Abstract
Hematopoietic stem cells (HSCs) arise, self-renew, or give rise to all hematopoietic lineages through the effects of transcription factors activated by signaling cascades. Lyl-1 encodes a transcription factor containing a basic helix-hoop-helix (bHLH) motif closely related to scl/tal, which controls numerous decisions in embryonic and adult hematopoiesis. We report here that Lyl-1 null mice are viable and display normal blood cell counts, except for a reduced number of B cells resulting from a partial block after the pro-B stage. Nevertheless, the deletion of Lyl-1 results in a diminution in the frequency of immature progenitors (Lin–, CD34–, sca-1+, c-kit+ [LSK], and LSK-side population [LSK-SP]) and in S12 colony-forming unit (CFU-S12) and long-term culture-initiating cell (LTC-IC) content in embryonic day 14 fetal liver (E14 FL) and adult bone marrow (BM). More important, Lyl-1–/– E14 FL cells and BM are severely impaired in their competitive reconstituting abilities, especially with respect to B and T lineage reconstitution. Thus, ablation of Lyl-1 quantitatively and functionally affects HSCs, a cell population that transcribes Lyl-1 more actively than their differentiated progenies. Our results demonstrate for the first time that Lyl-1 functions are important for HSC properties and B-cell differentiation and that they are largely distinct from scl functions.
Introduction
Hematopoiesis is maintained by a pool of hematopoietic stem cells (HSCs) defined by their capacity to self-renew and, hence, to maintain the pool and to differentiate into all mature progenies. These properties underlie their ability to reconstitute the hematopoietic system.1 HSCs are identified by molecular markers2 and are divided into long-term HSCs (LT-HSCs), short-term HSCs (ST-HSCs), and multilineage progenitors (MPPs).3,4 As they differentiate, HSCs give rise to committed progenitors, such as common lymphoid progenitors (CLPs) or common myeloid progenitors (CMPs), which generate mature cells within the different hematopoietic lineages.5
The action of HSCs to self-renew or to differentiate is a tightly controlled process linked to the induction or repression of some crucial genes.6 Gene-targeting experiments have provided support to this hypothesis by identifying transcription factors of various families or their coregulators that are implicated in HSC biology.7,8
The basic helix-loop-helix (bHLH) family of transcription factors regulates a wide range of developmental events.1,9 Some bHLHs, such as MyoD and NeuroD, display restricted tissue expression and control the differentiation of particular cellular lineages.10 They form transactivating dimers by heteromerization with ubiquitous bHLH proteins, termed E proteins, such as E2A, E12, and E47 genes.11,12 Conversely, the latter proteins are repressed by other HLH proteins, which often precludes differentiation.10,13,14
The tal/scl gene (hereafter referred to as scl) encodes a bHLH protein.15,16 It was identified through its implication in T-cell acute lymphocytic leukemia (T-ALL). Knock-out (KO) experiments17,18 show that scl is autonomously required for primitive and definitive hematopoiesis.19,20 Moreover, its enforced expression enhances megakaryocytopoiesis and erythropoiesis.21,22
The Lyl-1 gene encodes a bHLH protein closely related to scl.23 Its transcriptional activation upon translocation is also associated with T-ALL.24 SCL and LYL-1 bHLH regions show 82% of amino acid identity,24 suggesting that these 2 proteins share at least some target genes and biologic functions.25,26 However, LYL-1 and SCL diverge largely outside the bHLH region and display a distinct expression pattern in hematopoietic cells.23,27
The biologic functions of SCL prompted us to investigate those of LYL-1 in hematopoiesis. In contrast to scl–/– mice, Lyl-1–/– mice do not exhibit embryonic lethality but have a reduced number of B cells. Although the CLP compartment is normal, the immature B-cell compartments are reduced in adult mice. In addition, we show that Lyl-1 is highly expressed in stem/progenitor cells, correlating with an important role in the control of the size and function of this cell compartment. Similarly, the number of multipotent progenitor S12 colony-forming units (CFU-S12s) is reduced in Lyl-1–/– animals. Overall, these defects are distinct from those revealed upon scl KO, suggesting that scl and Lyl-1 exert specific functions during hematopoiesis.
Materials and methods
Generation of targeting constructs
With the use of an amplified fragment of the Lyl-1 gene as a probe, we screened a phage library made with genomic DNA of embryonic stem (ES) cells (a D3 derivative provided as a gift by Drs Bettenhausen and Achim Gossler [Institute for Molecular Biology, Hannover, Germany], unpublished data, 1993) and isolated 2 overlapping phage clones representing 27 kb of the mouse Lyl-1 locus.28 To generate the targeting vector, we constructed a LacZ/Neo gene cassette (pLRLacZpA/MCINeopA), which can be excised in multiple ways allowing in-frame cloning of the β-galactosidase (LacZ) reporter gene into coding regions. A 5.23-kb SmaI/SstI fragment containing the LacZ/Neo cassette replaced a 0.7-kb HpaI fragment of the Lyl-1 locus comprising the HLH domain and the entire 3′ end of the Lyl-1 gene. The 5′ arm of the targeting vector (HindIII/HpaI) spans 7.8 kb, and the 3′ arm (HpaI/EcoRI) spans 3.2 kb of the Lyl-1 locus. The linearized (HindIII) targeting vector was electroporated (Gene Pulser; Bio-Rad, Hercules, CA) into embryonic day 14.1 (E14.1) ES cells.29 Transfected ES cells were grown on a single monolayer of mitomycin C–treated G418-resistant embryonic fibroblast cells in the presence of G418 (200 μg/mL). G418-resistant ES cell clones (n = 147) were expanded and analyzed by Southern blot hybridization.30 Twenty-eight clones contained the targeted Lyl-1 locus. Clones 3, 4, and 10 were further characterized and used to generate chimeric mice. Mutant ES cells were injected into blastocysts of C57BL/6 mice, and the resultant male chimeras were crossed with C57Bl/6 females.
Animals
C57BL/6-Ly5.2 and C57BL/6-Ly5.1 mice (6 to 8 weeks old) were purchased from Janvier CERJ (Le Genest-St-Isle, France) and Charles River Laboratories (Les Oncins, France), respectively. For fetal liver (FL) experiments, the vaginal plug was taken as day 0 of gestation. Mice were genotyped by polymerase chain reaction (PCR) of tail DNA with primers hybridizing to exon 4 of Lyl-1 (amplifying the wild-type [wt] allele: forward, 5′-AAGCTGAGCAAGAACGAGGTGC-3′; reverse, 5′-TCTGCTCCAACTTGATGGGTCTC-3′) or to the LacZ (amplifying the mutant allele: forward, 5′-CGTCGTGACTGGGAAAACCC-3′; reverse, 5′-GGAACAAACGGCGGATTGAC-3′). PCR conditions were 94°C for 5 minutes; 35 cycles of 94°C for 30 seconds, 55°C for 1 minute, 72°C for 30 seconds; and 72°C for 7 minutes. Amplified bands were detected on a 1% agarose gel with ethidium bromide.
Hematologic parameters
A sample (50 μL) of peripheral blood (PB) was harvested from the retro-orbital sinuses of anesthetized mice. Total numbers of nucleated blood cells, erythrocytes, platelets, lymphocytes, and granulocytes were automatically measured with an MS9 counter (Melet Schloesing Technologies, Cergy-Pontoise, France) calibrated for mouse blood.
