × 105 cells/mL at day 2 and counted daily.
Results
Correlation of increased HIAP-1 and Tax expression in HTLV-1–transformed T cells
Systematic comparisons of the transcriptomes of HTLV-1–infected cells and uninfected counterparts indicate that several antiapoptotic mRNAs are overexpressed in HTLV-1–transformed cells. Using the suppression subtractive hybridization (SSH) technique49 and cDNA arrays, HIAP-1 transcripts were identified as 1 such mRNA.46,48,50 Increased levels of HIAP-1 mRNA in the presence of Tax could be confirmed in an apoptosis cDNA array analyzing Tesi RNA (T.R., unpublished data, May 2002). HIAP-1 is a member of the IAP (inhibitor of apoptosis protein) family, which binds and inhibits caspases 3, 7, and 9 and, hence, is antiapoptotic.51,52 Since HIAP-1 is overexpressed in some human malignancies,63,65 and is considered to be a possible oncogene,53 overexpression of HIAP-1 in HTLV-1–infected cells could be responsible for the reduced apoptotic sensitivity in these cells and may be required for malignant growth of ATL cells. To verify that the up-regulation of HIAP-1 is a general feature of HTLV-1–infected cells, RT-PCR and real-time RT-PCR analyses were performed. RNA was isolated from ATL-derived cells (Champ, StEd, ATL3, and JuanaW), HTLV-1–transformed cells (C91-PL and MT-2) and HTLV-1–negative CD4+ T-cell lines derived from acute lymphoblastic leukemia (ALL: Jurkat, Molt-4, and CCRF-CEM). In addition, RNA was prepared from an 8-week-old peripheral blood lymphocyte (PBL) culture of a patient with HTLV-1 (HAM/TSP) and from PBLs (PBL1-3) of 3 healthy donors. Specific transcripts were analyzed by RT-PCR and quantified by real-time PCR (Figure 1A-B). The results show that HIAP-1 mRNA is strongly increased in all HTLV-1–transformed and ATL cell lines compared with the HTLV-1–negative cell lines Jurkat, Molt-4, and CCRF-CEM. Even the HAM/TSP blood lymphocyte culture (Eva), which contains HTLV-1–transformed T-cells and remaining uninfected lymphocytes, revealed an increased HIAP-1 mRNA level. The HIAP-1 mRNA load in HTLV-1–transformed cells was in the same order of magnitude as the housekeeping gene β-actin; the mRNA copy per cell in ATL cultures was calculated to be between 94 (JuanaW) and 597 (ATL-3), whereas the copy numbers in ALL cell lines were below 12 per cell. The up-regulation of HIAP-1 coincides with Tax expression in HTLV-1–positive cells, as could be shown by real-time RT-PCR (Figure 1B). Immunoblotting revealed that the markedly elevated mRNA levels result in strongly increased amounts of HIAP-1 protein in the HTLV-1–positive lymphocytes (Figure 1C). By contrast, HIAP-1 protein was almost undetectable in the HTLV-1–negative controls. Thus, the antiapoptotic HIAP-1 protein is consistently overexpressed in all tested HTLV-1–positive cell lines.
Overexpression of HIAP-1 in HTLV-1–infected human T-cells. HIAP-1 and Tax expressions were analyzed in HTLV-1–transformed T-cell lines (C91-PL and MT-2), ATL-derived cultures (Champ, StEd, ATL-3, and JuanaW), HAM/TSP-derived cultures (Eva), in uninfected ALL-derived CD4+ control T-cell lines (CCRF-CEM, Molt-4, and Jurkat), and in PBLs isolated from 3 healthy donors (PBL1-PBL3). (A) Total RNA was extracted and subjected to RT-PCR analyses. Equal loading was controlled by β-actin RT-PCR. (B) The same cDNAs were used for real-time RT-PCR analyses of HIAP-1, Tax, and β-actin (internal standard) transcripts. The relative mRNA copy numbers of HIAP-1 and Tax mRNA were determined as multiples of the β-actin mRNA copy number. The columns represent the mean of 6 experiments; the error bars, mean error. (C) To detect HIAP-1 protein, immunoblots were stained using HIAP-1–and β-actin–specific monoclonal antibodies.