Bone marrow and fetal liver cell preparation
Cells from bone marrow (BM) and E14 FL were harvested in medium essential medium (MEM; Gibco BRL, Paisley, United Kingdom) supplemented with 10% fetal bovine serum (FBS; Invitrogen, Paisley, United Kingdom) by repeated flushing and passage through a 70-μm nylon mesh (Falcon 2350; Becton Dickinson Labware, Franklin Lakes, NJ). Erythrocytes were lysed in ammonium chloride potassium buffer.
Cell sorting and flow cytometry
BM and E14 FL cells were enriched for Lineage-negative (Lin–) cells after incubation with a cocktail of lineage antibodies (mAbs): anti–GR-1 (RB6-8C5), anti–MAC-1 (M1/70), anti-B220 (RA3-6B2), anti-CD4 (GK1.5), anti-CD8 (Lyt-1), and anti–erythroid TER-119 (Ter-119). Lin+ cells were depleted by the immunomagnetic bead technique (sheep anti–rat IgG; Dynal, Oslo, Norway). Then the Lin– fraction was incubated with a phycoerythrin (PE)–conjugated goat anti–rat IgG antibody and stained with a fluorescein-isothiocyanate (FITC) anti–Sca-1 mAb (E13-161-7) and an allophycocyanin (APC) anti–c-Kit mAb (2B8). This method allowed us to determine the frequency of Sca-1 and c-Kit cells among the Lin–, CD34–, sca-1+, c-kit+ (LSK) cells.31 CD34+ cells among LSKs were enumerated after labeling with a cocktail of biotin-lineage antibodies, a PE-anti–Sca-1, an APC-anti–c-Kit, and an FITC-anti–CD34 (RAM 34) mAb followed by phycoerythrin-cyanin-7 (PE-Cy-7) streptavidin.32,33 Isotype-matched antibodies were used as controls.
Side population (SP) analyses were performed with Hoechst 33342 according to Goodell et al.34 Controls were performed by incubating cells in the presence of 75 μM verapamil (Sigma, St Louis, MO). Cells were subsequently centrifuged and stained with LSK antibodies, as described.
Different subsets of B-cell lineage were sorted according to their expression markers using PE–anti-CD19 (1D3), APC–anti-IgM (II/41), biotin–anti–CD43-57, and APC–Cy-7–anti-B220 mAbs for the different B-cell populations35,36 of pro–B cells (B220+, CD19–, CD43+, IgM–), pre–B cells (B220+, CD19+, CD43+, IgM–), and immature B cells (B220+, CD19+, CD43–, IgM+). CLPs were phenotyped among the Lin– cells as IL7R+ (PE-SB/199), Sca-1+/low, and c-Kit+/low, as described by Kondo et al.5 β-Galactosidase (fluorescence-activated cell sorter [FACS]–Gal) expression was studied in the different B-cell populations described and in LSK cells stained with biotinylated lineage-specific mAbs followed by streptavidin PE–Cy-7, PE–Sca-1 mAb, and APC–Kit mAb. FACS-Gal assay was performed as previously described37 with fluorescein di-β-galactopyranoside (FDG; Molecular Probes, Invitrogen, Paisley, United Kingdom).
All antibodies were purchased from PharMingen (San Diego, CA), and 1 μg/mL of 7 amino actinomycin (7AAD; Sigma) was added 5 to 10 minutes before analysis to exclude dead cells. For cell cycle analysis of BM cells, Lin– cells were sorted and stained with 10 μg/mL Hoechst 33342 and 1 μg/mL Pyronine Y (Sigma), as described by Gothot et al.38 Different populations were sorted with a FACSDiva cell sorter (Becton Dickinson, Mountain View, CA) or were analyzed with an LSR II cytometer.
Quantitative reverse transcription–polymerase chain reaction on B-cell populations
Total RNA was harvested using Trizol reagent (Invitrogen). Samples were first treated with DNase (Ambion, Huntington, United Kingdom), heat denatured, and reverse transcribed using superscript II RNase H reverse transcriptase (Invitrogen). Real-time TaqMan PCR was carried out in the ABI Prism GeneAmp 5700 Sequence detection system (Perkin-Elmer Applied Biosystems, Foster City, CA). Lyl-1 expression level was normalized with tubulin expression. Probes and primers were: Lyl-1 sense, 5′-CAAGCCATAGTGAGCTGGACTTG-3′; Lyl-1 antisense, 5′-AGCGCTCACGGCTGTTG-3′; Lyl-1 probe, 5′-CTGCGGGCACCAACCCCAGA-3′; GAPDH sense, 5′-CCTGCACCACCAACTGCTTA-3′; GAPDH antisense, 5′-TCATGAGCCCTTCCACAATG-3′; GAPDH probe, 5′-CCTGGCCAAGGTCATCCATGACAA-3′.
Day 12 CFU-S assay
S12 colony-forming unit (CFU-S12) assay was performed as described by Ohta et al.39 Total BM and FL cells (5 × 104 per mouse) were injected into congenic lethally irradiated (9.5 Gy) recipients. Spleens were excised 12 days later and fixed in Bouin solution, and macroscopic surface colonies were scored.
Long-term culture-initiating cell assay
Long-term culture-initiating cell (LTC-IC) assay was performed as described by Ohta et al39 and Sutherland et al.40 Briefly, sorted LSK cells were placed on an S17 feeder layer in microwell plates and incubated at 33°C in a humidified incubator with 5% CO2 in air. Cultures were fed weekly by half-media exchange. The cultures were recovered after 5 weeks and assayed for the presence of myeloid culture colony-forming unit (CFU-C) using a methylcellulose medium (M3234; Stem Cell Technologies, Vancouver, BC, Canada) supplemented with cytokines. After 7 to 10 days of growth at 37°C, colonies were scored with an inverted microscope based on their morphology.
Competitive repopulation and secondary transplantation
C57Bl/6 (Ly 5.1) mice were used as recipients,41 whereas test BM and E14 FL cells were prepared from C57Bl/6 (Ly 5.2) donors. Recipients were lethally irradiated with two 5-Gy doses separated by a 3-hour interval and underwent cotransplantation with 5 × 105, 1 × 106, 5 × 106Lyl-1+/+ or Lyl-1–/– Ly5.2 BM or E14 FL cells, together with 1 × 106 Ly5.1 BM competitor cells injected through the retro-orbital sinus (ratios, 0.5:1, 1:1, and 5:1). Hematopoietic reconstitution was assessed 4 and 24 weeks after transplantation through analysis of PB collected from retro-orbital sinus and BM harvested by intrafemoral punction of anesthetized mice. Cells were stained with anti–CD45.2 FITC and were analyzed by flow cytometry Animals showing greater than 1% of Ly-5.2+ cells were considered positive for repopulation.
Secondary BM transplantations were also performed: 5 × 105,1 × 106, 5 × 106Lyl-1+/+ or Lyl-1–/– Ly 5.2 BM sorted cells from primary mice that underwent grafting 6 months earlier, together with 1 × 106 BM Ly5.1 competitor cells, were injected into secondary Ly5.1 lethally irradiated mice. Hematopoietic reconstitution was evaluated 4 and 24 weeks after the graft by flow cytometry, as described.
Statistical analysis
The Student t test was used with data obtained from at least 3 repeated experiments to determine the P value.