Overexpression of HIAP-1 in HTLV-1–infected human T-cells. HIAP-1 and Tax expressions were analyzed in HTLV-1–transformed T-cell lines (C91-PL and MT-2), ATL-derived cultures (Champ, StEd, ATL-3, and JuanaW), HAM/TSP-derived cultures (Eva), in uninfected ALL-derived CD4+ control T-cell lines (CCRF-CEM, Molt-4, and Jurkat), and in PBLs isolated from 3 healthy donors (PBL1-PBL3). (A) Total RNA was extracted and subjected to RT-PCR analyses. Equal loading was controlled by β-actin RT-PCR. (B) The same cDNAs were used for real-time RT-PCR analyses of HIAP-1, Tax, and β-actin (internal standard) transcripts. The relative mRNA copy numbers of HIAP-1 and Tax mRNA were determined as multiples of the β-actin mRNA copy number. The columns represent the mean of 6 experiments; the error bars, mean error. (C) To detect HIAP-1 protein, immunoblots were stained using HIAP-1–and β-actin–specific monoclonal antibodies.
To determine whether the increased HIAP-1 expression is caused by the viral transactivator, Jurkat cells were transfected with various amounts of a Tax expression plasmid. RT-PCR showed that Tax expression reproducibly resulted in the synthesis of significant amounts of endogenous HIAP-1 mRNA, whereas in empty vector–transfected Jurkat cells HIAP-1 transcripts were barely detectable (data not shown). Moreover, quantitating Tax and HIAP-1 transcripts by real-time RT-PCR (Figure 2A) revealed that linearly increasing Tax expression resulted in a linear increase of HIAP-1 mRNA synthesis. According to the Pearson correlational analysis, the correlation is significant at the .01 level (2-tailed). The impact of Tax on HIAP-1 transcription could also be reproduced in conditionally Tax-expressing Tesi cells (Figure 2B). The Tesi cell line was obtained by transforming human cord blood lymphocytes (CBLs) with a tetracycline repressible HTLV-1 Tax gene.27 Thus, the increased HIAP-1 levels consistently correlate with the expression of the viral transactivator Tax in different cell systems. These results provide a primary indication for Tax-mediated transactivation of the HIAP-1 gene.
Induction of HIAP-1 synthesis by HTLV-1 Tax. The impact of Tax on endogenous HIAP-1 expression was investigated in Jurkat T cells and in Tax-immortalized primary human T cells (Tesi). (A) Jurkat cells (107) were transfected with the indicated amounts of the Tax expression plasmid pcTax. Empty vector plasmid was added to obtain equal amounts of transfected DNA. After 48 hours RNA was extracted, cDNA prepared, and the amounts of HIAP-1 and β-actin transcripts (internal standard) were analyzed by real-time RT-PCR. The relative mRNA copy numbers of HIAP-1 and Tax mRNA were determined as multiples of the β-actin mRNA copy number. The correlation between Tax and HIAP-1 mRNA relative copy numbers is highly significant at the .01 level (2-tailed). (B) Human T cells transformed by a repressible TAX gene (Tesi) were kept under condition of induced (Tesi) and repressed Tax (Tesi + Tet [tetracycline]) expression. RNA was analyzed by RT-PCR and quantified by real-time RT-PCR. The relative mRNA copy numbers of HIAP-1 and Tax mRNA were determined as multiples of the β-actin mRNA copy number. The columns represent the mean of 3 experiments; the error bars indicate standard deviation.
Induction of HIAP-1 synthesis by HTLV-1 Tax. The impact of Tax on endogenous HIAP-1 expression was investigated in Jurkat T cells and in Tax-immortalized primary human T cells (Tesi). (A) Jurkat cells (107) were transfected with the indicated amounts of the Tax expression plasmid pcTax. Empty vector plasmid was added to obtain equal amounts of transfected DNA. After 48 hours RNA was extracted, cDNA prepared, and the amounts of HIAP-1 and β-actin transcripts (internal standard) were analyzed by real-time RT-PCR. The relative mRNA copy numbers of HIAP-1 and Tax mRNA were determined as multiples of the β-actin mRNA copy number. The correlation between Tax and HIAP-1 mRNA relative copy numbers is highly significant at the .01 level (2-tailed). (B) Human T cells transformed by a repressible TAX gene (Tesi) were kept under condition of induced (Tesi) and repressed Tax (Tesi + Tet [tetracycline]) expression. RNA was analyzed by RT-PCR and quantified by real-time RT-PCR. The relative mRNA copy numbers of HIAP-1 and Tax mRNA were determined as multiples of the β-actin mRNA copy number. The columns represent the mean of 3 experiments; the error bars indicate standard deviation.