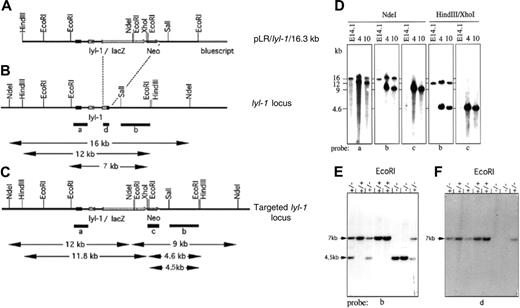
Gene targeting ofLyl-1. Structure of the gene targeting vector (A) and the genomic locus of Lyl-1 before (B) and after (C) homologous recombination. A 0.8-kb Hpa I fragment of the Lyl-1 gene, encoding the bHLH domain, was replaced by a LacZ/Neo gene cassette allowing expression of a Lyl-1LacZ fusion gene. Relevant restriction enzyme sites and the lengths of diagnostic fragments are shown. Southern blot analysis of homologous recombinant ES clones 4 and 10 and wt E41.1 cells (D) and mice tail DNA (E-F) confirmed the targeted inactivation of the Lyl-1 gene. Probes were as indicated.
Gene targeting ofLyl-1. Structure of the gene targeting vector (A) and the genomic locus of Lyl-1 before (B) and after (C) homologous recombination. A 0.8-kb Hpa I fragment of the Lyl-1 gene, encoding the bHLH domain, was replaced by a LacZ/Neo gene cassette allowing expression of a Lyl-1LacZ fusion gene. Relevant restriction enzyme sites and the lengths of diagnostic fragments are shown. Southern blot analysis of homologous recombinant ES clones 4 and 10 and wt E41.1 cells (D) and mice tail DNA (E-F) confirmed the targeted inactivation of the Lyl-1 gene. Probes were as indicated.
Results
Generation of Lyl-1–/– mice
To evaluate the functional role of Lyl-1 in hematopoiesis, the Lyl-1 gene was disrupted by homologous recombination in ES cells. A LacZ/Neo cassette was cloned in-frame into the fourth exon of the murine Lyl-1 gene, thus deleting the entire 3′ end including the HLH domain (Figure 1A-C; see also “Materials and methods”). This deleted region was similar to that targeted in the conditional scl KO.42 The β-galactosidase enzyme encoded by the LacZ gene was placed under the control of the Lyl-1 promoter. Lyl-1LacZ mutant mice were intercrossed, and tail DNA from the offspring was analyzed by Southern blotting to confirm the deletion of the 3′ end of the Lyl-1 gene (Figure 1E-F). Homozygous Lyl-1LacZ mice, hereafter called Lyl-1–/–, were viable and developed normally.
Lyl-1 is highly expressed in immature hematopoietic cells and pre–B cells
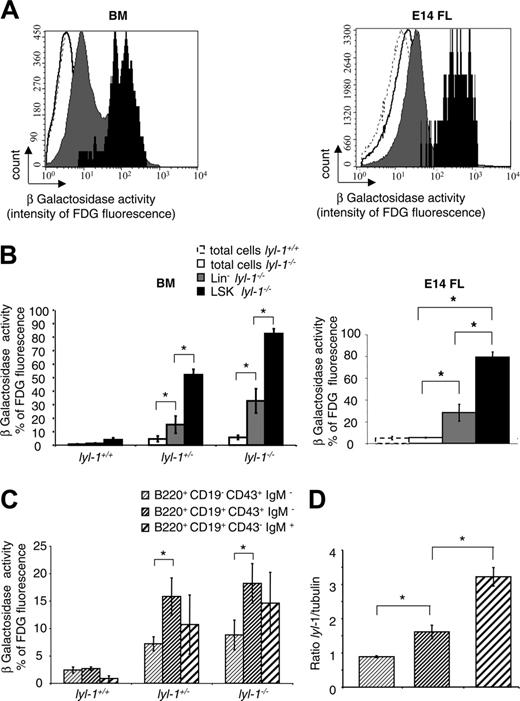
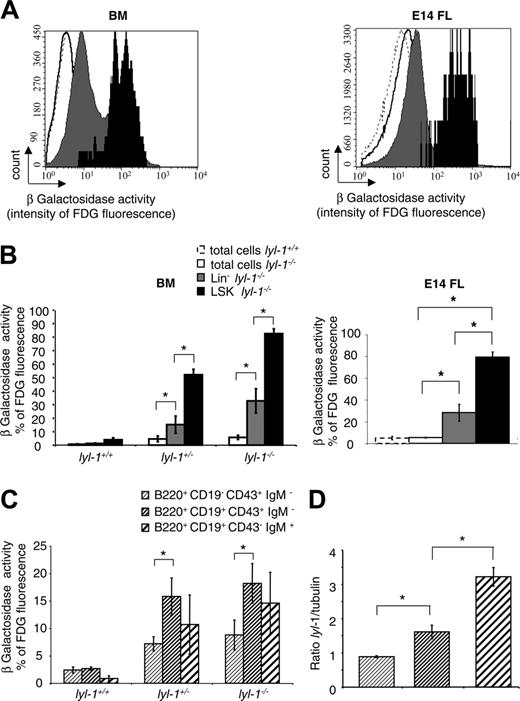
Expression of the LYL-1/LacZ fusion protein was assessed throughout the hematopoietic system using the FDG assay to reveal β-galactosidase activity.37 According to the percentage of β-galactosidase+ cells (Figure 2A) and the intensity of β-galactosidase (Figure 2B), LYL-1 was mainly expressed in BM and in E14 FL LSK cells compared with the Lin– and total cell populations.
In contrast to scl, lyl-1 is expressed in B cells.43,44 Therefore, we precisely determined its expression during B-cell differentiation by separating BM cells into pro–B, pre–B, and immature B cells. We found that the percentage of fluorescent cells was increased between the pro-B and pre-B stages (Figure 2C). By quantitative reverse transcription–polymerase chain reaction (RT-PCR), Lyl-1 mRNA in wt mice also increased between the pro-B and the pre-B stages (Figure 2D). These results demonstrate that Lyl-1 expression predominates in the primitive hematopoietic cell compartment and markedly increases during the first steps of B-cell maturation.
Expression of Lyl-1 in BM and E14 FL cells. Expression of Lyl-1 in total, Lin–, LSK BM, and E14 FL cells from wt and Lyl-1–/– mice was monitored by measuring β-galactosidase activity. Cells were stained with biotinylated anti-Lin, PE–anti-Sca-1 and APC–anti–c-Kit mAb and streptavidin PE–Cy-7 and were incubated with the FDG substrate. (A) Flow cytometric profiles depicting the intensity of fluorescence in each subpopulation of wt and Lyl-1–/– BM and E14 FL. (B) Histograms show the percentage of fluorescent cells in each subpopulation of BM from wt, heterozygote, and homozygote mice and of E14 FL cells of Lyl-1–/– mice. (C) Expression of Lyl-1 during B-cell differentiation in the BM from wt, heterozygote, and homozygote mice. B cells were separated into 3 different populations: pro–B (B220+ CD19–CD43+ IgM–), pre–B (B220+ CD19+ CD43+ IgM–), and immature B (B220+CD19+ CD43– IgM+) cells. Histograms represent the percentages of fluorescent cells in each B-cell subpopulation of wt, Lyl-1+/–, and Lyl-1–/– BM. (D) Lyl expression was measured by quantitative RT-PCR in pro–B (B220+ CD19–CD43+ IgM–), pre–B (B220+ CD19+ CD43+ IgM–), and immature B (B220+ CD19+ CD43–IgM+) cells. Histograms represent the ratio between Lyl-1 and GAPDH mRNA used as a housekeeping gene. (B-C) Mean of 3 independent experiments (*P < .05). Error bars indicate SEM.
Expression of Lyl-1 in BM and E14 FL cells. Expression of Lyl-1 in total, Lin–, LSK BM, and E14 FL cells from wt and Lyl-1–/– mice was monitored by measuring β-galactosidase activity. Cells were stained with biotinylated anti-Lin, PE–anti-Sca-1 and APC–anti–c-Kit mAb and streptavidin PE–Cy-7 and were incubated with the FDG substrate. (A) Flow cytometric profiles depicting the intensity of fluorescence in each subpopulation of wt and Lyl-1–/– BM and E14 FL. (B) Histograms show the percentage of fluorescent cells in each subpopulation of BM from wt, heterozygote, and homozygote mice and of E14 FL cells of Lyl-1–/– mice. (C) Expression of Lyl-1 during B-cell differentiation in the BM from wt, heterozygote, and homozygote mice. B cells were separated into 3 different populations: pro–B (B220+ CD19–CD43+ IgM–), pre–B (B220+ CD19+ CD43+ IgM–), and immature B (B220+CD19+ CD43– IgM+) cells. Histograms represent the percentages of fluorescent cells in each B-cell subpopulation of wt, Lyl-1+/–, and Lyl-1–/– BM. (D) Lyl expression was measured by quantitative RT-PCR in pro–B (B220+ CD19–CD43+ IgM–), pre–B (B220+ CD19+ CD43+ IgM–), and immature B (B220+ CD19+ CD43–IgM+) cells. Histograms represent the ratio between Lyl-1 and GAPDH mRNA used as a housekeeping gene. (B-C) Mean of 3 independent experiments (*P < .05). Error bars indicate SEM.
Hematologic examination of PB, BM, and spleen cells from Lyl-1–/– mice
We first examined the numbers of nucleated cells, red blood cells, and platelets in the PB of 8- to 12-week-old male and female mice. No difference was noticed between wt and Lyl-1–/– animals (Tables 1 and 2). Next, we performed flow cytometric analysis on PB, BM, and spleen cells. The numbers of myeloid, erythroid, and T cells were indistinguishable between wt and KO mice. However, a 1.5- to 3-fold decrease in the number of B220+ cells in the 3 analyzed tissues was observed (Tables 1 and 2).
Analysis of B lineage in the bone marrow of Lyl-1–/– mice
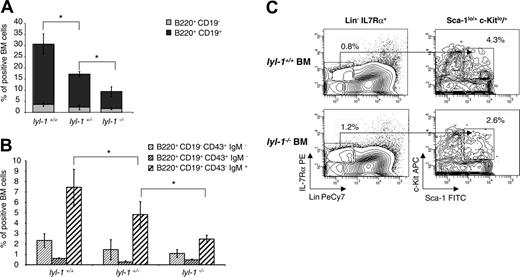
We performed phenotypic analysis along the B-cell pathway to characterize the differentiation stage eventually affected by Lyl-1 inactivation. A marked and significant decrease in the B220+ CD19+ cell population was found in the BM of heterozygote and homozygote mice (Figure 3A). Further phenotyping of the CD19+ cells showed a 2-fold reduction in immature B-cell compartments in homozygote mice and an intermediate reduction in heterozygote mice (Figure 3B), suggesting a gene dosage effect. In contrast, the earlier stages of lymphoid differentiation were not affected by Lyl-1 invalidation, as shown by the percentage of CLP (Figure 3C) and pro–B cells.
Thus, the relatively high expression of Lyl-1 at the RNA and protein levels (LYL-1/LacZ fusion protein) in pre–B cells correlates with a decreased number of these cells after this stage in KO mice.
Lyl-1 disruption diminishes the frequency of immature multipotent hematopoietic cells and HSCs in E14 FL and adult BM
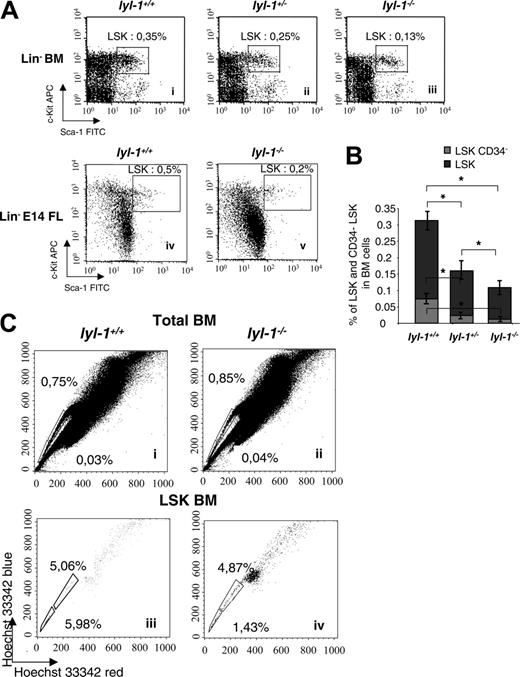
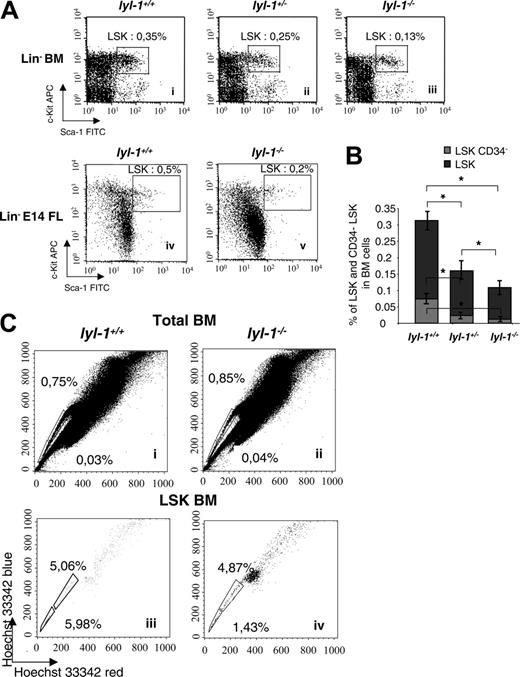
We used flow cytometric analyses to quantify the proportion of immature hematopoietic progenitors and HSCs in the BM of adult mice and E14 fetal livers. The number of LSK cells was reduced approximately 2-fold in Lyl-1–/– compared with wt mice (0.11% ± 0.015%) in Lyl-1–/– BM compared with 0.31% ± 0.03% in Lyl-1+/+ BM and 0.29% ± 0.013% in E14 FL Lyl-1–/– compared with 0.66% ± 0.027% in Lyl-1+/+ E14 FL (Figure 4A). However, the LSK population is heterogeneous, and the less mature cells are devoid of CD34.32,33 We thus examined the percentage of CD34– cells within the LSK populations and found that their number was markedly decreased in Lyl-1–/– BM (0.012% ± 0.0017% in lyl-1–/– vs 0.074% ± 0.008% in Lyl-1+/+ mice; Figure 4B). Importantly, this decrease (6-fold) was even more significant than that seen for the total LSK population (2-fold). In all experiments, heterozygote Lyl-1+/–mice harbored an intermediate number of LSKs (Figure 4A-B), suggesting that the effect of Lyl-1 on the HSCs might be dose dependent.