Stimulation of the HIAP-1 promoter via NF-κB by the viral transactivator Tax
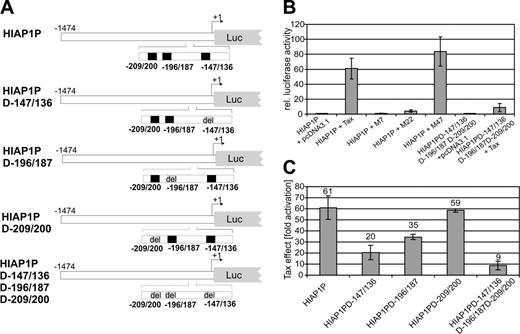
To test whether promoter transactivation by Tax causes HIAP-1 overexpression, corresponding sequences (–1474 to +6) were PCR-amplified from genomic DNA and cloned into a luciferase reporter plasmid (Figure 3A). Luciferase activity, determined from transfected Jurkat T lymphocytes, revealed a strong stimulation (60-fold) of the HIAP-1 promoter in the presence of Tax (Figure 3B). To narrow down transactivation-relevant signaling pathways, Tax mutants M7, M22, and M4766 were cotransfected along with the HIAP-1 promoter construct and luciferase activities were determined. M22, which activates the CREB transcriptional pathway,66 but is incapable of stimulating NF-κB transcription factors due to a defect in associating with IKKγ,19 is greatly impaired in HIAP-1 promoter transactivation. The Tax mutant M47 could transactivate the HIAP-1 promoter like TaxWT. This mutant can activate NF-κB but is impaired in stimulating the CREB and SRF pathways due to its inability to interact with the p300- and CBP-associated factor (PCAF).67,68 M7, which is inactive in transactivation, had no effect on the HIAP-1 promoter. These results point to a Tax transactivation of the HIAP-1 promoter via NF-κB. The promoter contains 3 potential NF-κB binding sites.57 To verify the role of NF-κB in transcriptional transactivation, the promoter mutant HIAP1PD-147/136D-196/187D-209/200 was constructed, in which these sites were deleted (Figure 3A). Transfections of this deletion mutant showed drastically reduced Tax stimulation (Figure 3B). A residual transactivation by Tax hints at a minor contribution of other transcription factors. This is also suggested by the small induction of the HIAP-1 promoter by the NF-κB–deficient Tax mutant M22. This may resemble the physiologic induction of HIAP-1, which may rely on AP-1, NF-AT, and CREB57 in addition to NF-κB.69 These are known targets of Tax transactivation10,21,25 and, thus, it is conceivable that the residual transactivation of the HIAP-1 promoter by Tax may be due to the action of transcription factors AP-1, NF-AT, and/or CREB. To address the significance of the individual NF-κB binding sites, we analyzed HIAP-1 promoter mutants (HIAP1PD-147/136, HIAP1PD-196/187, and HIAP1PD-209/200), deleted in single NF-κB binding sites (Figure 3A). The deletion of the sites HIAP1PD-147/136 and HIAP1PD-196/187 resulted in a significant reduction of Tax's capacity to stimulate the promoter, whereas the deletion of the NF-κB binding site HIAP1PD-209/200 resulted only in a marginal reduction. Therefore, the HIAP1PD-147/136 NF-κB binding site is most important for transactivation by Tax. In summary, the viral transactivator Tax efficiently stimulated the HIAP-1 promoter mainly through NF-κB activation and, thus, could cause the observed HIAP-1 overexpression.
Transactivation of the HIAP-1 promoter by the HTLV-1 Tax protein. (A) Overview of the HIAP-1 promoter and deletion variants. The potential NF-κB binding sites are indicated in black, deletions in gray. (B) Tax transactivation of the HIAP-1 promoter. Jurkat T lymphocytes were cotransfected with the HIAP-1 promoter (HIAP1P) or the mutant (HIAP1PD-147/136D-196/187D-209/200), in which 3 NF-κB sites were deleted, together with TaxWT, Tax mutants (M7, M22, and M47), or empty expression vector (pcDNA3.1). Luciferase activity was determined and normalized to the negative control (HIAP1P + pcDNA3.1). The columns represent the mean of 3 independent experiments and standard deviation. (C) Tax transactivation of the NF-κB site deletion variants of the HIAP-1 promoter. Indicated promoter constructs were transfected in the presence and absence of Tax, luciferase activity was analyzed, and the fold activation of each construct by Tax was determined. The bars represent the mean of 2 independent experiments; error bars indicate the mean error.
Transactivation of the HIAP-1 promoter by the HTLV-1 Tax protein. (A) Overview of the HIAP-1 promoter and deletion variants. The potential NF-κB binding sites are indicated in black, deletions in gray. (B) Tax transactivation of the HIAP-1 promoter. Jurkat T lymphocytes were cotransfected with the HIAP-1 promoter (HIAP1P) or the mutant (HIAP1PD-147/136D-196/187D-209/200), in which 3 NF-κB sites were deleted, together with TaxWT, Tax mutants (M7, M22, and M47), or empty expression vector (pcDNA3.1). Luciferase activity was determined and normalized to the negative control (HIAP1P + pcDNA3.1). The columns represent the mean of 3 independent experiments and standard deviation. (C) Tax transactivation of the NF-κB site deletion variants of the HIAP-1 promoter. Indicated promoter constructs were transfected in the presence and absence of Tax, luciferase activity was analyzed, and the fold activation of each construct by Tax was determined. The bars represent the mean of 2 independent experiments; error bars indicate the mean error.
Apoptosis induction and proliferation prevention of HTLV-1–transformed cells through RNAi-suppressed HIAP-1
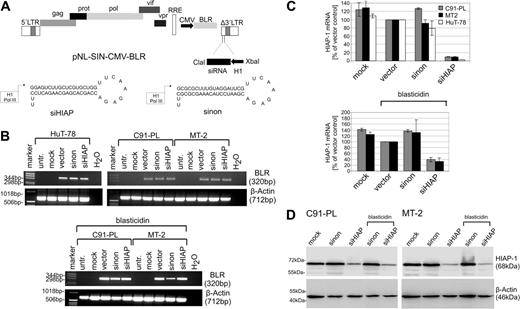
To investigate the significance of HIAP-1 overexpression in HTLV-1–transformed cells, we expressed specific shRNAs using lentiviral vectors. Double-stranded oligonucleotides encoding shRNAs specific for HIAP-1 and a nonsense shRNA60 were assembled into a RNA polymerase III expression cassette and cloned into the lentiviral vector pNL-SIN-CMV-BLR62 (Figure 4A), which encodes blasticidin S deaminase (BLR) as a selectable marker. The nonsense small interfering RNA (siRNA) was selected as control since it was proven to not reduce any gene expression.60 VSV-G–pseudotyped viruses were produced in 293T cells by cotransfecting the shRNA-containing lentiviral vector backbone together with expression plasmids for Rev, Tat, and VSV-G.
Suppression of HIAP-1 by lentiviral transduction of a shRNA expression cassette into HTLV-1–transformed cells. (A) Physical map of the shRNA lentiviral vector (pNL-SIN-CMV-BLR) and the inserted shRNA precursor specific for HIAP-1 and the nonsense precursor. (B) The HTLV-1–transformed T-cell lines (MT-2 and C91-PL) and HTLV-1–negative control cells (HuT-78) were infected with lentiviruses encoding shRNAs specific for HIAP-1 (siHIAP), nonsense shRNAs (sinon), empty lentiviruses (vector), and were mock-infected. The RNA of the cells was extracted, and subjected to RT-PCR using blasticidin S deaminase primers and β-actin primers as a control. (C) Quantitation of HIAP-1 expression by real-time RT-PCR. The HIAP-1 expression was normalized to the empty vector control. The bars represent the mean of 2 independent experiments and the mean error. (D) To detect RNAi-mediated reduction of HIAP-1 protein content, proteins were prepared from the transduced cells and analyzed by immunoblots. Specific bands were detected by HIAP-1– and β-actin–specific monoclonal antibodies.
Suppression of HIAP-1 by lentiviral transduction of a shRNA expression cassette into HTLV-1–transformed cells. (A) Physical map of the shRNA lentiviral vector (pNL-SIN-CMV-BLR) and the inserted shRNA precursor specific for HIAP-1 and the nonsense precursor. (B) The HTLV-1–transformed T-cell lines (MT-2 and C91-PL) and HTLV-1–negative control cells (HuT-78) were infected with lentiviruses encoding shRNAs specific for HIAP-1 (siHIAP), nonsense shRNAs (sinon), empty lentiviruses (vector), and were mock-infected. The RNA of the cells was extracted, and subjected to RT-PCR using blasticidin S deaminase primers and β-actin primers as a control. (C) Quantitation of HIAP-1 expression by real-time RT-PCR. The HIAP-1 expression was normalized to the empty vector control. The bars represent the mean of 2 independent experiments and the mean error. (D) To detect RNAi-mediated reduction of HIAP-1 protein content, proteins were prepared from the transduced cells and analyzed by immunoblots. Specific bands were detected by HIAP-1– and β-actin–specific monoclonal antibodies.