SP cells were also characterized by their ability to efflux the Hoechst 33342 dye.34,45 No significant difference was seen between Lyl-1–/– and wt BM (0.8% ± 0.3% vs 1.0% ± 0.36%; Figure 4C). However, SP cells include differentiated cells46,47 and long-term reconstitution cells (LT-RCs) that are highly enriched in the SP population with an LSK phenotype.45,48 Thus, we quantified LSK-SP cells and found that their percentage was decreased in the BM of Lyl-1–/– mice (8.9% ± 3.2% in Lyl-1+/+ BM vs 3.6% ± 1.2% in Lyl-1–/– BM; Figure 4C). Furthermore, LSK-SP cells endowed with the highest potential for long-term reconstitution incorporate the lowest level of Hoechst and, hence, are detected in the Hoechstlow population (Figure 4Ciii-iv, end of the tail).49 Importantly, we noticed a more pronounced decrease in LSK-Hoechstlow SP cells in Lyl-1–/– mice (1.47% ± 0.4% vs 5.66% ± 1.7% in Lyl-1+/+ mice) that was on the same order of magnitude as that observed for CD34– LSK cells. The same approach was not performed with E14FL cells because the presence and the significance of SP cells at this developmental stage remain controversial.50,51 Altogether these phenotypic analyses suggest that the HSC compartment with an LT-RC phenotype is quantitatively altered in Lyl-1–/– mice.
Lyl-1 disruption impairs CFU-S12 formation and LTC-IC
To determine the functional effects of Lyl-1 disruption, we performed spleen colony-forming unit (CFU-S12) and LTC-IC assays, 2 cell populations roughly corresponding to short-term repopulating cells (ST-RCs). The number of CFU-S12s was 1.5-fold decreased in the BM and E14FL from Lyl-1–/– mice compared with wt mice (Table 3). This contrasted with a nonaltered number of myeloid colony-forming cells (CFCs) (data not shown). LTC-ICs were quantitatively studied by limiting dilution assays, and a 3-fold reduction was observed in BM from Lyl-1–/– mice (Table 4). Furthermore, the number of myeloid CFCs per LTC-IC was decreased more than 7-fold in Lyl-1–/– BM, suggesting that Lyl-1 disruption profoundly affects the more primitive LTC-ICs (Table 4). Together these results indicate that cells defining ST-RC are depleted of Lyl-1–/– BM.
Lyl-1 is required for full-scale hematopoietic reconstitutive activity of HSCs
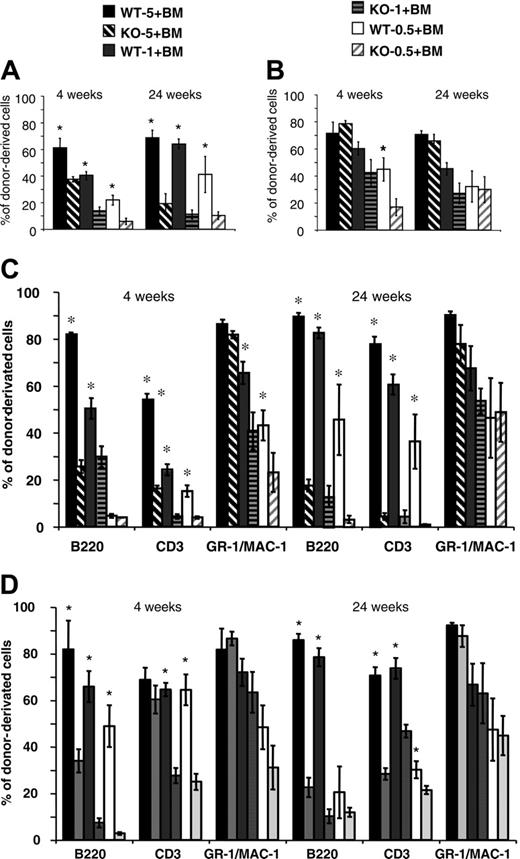
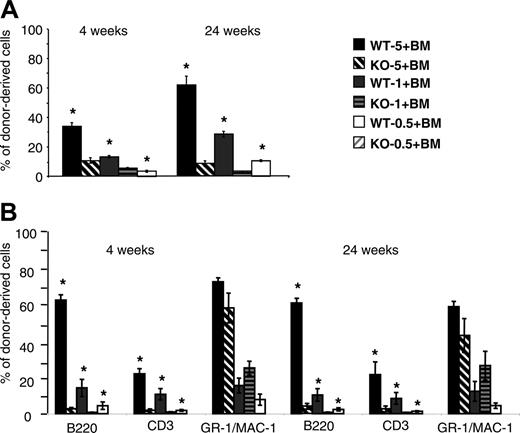
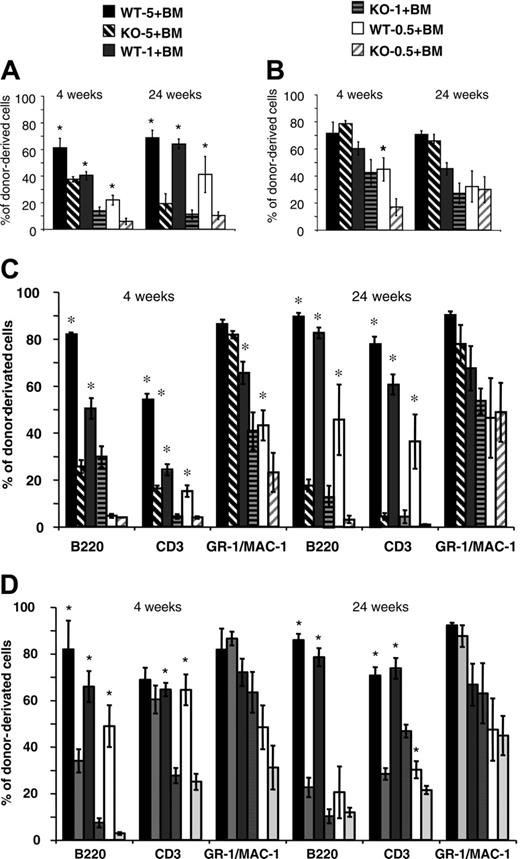
Competitive reconstitution assays were performed to compare the relative repopulating ability of cells from Lyl-1–/– and wt mice. Different ratios (0.5:1, 1:1, 5:1) of BM cells from Ly5.2 Lyl-1–/– or Lyl-1+/+ mice were injected, together with a fixed number of Ly5.1 BM competitor cells, into irradiated Ly5.1 recipients. The contribution of Lyl-1+/+ and Lyl-1–/– Ly5.2 cells to PB leukocytes and BM was assessed after 4 and 24 weeks. A much lower contribution of Lyl-1–/– cells compared with Lyl-1+/+ was observed in PB (Figure 5A). In addition, the contribution of donor-derived cells increased between weeks 4 and 24 for wt but not for Lyl-1–/– BM. Whatever the ratio used and the time point considered, the reconstitution derived from the Lyl-1–/– graft was decreased 1.6- to 5.7-fold (Figure 5A). Strikingly, 5 × 106Lyl-1–/– BM cells were less efficient than 5 × 105 wt BM cells to ensure long-term reconstitution, indicating that reconstitution capacity was more than 10-fold decreased in the absence of Lyl-1. However, analysis of donor-derived contributions to various hematopoietic lineages showed major differences between the myeloid and the lymphoid lineages, with the ability of Lyl-1–/– BM to reconstitute the lymphoid (B and T) compartments drastically reduced, whereas reconstitution of the myeloid lineages was impaired only for a short period (Figure 5C).