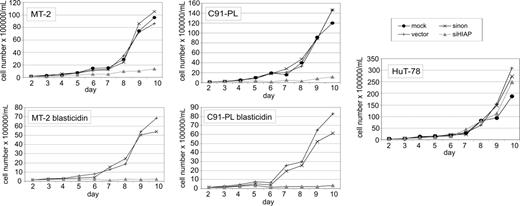
To analyze the consequences of suppressed HIAP-1 synthesis in HTLV-1–transformed lymphocytes, the cell lines MT-2 and C91-PL were infected with lentiviruses expressing shRNAs specific for HIAP-1 (siHIAP). As controls, the same vector expressing a nonsense shRNA (sinon), empty lentivirus vectors (vector), and mock infections were done in parallel. As a HIAP-1–positive HTLV-1–negative control T-cell line HuT-78, which is derived from a Sezary syndrome, was also infected. According to real-time RT-PCR analyses, these cells contained increased HIAP-1 expression levels comparable with those of HTLV-1–infected lymphocytes (relative HIAP-1 mRNA copy no. of HuT-78 = 1.5). For this cell line an autocrine stimulation by TNFα was shown, which probably caused the HIAP-1 up-regulation.70 Transduction experiments were performed in duplicate; cells were treated with blasticidin S hydrochlorid in part. Equal transduction efficiencies of the lymphocyte cultures were verified by RT-PCR analysis of BLR mRNA (Figure 4B). The effect of the transduced shRNA on HIAP-1 expression was analyzed by real-time RT-PCR 5 days after shRNA gene transduction. It indicated strongly reduced levels of HIAP-1 mRNA in all cells containing siHIAP (Figure 4C). Immunoblots revealed that treatment with siHIAP-expressing lentiviruses had resulted in strongly reduced levels of HIAP-1 protein (Figure 4D). To investigate the impact on cell proliferation, growth rates of the cultures were determined. Cells were counted every day starting at day 2 after infection (Figure 5). Accordingly, transduction with siHIAP-expressing lentiviruses resulted in drastically reduced proliferation of HTLV-1–infected T cells. By contrast, all control cells proliferated well, indicating that neither the expression of a nonsense shRNA nor the infection with the lentiviral vector harmed the cells. In particular the siHIAP genetransduced HTLV-1–negative cell line HuT-78 did not exhibit any changes in its growth behavior, indicating that the shRNA specific for HIAP-1 was not toxic. Thus, HIAP-1 suppression resulted in poor growth of HTLV-1–transformed cells but not in other leukemic T-cell lines.
Poor proliferation of siHIAP1-containing HTLV-1–transformed cells. The HTLV-1–transformed T-cell lines (MT-2 and C91-PL) and HTLV-1–negative control cells (HuT-78) were infected with lentiviruses expressing shRNAs specific for HIAP-1 (siHIAP), nonsense shRNAs (sinon), empty lentiviruses (vector), and were mock-infected. Two days after infection, the cell density was adjusted to 1.5 × 105 cells/mL and then counted at the indicated time points. The figure shows growth curves obtained for unselected cultures and for cultures treated with blasticidin S hydrochlorid (selected). Mock infected control cultures died in the presence of blasticidin S hydrochlorid within 4 to 6 days (not shown).
Poor proliferation of siHIAP1-containing HTLV-1–transformed cells. The HTLV-1–transformed T-cell lines (MT-2 and C91-PL) and HTLV-1–negative control cells (HuT-78) were infected with lentiviruses expressing shRNAs specific for HIAP-1 (siHIAP), nonsense shRNAs (sinon), empty lentiviruses (vector), and were mock-infected. Two days after infection, the cell density was adjusted to 1.5 × 105 cells/mL and then counted at the indicated time points. The figure shows growth curves obtained for unselected cultures and for cultures treated with blasticidin S hydrochlorid (selected). Mock infected control cultures died in the presence of blasticidin S hydrochlorid within 4 to 6 days (not shown).
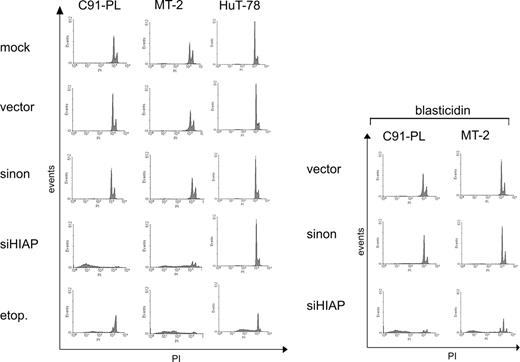
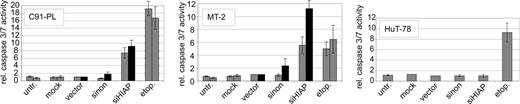
Since HIAP-1 is an antiapoptotic effector, increased apoptosis is a probable reason for the poor survival. Thus, the apoptosis rates were determined with PI staining of nuclear DNA.59 In this assay apoptotic cells can be identified by a DNA content peaking below the G1 peak (sub-G1 fraction). According to this criterion, control cells underwent no or only little apoptosis (Figure 6), similar to untreated cells (data not shown). By contrast, siHIAP-containing HTLV-1–infected cells, but not HTLV-1–negative control cells, showed extensive apoptosis. The effect of the shRNA resembles the positive control, in which uninfected cells were treated with etoposide (etop.), a strong apoptosis inducer. In agreement with unchanged proliferation, the shHIAP-treated HTLV-1–negative cell line HuT-78 did not exhibit apoptosis, indicating that in this cutaneous lymphoma-derived cell line the expression of HIAP-1 is not essential for preventing apoptosis. In siHIAP-containing cells, apoptosis was induced independent of blasticidin treatment. Since HIAP-1 functions by binding and inhibiting caspases 3/7,51,52 their activity was determined. Moreover, caspases 3/7 activity is a central event in apoptosis induction71 and, thus, allows indirect measurement of apoptosis rates. Cells were lysed and incubated with a specific substrate which releases aminoluciferin if cleaved by these caspases. Aminoluciferin, as indicator of the caspase activity, was measured in a luciferase reaction. This experiment revealed a strong increase of caspases 3/7 activity in the siHIAP-containing HTLV-1–positive cells but not in the HTLV-1–negative leukemic cell line HuT-78 (Figure 7). The increased caspase activation in selected cells might be due to proapoptotic signals induced by blasticidin selection. Thus, repression of HIAP-1 in HTLV-1–transformed lymphocytes induces caspases 3/7 activity and endogenous apoptosis and so results in the dramatic loss of cellular proliferation. In summary, the stimulation of HIAP-1 by Tax results in overexpression, which protects HTLV-1–transformed lymphocytes from endogenous apoptosis and, thus, is essential for the survival of these cells.
Apoptosis induction in HTLV-1–transformed cells treated with shRNA specific for HIAP-1. The HTLV-1–transformed T-cell lines (MT-2 and C91-PL) and HTLV-1–negative control cells (HuT-78) were infected with lentiviruses containing shRNAs specific for HIAP-1 (siHIAP), nonsense shRNAs (sinon), empty lentiviruses (vector), and were mock-infected. (A) The apoptosis rate was determined by PI staining of nuclear DNA. The cells in the sub-G1 fraction represent apoptotic cells. As an apoptosis control, uninfected cells were treated with 1μg/mL etoposide (etop.) for 24 hours.
Apoptosis induction in HTLV-1–transformed cells treated with shRNA specific for HIAP-1. The HTLV-1–transformed T-cell lines (MT-2 and C91-PL) and HTLV-1–negative control cells (HuT-78) were infected with lentiviruses containing shRNAs specific for HIAP-1 (siHIAP), nonsense shRNAs (sinon), empty lentiviruses (vector), and were mock-infected. (A) The apoptosis rate was determined by PI staining of nuclear DNA. The cells in the sub-G1 fraction represent apoptotic cells. As an apoptosis control, uninfected cells were treated with 1μg/mL etoposide (etop.) for 24 hours.
Induction of caspase activity in HTLV-1–transformed cells treated with shRNA specific for HIAP-1. The HTLV-1–transformed T-cell lines (MT-2 and C91-PL) and HTLV-1–negative control cells (HuT-78) were infected with lentiviruses containing shRNAs specific for HIAP-1 (siHIAP), nonsense shRNAs (sinon), empty lentiviruses (vector), and were mock-infected. In these cultures the activity of caspases 3/7 was determined by means of a substrate that releases aminoluciferin upon cleavage by these caspases. Aminoluciferin, which is proportional to the amount of active caspases 3/7, was measured in a luciferase reaction. The relative caspases 3/7 activity in cells was calculated as multiple of the empty vector control. As an apoptosis control, uninfected cells were treated with 1 μg/mL etoposide (etop.) for 24 hours. ▦ indicates unselected cultures; ▪, cultures treated with blasticidin.