Analysis of B lymphopoiesis in Lyl-1 KO mice. (A) Quantification of B cells in total BM from wt, Lyl-1+/– and Lyl-1–/– mice. The proportion of CD19+ cells among B220+ cells is shown. (B) Quantification of pro–B (B220+ CD19–D43+ IgM–), pre–B (B220+ CD19+ CD43+ IgM–), and immature B (B220+ CD19+ CD43–IgM+) cells in BM from wt, Lyl-1+/–, and Lyl-1–/– mice. (A-B) Mean ± SEM of 3 independent experiments (*P < .05). (C) Comparison of the quantity of CLPs in the BM of wt and Lyl-1–deficient mice. BM cells were incubated with a cocktail of biotin anti-Lin, PE–anti–IL-7R, FITC–anti–Sca-1, and APC–anti–c-Kit mAb in a first step and were stained with streptavidin-PE–Cy-7 in a second step. Shown are representative flow cytometric profiles determining the frequency of CLP cells obtained (Lin–, IL7R+, sca-1+/low, c-Kit+/low).
Analysis of B lymphopoiesis in Lyl-1 KO mice. (A) Quantification of B cells in total BM from wt, Lyl-1+/– and Lyl-1–/– mice. The proportion of CD19+ cells among B220+ cells is shown. (B) Quantification of pro–B (B220+ CD19–D43+ IgM–), pre–B (B220+ CD19+ CD43+ IgM–), and immature B (B220+ CD19+ CD43–IgM+) cells in BM from wt, Lyl-1+/–, and Lyl-1–/– mice. (A-B) Mean ± SEM of 3 independent experiments (*P < .05). (C) Comparison of the quantity of CLPs in the BM of wt and Lyl-1–deficient mice. BM cells were incubated with a cocktail of biotin anti-Lin, PE–anti–IL-7R, FITC–anti–Sca-1, and APC–anti–c-Kit mAb in a first step and were stained with streptavidin-PE–Cy-7 in a second step. Shown are representative flow cytometric profiles determining the frequency of CLP cells obtained (Lin–, IL7R+, sca-1+/low, c-Kit+/low).
Intrafemoral punctions were performed to analyze reconstitution of the BM tissue. Contributions of mutant cells were also decreased in comparison with wt cells. However, the contribution of Lyl-1–/– cells was higher for BM than for PB leukocytes (for instance, compare the injection of 5 × 106 BM cells at 24 weeks; Figure 5A-B). This was related to the major defect of Lyl-1–/– BM cells to reconstitute B- and T-cell lineages, whereas myeloid cells were only marginally affected except in the short term (Figure 5D). Similar results were obtained with E14 FL (Figure S1, available at the Blood website; see the Supplemental Figure link at the top of the online article).
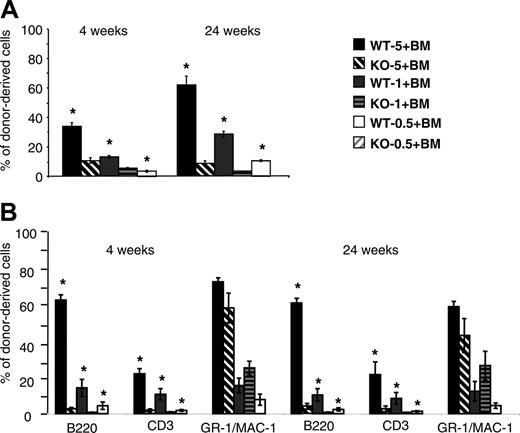
Secondary transplantations were performed to better evaluate the Lyl-1–/– HSC functions. BM cells for primary reconstituted mice were harvested at 4 months, sorted on expression of the CD45.2 marker, and injected together with Ly5.1 BM competitor cells into secondary irradiated Ly5.1 recipients. Percentages of donor-derived PB in secondary hosts were analyzed. Whatever the number of cells injected, the proportion of Lyl-1–/– cells was markedly altered, especially after 24 weeks (Figure 6A). The reconstitution of lymphoid compartments was even more drastically reduced than it was in primary hosts. As for primary engraftment, myeloid reconstitution was not affected by Lyl-1 deletion when 1 or 5 × 106 cells were injected (Figure 6B). Furthermore, no contribution to lymphoid and myeloid hematopoiesis was observed when a low number of Lyl-1–/– BM cells was transplanted as a secondary procedure (5 × 105Lyl-1–/–cells, whereas moderate chimerism (approximately 10% at 24 weeks) was observed for wt cells. Taken together, these results demonstrate that Lyl-1–/– BM cells show a severe defect in their ability to reconstitute the BM and PB of both primary and secondary hosts with a markedly predominant effect on the lymphoid lineages.
Flow cytometric analysis of BM and FL from Lyl-1–/–, lyl-1+/–, and wild-type mice. (A) Analysis of the Lin– Sca-1+ c-Kit+ population in the bone marrow of Lyl-1+/+ (i), Lyl-1+/– (ii), and Lyl-1–/– (iii) mice and in the E14 FL from Lyl-1+/+ (iv) and Lyl-1–/– (iv) mice. (B) Proportions of CD34– cells in the LSK population of BM from Lyl-1+/+, Lyl-1+/–, and Lyl-1–/– mice. Graphs represent mean ± SEM of 3 independent experiments (*P < .05). (C) Analysis of side population in the total BM of Lyl-1+/+ (i) and Lyl-1–/– (ii) mice. BM cells were stained with Hoechst 33342. Side population was also quantified in the Lin– cell population. BM cells from Lyl-1+/+ (iii) and Lyl-1–/– (iv) mice were stained with Hoechst 33342 and with mAb against cell surface markers. Flow cytometric profiles are representative of 3 independent experiments.
Flow cytometric analysis of BM and FL from Lyl-1–/–, lyl-1+/–, and wild-type mice. (A) Analysis of the Lin– Sca-1+ c-Kit+ population in the bone marrow of Lyl-1+/+ (i), Lyl-1+/– (ii), and Lyl-1–/– (iii) mice and in the E14 FL from Lyl-1+/+ (iv) and Lyl-1–/– (iv) mice. (B) Proportions of CD34– cells in the LSK population of BM from Lyl-1+/+, Lyl-1+/–, and Lyl-1–/– mice. Graphs represent mean ± SEM of 3 independent experiments (*P < .05). (C) Analysis of side population in the total BM of Lyl-1+/+ (i) and Lyl-1–/– (ii) mice. BM cells were stained with Hoechst 33342. Side population was also quantified in the Lin– cell population. BM cells from Lyl-1+/+ (iii) and Lyl-1–/– (iv) mice were stained with Hoechst 33342 and with mAb against cell surface markers. Flow cytometric profiles are representative of 3 independent experiments.