Induction of caspase activity in HTLV-1–transformed cells treated with shRNA specific for HIAP-1. The HTLV-1–transformed T-cell lines (MT-2 and C91-PL) and HTLV-1–negative control cells (HuT-78) were infected with lentiviruses containing shRNAs specific for HIAP-1 (siHIAP), nonsense shRNAs (sinon), empty lentiviruses (vector), and were mock-infected. In these cultures the activity of caspases 3/7 was determined by means of a substrate that releases aminoluciferin upon cleavage by these caspases. Aminoluciferin, which is proportional to the amount of active caspases 3/7, was measured in a luciferase reaction. The relative caspases 3/7 activity in cells was calculated as multiple of the empty vector control. As an apoptosis control, uninfected cells were treated with 1 μg/mL etoposide (etop.) for 24 hours. ▦ indicates unselected cultures; ▪, cultures treated with blasticidin.
Discussion
Here we show that the antiapoptotic HIAP-1 is essential for the survival of HTLV-1–transformed cells and is strongly stimulated by the viral transactivator Tax. These findings suggest that HIAP-1 stimulation plays a key role in HTLV-1 persistence, transformation, and pathogenesis.
Lentiviral transduction of shRNA is a novel approach for targeting cellular genes in HTLV-1–transformed T-lymphocytes. The results obtained show that the role of Tax-transactivated genes in viral transformation can be successfully studied. In addition, this approach allows for efficient targeting of IAPs in lymphocytes. This may be of interest since IAPs are being explored as potential targets for the development of new antitumor therapeutics.63,65
Like HTLV-1, other viruses have developed strategies to modulate cellular apoptosis control genes or influence their function.72 Baculoviruses and African swine fever viruses encode proteins with homology to cellular IAPs. In particular, many tumor viruses have developed the ability to inhibit apoptotic signaling pathways by expressing genes with homologies to cellular antiapoptotic effectors, (for example, oncogenic γ-herpesviruses and adenoviruses). The presence of antiapoptotic signaling in many different and unrelated virus families hints at the importance of apoptosis induction as a general innate defense mechanism of the host cell against viral infection and transformation, which has to be neutralized by the virus.72
Tax may replace cellular signals required for the induction of HIAP-1 synthesis. This is suggested by the stimulation of endogenous HIAP-1 expression in Jurkat T cells by transfected Tax expression plasmids and in Tesi Tax-repressible cells. Moreover, dose-response experiments revealed a significant positive direct correlation of Tax and HIAP-1 mRNA loads within the experimental limits. In some ATL cell lines expressing very high doses of Tax, the reduced Tax dose-response effects on HIAP-1 mRNA load suggest that the Tax stimulation of the HIAP-1 promoter can reach a saturation plateau. Thus, it is likely that HTLV-1 also enhances HIAP-1 expression in patients. This notion is further supported by the up-regulation of HIAP-1 in a fresh culture of HAM/TSP patient lymphocytes. Furthermore, many of the HTLV-1–positive cell lines with up-regulated HIAP-1 resemble primary transformed human T cells in several features, among them the dependence of exogenous IL-2. The increase of protein and RNA in the presence of Tax was accompanied by the up-regulation of the HIAP-1 promoter, thus suggesting that Tax acts by stimulating the initiation of mRNA synthesis. Promoter and Tax mutants indicated that Tax transactivates the HIAP-1 promoter mainly via NF-κB. This transcription factor is also involved in the physiologic activation of the promoter by the CD40 ligand and TNFα.57 Thus, our results also show that HIAP-1 is an important target of the Tax-stimulated NF-κB pathway. The failure to stimulate HIAP-1 therefore may explain the increased apoptosis and caspase activation observed in HTLV-1–transformed cells, in which NF-κB activation was inhibited.73,75 This HIAP-1 effect could even be supported by Bcl-xL, which is moderately stimulated by Tax also via the NF-κB pathway.39 Physiologically, the HIAP-1 gene is activated by lymphocyte receptors generating survival signals in long-term growing T lymphocytes, (eg, CD40 receptor).57 Thus, Tax mimics the survival signal mediated by the CD40 receptor. In that respect it resembles the oncoprotein LMP1 of Epstein-Barr virus, which is required for lymphocyte transformation.76 Therefore, HIAP-1 seems to be a target for cellular survival pathways, which are exploited by viral oncoproteins.
The suppression of HIAP-1 by RNAi led to apoptosis of HTLV-1–transformed cells, indicating its requirement for opposing apoptosis signals in infected T cells. A proapoptotic signal is generated in HTLV-1–transformed cells by the simultaneous expression of FasL with its cognate death receptor.77 Moreover, the transforming features of Tax may induce the activity of antioncogenes, which also stimulate proapoptotic pathways. Disabling the cell's apoptotic response by HIAP-1 could therefore be a prerequisite for transformation. In this respect, it could cooperate with the p53-inhibitory function of Tax.36 HIAP-1 may also function to prevent external proapoptotic signals. This may explain Tax's capacity to protect cells from apoptosis and high resistance of HTLV-1–transformed cells against various proapoptotic stimuli, including FasL.42,78,80 Apoptotic triggering by FasL and granzyme B is essential for the elimination of virus-infected cells by cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells.81 Apoptotic cascades stimulated by these molecules involve caspases 3, 7, and 9, which are inhibited by HIAP-1.51,52 Thus, the transactivation of HIAP-1 by Tax may contribute to immune evasion and to apoptotic resistance in HTLV-1–transformed cells, which could be essential for the survival of infected cells, for viral persistence and pathogenesis. The HIAP-1 gene could therefore be a target for the antiviral therapy.
HIAP-1 overexpression was consistently found in all cultured ATL cells but not in 3 ALL-derived CD4+ T-cell leukemic lines. These observations are in accordance with the result of systematic analysis of 60 cancer cell lines for HIAP-1 expression.82 It reveales that HIAP-1 expression is more restricted than the expression of its closest relatives XIAP and HIAP-2/cIAP1, and it is rare in leukemia cell lines. Among the 6 leukemia cell lines (HTLV-1–negative) tested, only a single cell line contained detectable protein levels. Thus, the up-regulation in the ATL cell lines may be a specific feature of a defined subgroup of leukemic cell lines. The consistent up-regulation of HIAP-1 in ATL cells may also suggest that it is up-regulated in leukemic cells in patients. Since HIAP-1 is efficient in blocking apoptotic signals and is overexpressed in different human cancers,63,65 its antiapoptotic activity may be required for malignant growth. Thus, it could also be relevant for the survival of ATL cells in patients. Evidence that IAPs play an important role in the chemotherapy resistance of leukemia cells65,82 suggests that it may also contribute to the high resistance to apoptosis, observed in ATL cells, which is responsible for the poor success of chemotherapy.44 Thus, interfering with HIAP-1 function could improve the efficacy of chemotherapeutics in ATL treatment.
Prepublished online as Blood First Edition Paper, February 7, 2006; DOI 10.1182/blood-2005-08-3138.
Supported by the Deutsche Forschungsgemeinschaft (SFB466-C3, GRK-1071) and the Wilhelm Sander-Stiftung (2004.019.1).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Klemens Pichler for helpful discussions. The technical assistance of Kirsten Fraedrich and Birthe Müller is greatly appreciated.


![Figure 2. Induction of HIAP-1 synthesis by HTLV-1 Tax. The impact of Tax on endogenous HIAP-1 expression was investigated in Jurkat T cells and in Tax-immortalized primary human T cells (Tesi). (A) Jurkat cells (107) were transfected with the indicated amounts of the Tax expression plasmid pcTax. Empty vector plasmid was added to obtain equal amounts of transfected DNA. After 48 hours RNA was extracted, cDNA prepared, and the amounts of HIAP-1 and β-actin transcripts (internal standard) were analyzed by real-time RT-PCR. The relative mRNA copy numbers of HIAP-1 and Tax mRNA were determined as multiples of the β-actin mRNA copy number. The correlation between Tax and HIAP-1 mRNA relative copy numbers is highly significant at the .01 level (2-tailed). (B) Human T cells transformed by a repressible TAX gene (Tesi) were kept under condition of induced (Tesi) and repressed Tax (Tesi + Tet [tetracycline]) expression. RNA was analyzed by RT-PCR and quantified by real-time RT-PCR. The relative mRNA copy numbers of HIAP-1 and Tax mRNA were determined as multiples of the β-actin mRNA copy number. The columns represent the mean of 3 experiments; the error bars indicate standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/11/10.1182_blood-2005-08-3138/4/m_zh80110696140002.jpeg?Expires=1769095212&Signature=VsUhC1uO6DehQh6hrfAojM~zCrcoxR9K9W3PGq7U6mEO7etZukXpim~s7rUsrPlcGO4a8Y2HSYwJU3aTVyuZVPspwVlPzi0XHk2Qb~UZ5XJuRJY8OLAqznk1YV8Tdf3LSwyuiO5p4BKv3iJ-JxghCc-sbqah-HRtMfifwxvCaLQPuux8L26~ACKRmc-1XCZkYR47kBeZrydgnB6rFnGnsgEXG6sVSti3zI4g3GwQaZjqxJW7mWhvekPzn6YYDQTSlfUaUdVPtF~g3ktfiGjZ64IvbICYLUrUhMEg7tiAKzJQYHXvCzglLupJzErpT9lpo4qEJV2pcdXs6y3cDZ-5iw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)