Normal cell cycle distribution in Lyl-1–/– BM and E14 FL progenitors
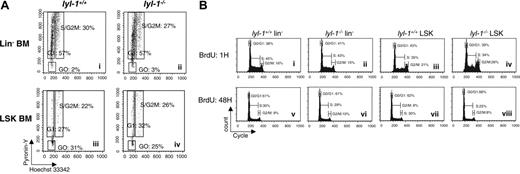
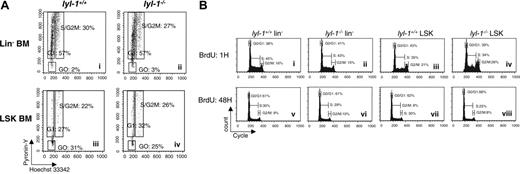
To examine whether hematopoietic defects in Lyl-1–/– BM or E14 FL reflected impaired proliferation, we studied the cell cycle status of multipotent progenitors from these 2 tissues. To this end, RNA and DNA contents of Lin– and LSK cells were measured by pyronin-Y (PY) and Hoechst 33342 (Ho) staining, respectively, and the fraction of cells in G0, G1, or S/G2-M was determined.38 No difference in cell cycle distribution was observed between multipotent hematopoietic progenitor cell populations enriched from the BM of Lyl-1–/– mice and their wt littermates (Figure 7A). Similarly, no difference in cell cycle and bromodeoxyuridine (BrdU) incorporation was seen for E14 FL LSK (Table 5 and Figure 7B).
Discussion
Understanding how HSCs are generated, maintained, or fated to differentiate is of interest for their convenient clinical use.52 In the present study, we studied the role of the LYL-1 bHLH transcription factor in hematopoiesis by means of gene disruption. We report that Lyl-1–/– mice are viable and fertile. Adult Lyl-1–/– mice show seemingly normal hematopoiesis, except for a marked reduction in the number of B cells in PB, BM, and spleen and a reduced number of phenotypically characterized stem/progenitor cells. Characterization of the B-cell defect in Lyl-1–/– mice indicates a partial block in differentiation after the pro-B stage. Further studies indicate that the number of primitive progenitors (CFU-S12) is also reduced upon Lyl-1 disruption. Most important, competitive reconstitution experiments demonstrate that BM or E14 FL cells from mutant mice display a weakened ability to reconstitute short- and long-term hematopoiesis by affecting nearly exclusively the lymphoid lineages. Secondary transplantation experiments confirm both defects. These results point out an important role for Lyl-1 in hematopoiesis.
Chimerism analysis after competitive repopulation assay with Lyl-1–/– and Lyl-1+/+ BM cells. Ly5.2 BM Lyl-1+/+ (wt-5, wt-1, and wt-0.5, respectively) or Lyl-1–/– (KO-5, KO-1, and KO-0.5, respectively) cells (5 × 106, 1 × 106, and 5 × 105) were mixed with 1 × 106 Ly5.1 BM cells and transplanted into lethally irradiated C57B6 Ly5.1 mice. Diagrams represent the percentage of donor-derived cells in PB (A) and BM (B) of reconstituted mice (6 mice per group) at 4 and 24 weeks after injection. An example of 2 independent experiments is shown. Data represent mean ± SEM (*P < .05). The contribution of donor-derived cells to each reconstituted lineage is determined by analyzing the percentage of CD45.2+, B220+; CD45.2+, CD3+; and CD45.2+, GR-1/MAC-1+ cells in PB (C) and in BM (D). Data represent mean ± SEM of 2 independent experiments (*P < .05).
Chimerism analysis after competitive repopulation assay with Lyl-1–/– and Lyl-1+/+ BM cells. Ly5.2 BM Lyl-1+/+ (wt-5, wt-1, and wt-0.5, respectively) or Lyl-1–/– (KO-5, KO-1, and KO-0.5, respectively) cells (5 × 106, 1 × 106, and 5 × 105) were mixed with 1 × 106 Ly5.1 BM cells and transplanted into lethally irradiated C57B6 Ly5.1 mice. Diagrams represent the percentage of donor-derived cells in PB (A) and BM (B) of reconstituted mice (6 mice per group) at 4 and 24 weeks after injection. An example of 2 independent experiments is shown. Data represent mean ± SEM (*P < .05). The contribution of donor-derived cells to each reconstituted lineage is determined by analyzing the percentage of CD45.2+, B220+; CD45.2+, CD3+; and CD45.2+, GR-1/MAC-1+ cells in PB (C) and in BM (D). Data represent mean ± SEM of 2 independent experiments (*P < .05).
Analysis of the donor cell contribution after secondary transplantation of BM cells from primary animals grafted with Lyl-1+/+ or Lyl-1–/– BM cells. (A) Analysis of chimerism measured by the percentage of CD45.2+ cells in the blood after secondary transplantation. Secondary Ly5.1 recipient mice were injected with the same ratio as in primary graft (wt-5, wt-1, wt-0.5, KO-5, KO-1, and KO-0.5) of sorted Ly5.2 primary BM cells, and global chimerism was analyzed in PB 4 and 24 weeks after secondary transplantation. (B) The contribution of donor-derived cells to each reconstituted lineage is determined by analyzing the percentage of CD45.2+, B220+; CD45.2+, CD3+; and CD45.2+, GR-1/MAC-1+ cells in the PB of secondary recipients. Data represent mean ± SEM of 2 independent experiments (*P < .05).
Analysis of the donor cell contribution after secondary transplantation of BM cells from primary animals grafted with Lyl-1+/+ or Lyl-1–/– BM cells. (A) Analysis of chimerism measured by the percentage of CD45.2+ cells in the blood after secondary transplantation. Secondary Ly5.1 recipient mice were injected with the same ratio as in primary graft (wt-5, wt-1, wt-0.5, KO-5, KO-1, and KO-0.5) of sorted Ly5.2 primary BM cells, and global chimerism was analyzed in PB 4 and 24 weeks after secondary transplantation. (B) The contribution of donor-derived cells to each reconstituted lineage is determined by analyzing the percentage of CD45.2+, B220+; CD45.2+, CD3+; and CD45.2+, GR-1/MAC-1+ cells in the PB of secondary recipients. Data represent mean ± SEM of 2 independent experiments (*P < .05).
Lyl-1 inactivation primarily affected reconstitution of the B and T lineages in the blood and in the BM of primary and secondary recipients. It is possible that the specific role of Lyl-1 in B cells contributes to the defect in lymphoid reconstitution and is responsible for the partial impairment of differentiation after the pro-B stage. In contrast, it has been reported that T cells do not express Lyl-1 mRNA.23 Surprisingly, the production of T cells appeared to be affected to an extent comparable to that of B cells in reconstituted mice, whereas it was unaffected in Lyl-1–/– control mice. These results suggest that the impaired T lymphopoiesis in mice engrafted with Lyl-1–/– cells must stem from functional defects either in HSCs or later during differentiation, even if the number of phenotypically characterized CLPs appeared normal in Lyl-1–/– mice. Conversely, the block at the pre-B or immature B stage in Lyl-1–/– mice may reflect a distinct defect. It could come as a surprise that a defect occurred specifically in reconstituted mice but not in control mice entirely devoid of Lyl-1. However, the requirement for some genes can arise from competition effects and, hence, is most obviously (or even only) revealed in chimeric animals when mutant and wt cells are simultaneously present. This has been strikingly illustrated in Drosophila wing cell mutants for the bHLH protein Myc.53 Given that our experiments showing impaired lymphoid reconstitution cells are based on a competition between wt and Lyl-1–/– cells, it is conceivable that HSCs and CLPs lacking Lyl-1 are somehow “outcompeted” by their wt counterparts. We can hypothesize that this major defect of lymphoid reconstitution in Lyl-1–/– mice may be related to a lack of compensation of LYL-1 function by SCL because SCL is not expressed in lymphoid cells.43,44
Cell cycle analysis of Lin– and LSK cell population from BM and E14 FL of Lyl-1–/– mice. (A) Cell cycle of BM cells was analyzed with DNA dye Hoechst (Ho) and RNA dye pyronin (PY). Lin– cells were sorted and stained with Ho and PY and then were stained with anti–Sca-1–FITC and anti–c-Kit–APC. Shown are representative flow cytometric profiles of 5 independent experiments. (B) Cell cycle of E14 FL cells was analyzed using BrdU dye. Lin– and LSK cells were sorted and stained with BrdU for 1 hour (i-iv) and 48 hours (v-viii). Three independent experiments were performed with 3 mice per genotype.
Cell cycle analysis of Lin– and LSK cell population from BM and E14 FL of Lyl-1–/– mice. (A) Cell cycle of BM cells was analyzed with DNA dye Hoechst (Ho) and RNA dye pyronin (PY). Lin– cells were sorted and stained with Ho and PY and then were stained with anti–Sca-1–FITC and anti–c-Kit–APC. Shown are representative flow cytometric profiles of 5 independent experiments. (B) Cell cycle of E14 FL cells was analyzed using BrdU dye. Lin– and LSK cells were sorted and stained with BrdU for 1 hour (i-iv) and 48 hours (v-viii). Three independent experiments were performed with 3 mice per genotype.
Several defects may account for impaired HSC function. Our in vivo experiments indicate that the homing of BM and E14 FL cells is not affected by Lyl-1 deletion (data not shown). In addition, pyronin-Hoechst and BrdU staining experiments showed that mutant HSCs cycled like their wt counterparts. Similarly, an impaired release of the Lyl-1–/– HSC progeny out of the BM appears unlikely because the defect in lymphoid reconstitution was similarly observed in BM and in PB. In addition, we were unable to detect any increase in apoptosis of Lyl-1–/– HSCs.
Three lines of evidence suggest that Lyl-1 KO does not induce a pure lymphoid differentiation defect, but it also quantitatively affects the HSC compartment. First, marked reductions in CD34– LSKs and SP-LSKs, 2 cell populations closely related to LT-RCs, were found by immunophenotyping. Second, reduced numbers of CFU-S12 and LTC-IC were demonstrated by functional assay. Third, no contribution of Lyl-1–/– cells to hematopoiesis was observed in secondary hosts when a low number of cells (5 × 105) was injected. These results demonstrate that the compartment of stem/progenitor cells was quantitatively altered in Lyl-1–/– mice. The marked defect in the lymphoid potential of Lyl-1–/– HSCs during reconstitution may be associated with an increased potential for myeloid differentiation. Further studies using serial transplantations and limiting dilution experiments will be necessary to precisely elucidate the HSC defect induced by Lyl-1 gene disruption.
Given the close homology between Lyl-1 and scl and their shared implication in T-ALL, a comparison of their roles in hematopoiesis is of interest.25,26,44 Obviously, the essential role of scl in embryonic hematopoiesis is not shared by Lyl-1. Moreover, in committed cells, conditional KO experiments in mice after transplantation showed that scl was essential for the completion of megakaryocytic and erythroid differentiation,42 whereas the number of differentiated erythroid cells and platelets was normal in Lyl-1–/– mice. In fact, an inducible deletion of scl led to a complete absence of BFU-E in the BM of transgenic mice,54 whereas our methylcellulose experiments showed that the BFU-E number was increased in Lyl-1–/– mice, possibly as a result of the enhanced expression of scl (data not shown). Similarly, both Lyl-1 ablation and constitutive enforced scl expression impaired B-cell differentiation,55 again suggesting opposite roles for these 2 genes.
However, the scl and Lyl-1 roles in some cell types are not always completely distinct. Indeed, the number of CFU-S12 cells was also reduced 2-fold in both mutants.54 Moreover, although scl was initially reported to be dispensable in adult HSCs,42 a more recent study provided evidence that scl-deficient HSCs in BM had poor short-term reconstitutive ability. Interestingly, both studies with scl–/– mice used KO conditional experiments, but the phenotype was revealed when scl was ablated in BM cells before their engraftment into lethally irradiated mice, an experimental setting close to our reconstitution experiments.56 Finally, overexpression of scl in transgenic mice led to an expansion of the SP population in BM presumably endowed with HSC activity.44,57 These results suggest that scl and Lyl-1 play a related, though not fully redundant, role in adult HSCs, whereas scl performs unique functions in embryonic hematopoiesis and erythroid and megakaryocytic differentiation. Ongoing experiments using scl/Lyl-1 double KO mice will clarify the role of Lyl-1 and scl in immature hematopoietic cells, especially with respect to the control of their number.
At a molecular level, how can we interpret the distinct but possibly overlapping functions of these 2 related transcription factors? SCL is thought to induce erythroid terminal differentiation by acting as a transcriptional activator upon the recruitment of coactivators,25,58 although some steps of erythropoiesis can be promoted by an SCL protein unable to bind DNA.19,59 In contrast, in undifferentiated erythroleukemia cells and presumably in T-ALL,60 SCL is likely to dampen differentiation by acting rather as a transcriptional repressor, in part through the recruitment of corepressors61 and possibly through an Id (inhibitor of DNA binding)–like effect.62 Such an ambivalent role for SCL, which makes it a molecular switch endowed with antidifferentiation and prodifferentiation activities, is not unique in its subgroup of bHLH proteins, as exemplified by MyoD.63 The near-perfect similarity and functional interchangeability25,26 between the SCL and LYL-1 bHLH regions suggests that these 2 transcription factors have at least overlapping target genes in HSCs, where they may act as transcriptional repressors. However, in certain committed cells, SCL, but not LYL-1, would switch to transcriptional activation.58,61 Differences between the 2 proteins outside the bHLH domain may underlie this molecular specialization. The effects of LYL-1 on B-cell differentiation may be an exception to this rule. Indeed, the block at the pre-B stage we observed in Lyl-1–/– KO mice resembled the phenotype associated with the deletion of E47, a dimerization partner of LYL-1 harboring an activation domain.64 This suggests that one transactivating complex required for B cells to undergo differentiation beyond the pro-B stage contains the E47/LYL-1 proteins as a DNA-binding heterodimer. Future studies will further examine how Lyl-1 regulates the fate of B and hematopoietic stem cells in molecular terms.
Prepublished online as Blood First Edition Paper, March 2, 2006; DOI 10.1182/blood-2005-08-3145.
Supported by INSERM, the Association pour la Recherche sur le Cancer, the Fondation pour la Recherche Médicale, the Société Française d'Hématologie, the Deutsche Forschungsgemeinschaft, and the Wellcome Trust.
C.C. performed the experiments (murine transplantation, in vitro experiments, FACS experiments, mice breeding), analyzed the data, and wrote the manuscript; Y.L. was responsible for the FACS experiments and their analyses; O.A. and W.V. discussed and criticized the data and assisted in writing the paper and responding to reviewers; A.F., D.S., and I.G. helped to analyze data; C.L., A.L.K., I.P., and F. Svinartchouk performed many in vivo experiments; E.S. and F. Sablitzky generated the Lyl-1 knock-in mice; and A.B.-G. and D.D. supervised the work.
A.B.-G. and D.D. contributed equally to this study.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr M Serwe for his help in establishing the KO mice and Françoise Wendling for discussions and improvements to the manuscript, including English corrections. We thank Patrice Ardouin, Annie Rouches, and all the personnel of the animal facilities for their valuable help in maintaining the mice strains. We extend our special respects to Patrice Ardouin.